Enhanced Performance of TiO2 Composites for Solar Cells and Photocatalytic Hydrogen Production
Abstract
1. Introduction
2. Experiment
2.1. Materials
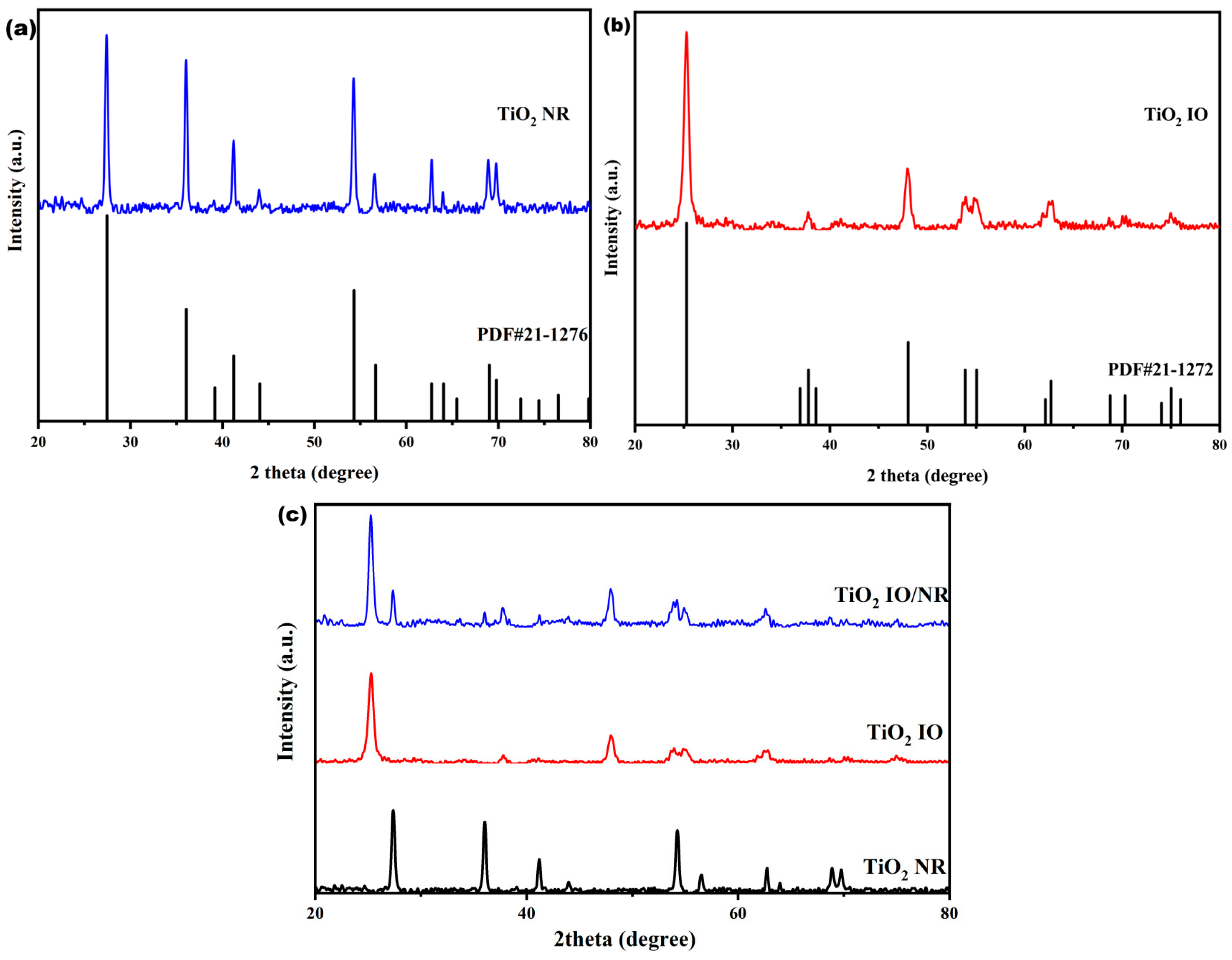
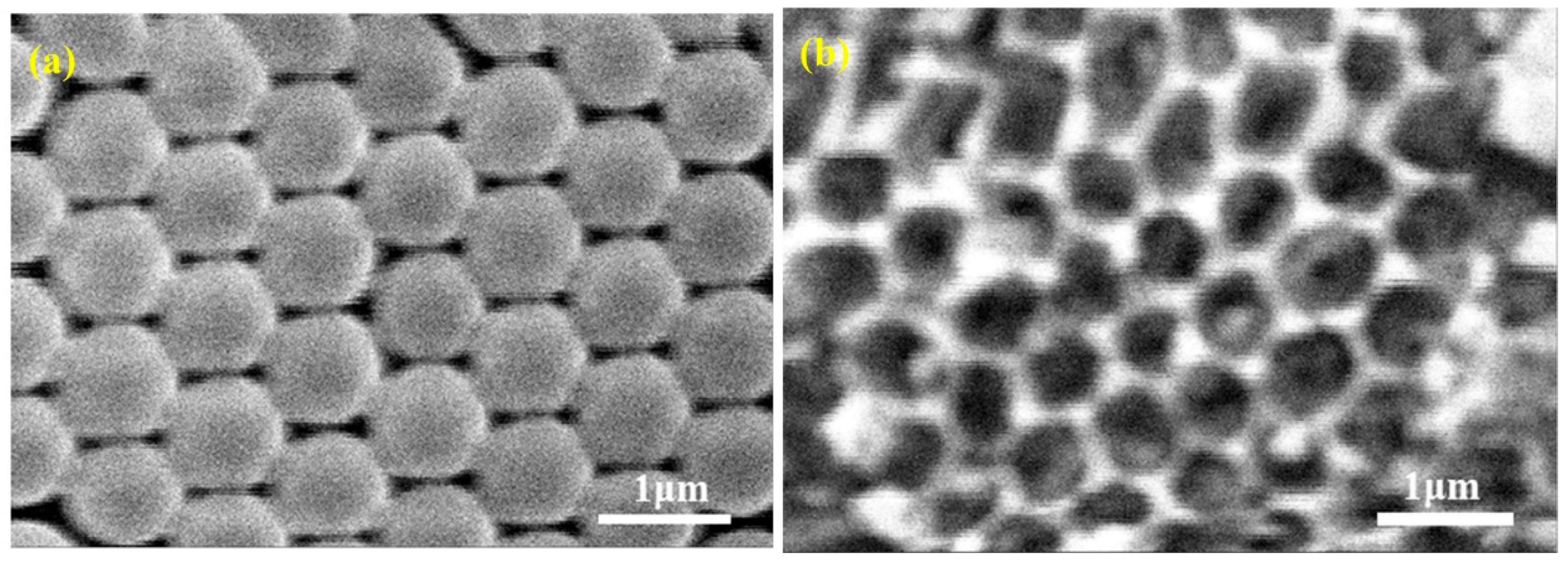
2.2. Preparation of TiO2 Inverse Opal
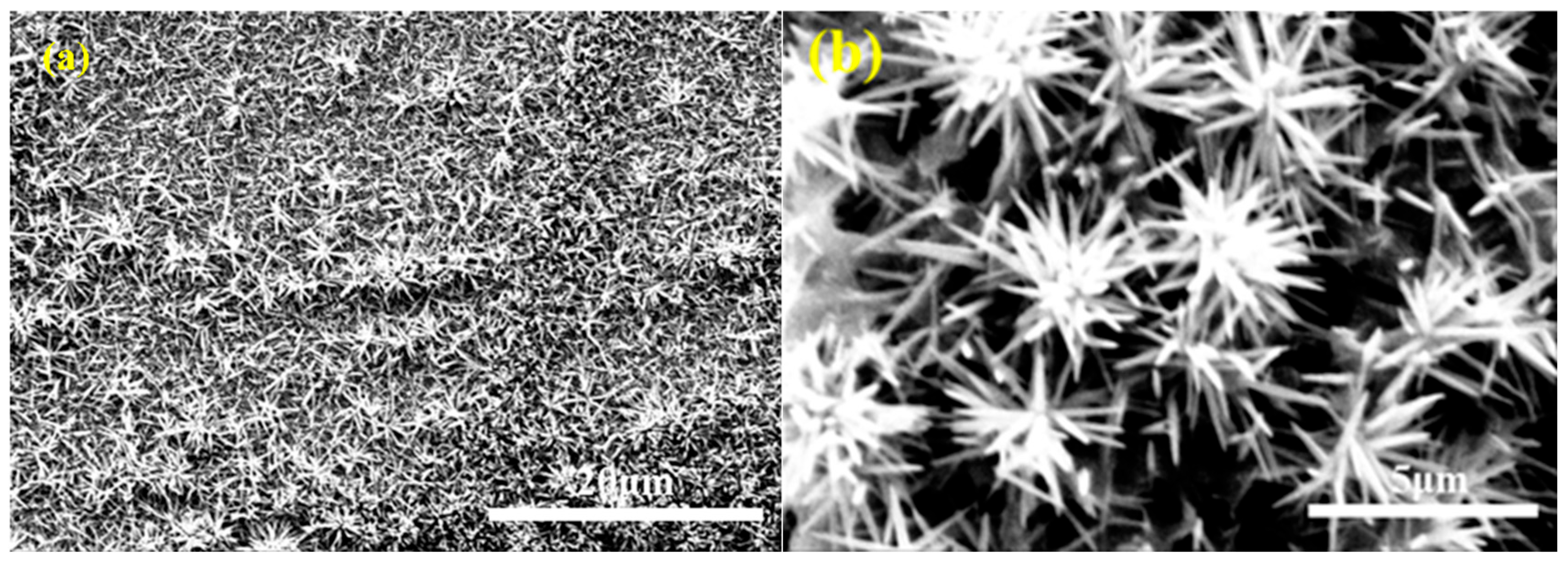
2.3. Preparation of TiO2 Nanorods
2.4. Deposition of Quantum Dots
2.5. Preparation of Counter Electrode
2.6. Assembly and Characterization of Solar Cells
2.7. Photocatalytic Hydrogen Generation
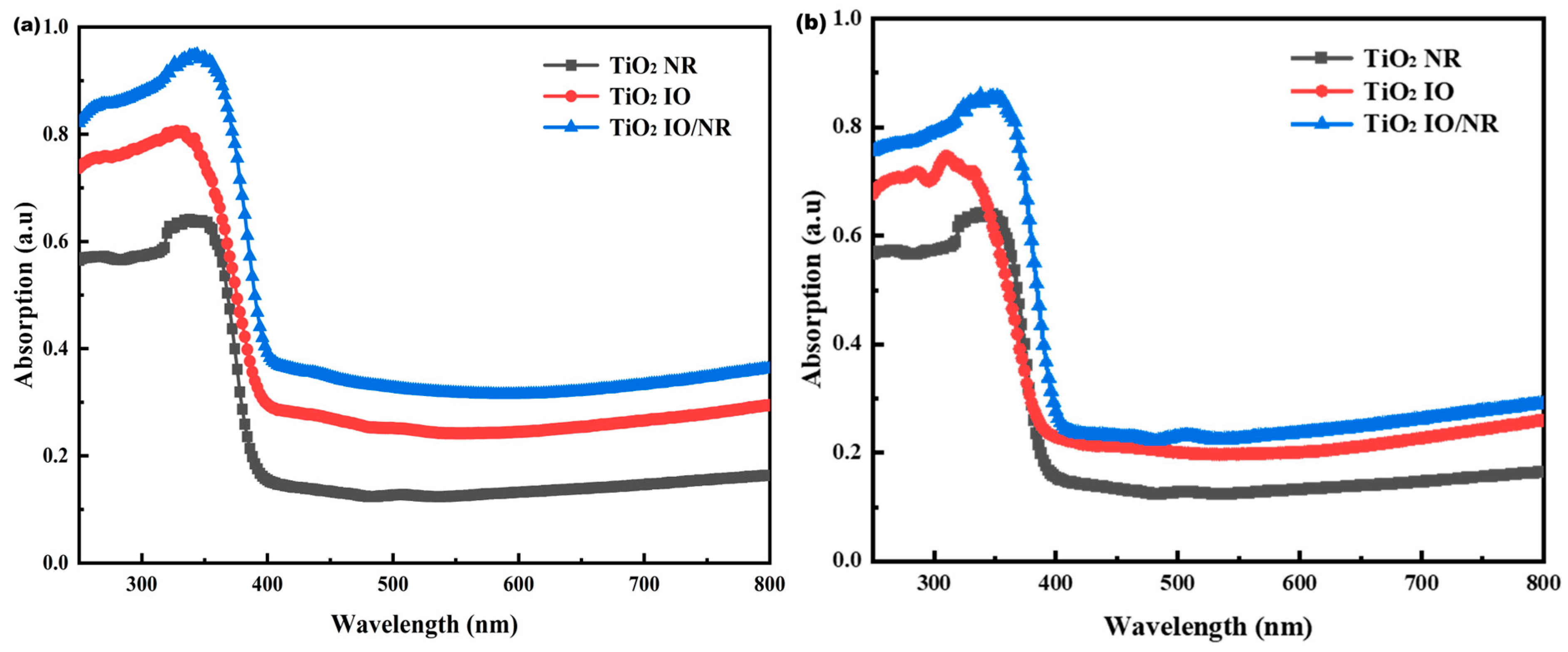
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSSC | Dye-sensitized solar cell |
| QDSSC | Quantum-dot sensitized solar cell |
| IO | Inverse opal |
| NR | Nanorod |
| PCE | Power conversion efficiency |
| QD | Quantum dot |
References
- Kokkonen, M.; Talebi, P.; Zhou, J.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; et al. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Chang, Y.C.; Zeng, C.J.; Chen, C.Y.; Tsay, C.Y.; Lee, G.J.; Wu, J.J. NiS/Pt loaded on electrospun TiO2 nanofiber with enhanced visible-light-driven photocatalytic hydrogen production. Mater. Res. Bull. 2023, 157, 112041. [Google Scholar] [CrossRef]
- Chang, W.; Wang, H.; Wu, T.; Zhang, S.; Li, Y.; Wang, J.; Yang, Y.; Wang, L. Interfacial lattice matched sub-2 nm RuO2 on rutile TiO2 nanorod for visible light driven CO2 conversion to CH4. J Mater. Sci. Technol. 2025, 235, 28–36. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, C.; Yang, C.; Wang, X.; Wan, Z.; Zhao, E.; Xiao, Y.; Zhao, W.; Ma, M.; Chen, D.; et al. A review on conductive polymers-modified TiO2 photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2025, 13, 116518. [Google Scholar] [CrossRef]
- Gao, D.; Xu, J.; Yu, H.; Liu, Y.; Yu, J. Hydroxyl-enriched highly crystalline TiO2 suspensible photocatalyst: Facile synthesis and superior H2-generation activity. Chem. Commun. 2021, 57, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- O'regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic acid pre-adsorption raises the efficiency of cosensitized solar cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency dye-sensitized solar cells by co-sensitizing novel thieno[3,2-b]indole-based organic dyes with a promising porphyrin sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]
- Chang, Y.; Han, W.; Cui, S.; Cai, A. Cellulose-inspired synthesis of hierarchically nanostructured TiO2 with high photocatalytic activity. Chem. Phys. Lett. 2020, 745, 137249. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2016, 26, 3262–3291. [Google Scholar] [CrossRef]
- Sánchez-Godoy, H.E.; López-Luke, T.; Zarazúa, I.; Herrera-Rodríguez, A.; Castañeda-Contreras, J.; Rodríguez-Rojas, R.A. Enhanced photovoltaic efficiency in TiO2-based CdS quantum dot-sensitized solar cells by the introduction of ZnO:Al3+ nanoparticles. Sol. Energy 2024, 281, 112818. [Google Scholar] [CrossRef]
- Zeng, H.H.; Rasal, A.S.; Abate, M.A.; Ghule, A.V.; Chang, J. Surface and interfacial engineering for aqueous-processed quantum dots-sensitized solar cell with efficiency approaching 11%. Chem. Eng. J. 2024, 495, 153702. [Google Scholar] [CrossRef]
- Sargin, I.; Yanalak, G.; Arslan, G.; Patir, I.H. Green synthesized carbon quantum dots as TiO2 sensitizers for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 21781–21789. [Google Scholar] [CrossRef]
- Song, H.; Lin, Y.; Zhang, Z.; Rao, H.; Wang, W.; Fang, Y.; Pan, Z.; Zhong, X. Improving the Efficiency of Quantum Dot Sensitized Solar Cells beyond 15% via Secondary Deposition. J. Am. Chem. Soc. 2021, 143, 4790–4800. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lin, Y.; Zhou, M.; Rao, H.; Pan, Z.; Zhong, X. Zn-Cu-In-S-Se Quinary “Green” Alloyed Quantum-Dot-Sensitized Solar Cells with a Certified Efficiency of 14.4%. Angew. Chem. Int. Ed. 2021, 60, 6137–6144. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Aydil, E.S. Growth of Oriented Single-Crystalline Rutile TiO2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef]
- Pawar, S.A.; Patil, D.S.; Lokhande, A.C.; Gang, M.G.; Shin, J.C.; Patil, P.S.; Kim, J.H. Chemical synthesis of CdS onto TiO2 nanorods for quantum dot sensitized solar cells. Opt. Mater. 2016, 58, 46–50. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Yao, M.; Yao, X. Band structure design of semiconductors for enhanced photocatalytic activity: The case of TiO2. Prog. Nat. Sci. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, Y.; He, Y.; Zhang, J.; Lin, K.; Song, W.; Yue, X.; Wang, D.; Wu, A.; Tian, C. Ag-Doped hollow Multi-Shelled structure TiO2 for highly selective photocatalytic CO2 reduction. J. Colloid Interface Sci. 2025, 694, 137684. [Google Scholar] [CrossRef]
- Wu, M.C.; Lin, T.H.; Hsu, K.H.; Hsu, J.F. Photo-induced disinfection property and photocatalytic activity based on the synergistic catalytic technique of Ag doped TiO2 nanofibers. Appl. Surf. Sci. 2019, 484, 326–334. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, L.; Liu, Y.; Gao, S.; Yu, X.; Xiong, Y. Double-layer TiO2 inverse opal-based quantum dot-sensitized solar cells. J. Solid State Electrochem. 2021, 25, 291–299. [Google Scholar] [CrossRef]
- Zhang, X.; John, S. Enhanced photocatalysis by light-trapping optimization in inverse opals. J. Mater. Chem. A 2020, 8, 18974–18986. [Google Scholar] [CrossRef]
- Senić, Ž.; Bauk, S.; Vitorović-Todorović, M.; Pajić, N.; Samolov, A.; Rajić, D. Application of TiO2 nanoparticles for obtaining self decontaminating smart textiles. Sci. Technol. Rev. 2011, 61, 63–72. [Google Scholar]
- Lee, Y.L.; Lo, Y.S. Highly efficient quantum-dot-sensitized solar cell based on co-sensitization of CdS/CdSe. Adv. Funct. Mater. 2009, 19, 604–609. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, H.; Cheng, K.; Hou, Y.; Hua, J.; Zhong, X. Highly efficient inverted type-I CdS/CdSe core/shell structure QD-sensitized solar cells. ACS Nano 2012, 6, 3982–3991. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, S.; Sayyad, M.H.; Khan, N.; Manzoor, T.; Nasr, N.; Toor, R.A.; Shah, S.A.A.; Guo, Z. Comparative photovoltaic and impedance spectroscopic study on carbon counter electrode based CdS quantum dot sensitized solar cell using polysulfide and iodide/triiodide as redox liquid electrolytes. Mat. Sci. Eng. B-Adv. 2021, 273, 115437. [Google Scholar] [CrossRef]
- Li, D.; Cheng, L.; Zhang, Y.; Zhang, Q.; Huang, X.; Luo, Y.; Meng, Q. Development of Cu2S/carbon composite electrode for CdS/CdSe quantum dot sensitized solar cell modules. Sol. Energy Mater. Sol. Cells 2014, 120 Pt B, 454–461. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, J.; Liu, F.; Wei, J.; Hu, L.; Dai, S. Metal chalcogenide complex-mediated fabrication of Cu2S film as counter electrode in quantum dot sensitized solar cells. Sci. China Chem. 2013, 56, 977–981. [Google Scholar] [CrossRef]
- Snaith, H.J. The perils of solar cell efficiency measurements. Nat. Photonics 2012, 6, 337–340. [Google Scholar] [CrossRef]
- Pazoki, M.; Cappel, U.B.; Johansson, E.M.J.; Hagfeldt, A.; Boschloo, G. Characterization techniques for dye-sensitized solar cells. Energy Environ. Sci. 2017, 10, 672–709. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X.; Jiang, J.Y. Solar Cell Efficiency Tables (Version 65). Prog. Photovolt. Res. Appl. 2025, 33, 3–15. [Google Scholar] [CrossRef]
- Snaith, H.J. How should you measure your excitonic solar cells? Energy Environ. Sci. 2012, 5, 6513. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; Gao, X.; Gan, X.; Weng, B.; Li, L.; Yuan, Z.; Shi, Z.; Hwang, Y.H. Branched double-shelled TiO2 nanotube networks on transparent conducting oxide substrates for dye sensitized solar cells. J. Mater. Chem. 2012, 22, 23411. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, L.; Gao, S.; Zheng, D.; Yu, X.; Wu, Q.; Yang, Q.; Zhu, D.; Yang, W.; et al. Highly efficient quantum-dot-sensitized solar cells with composite semiconductor of ZnO nanorod and oxide inverse opal in photoanode. Electrochim. Acta 2022, 412, 140145. [Google Scholar] [CrossRef]
- Osterloh, F.E.; Daemi, S.; Kundmann, A.; Becker, K.; Cheng, Y. Surface Photovoltage Spectroscopy on BiVO4, Gallium Phosphide, and CuGa3Se5 Photoelectrodes in Contact with Aqueous Electrolytes. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2022; p. 1575. [Google Scholar]
- Diguna, L.J.; Shen, Q.; Kobayashi, J.; Toyoda, T. High efficiency of CdSe quantum-dot-sensitized TiO2 inverse opal solar cells. Appl. Phys. Lett. 2007, 91, 023116. [Google Scholar] [CrossRef]
- Alavi, M.; Rahimi, R.; Maleki, Z.; Hosseini-Kharat, M. Improvement of Power Conversion Efficiency of Quantum Dot-Sensitized Solar Cells by Doping of Manganese into a ZnS Passivation Layer and Cosensitization of Zinc-Porphyrin on a Modified Graphene Oxide/Nitrogen-Doped TiO2 Photoanode. ACS Omega 2020, 5, 11024–11034. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; An, L.; Xue, G.; Li, X. Wavefunction engineering for efficient photoinduced-electron transfer in CuInS2 quantum dot-sensitized solar cells. Nanotechnology 2020, 31, 215408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.; Li, L.; Liu, S.; Li, J.; Lu, L.; Li, X.; Liu, W.; Liu, R.; Chen, J.; et al. Interfacial engineering of ZnS passivating contacts for crystalline silicon solar cells achieving 20% efficiency. Mater. Today Energy 2023, 35, 101336. [Google Scholar]
- Shin, J.H.; Kang, J.H.; Jin, W.M.; Park, J.H.; Cho, Y.S.; Moon, J.H. Facile synthesis of TiO2 inverse opal electrodes for dye-sensitized solar cells. Langmuir 2011, 27, 856–860. [Google Scholar] [CrossRef]
- Seo, Y.G.; Woo, K.; Kim, J.; Lee, H.; Lee, W. Rapid fabrication of an inverse opal TiO2 photoelectrode for DSSC using a binary mixture of TiO2 nanoparticles and polymer microspheres. Adv. Funct. Mater. 2011, 21, 3094–3103. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Garcia-Belmonte, G.; Boschloo, G.; Hagfeldt, A. Influence of electrolyte in transport and recombination in dye-sensitized solar cells studied by impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2005, 87, 117–131. [Google Scholar] [CrossRef]
- Zarazúa, I.; Esparza, D.; López-Luke, T.; Ceja-Fdez, A.; Reyes-Gomez, J.; Mora-Sero, I.; De la Rosa, E. Effect of the electrophoretic deposition of Au NPs in the performance CdS QDs sensitized solar cells. Electrochim. Acta 2016, 188, 710–717. [Google Scholar] [CrossRef]
- Chen, T.P.; Young, S.J.; Chang, S.J.; Hsiao, C.H.; Wu, S.L. Photoelectrical and low-frequency noise characteristics of ZnO nanorod photodetectors prepared on flexible substrate. IEEE Trans. Electron. Dev. 2012, 60, 229–234. [Google Scholar] [CrossRef]
- Wang, Q.; Ito, S.; Grätzel, M.; Fabregat-Santiago, F.; Mora-Seró, I.; Bisquert, J.; Bessho, T.; Imai, H. Characteristics of high efficiency dye-sensitized solar cells. J. Phys. Chem. B 2006, 110, 25210–25221. [Google Scholar] [CrossRef]
- Reli, M.; Nadrah, P.; Edelmannová, M.F.; Ricka, R.; Škapin, A.S.; Štangar, U.L.; Kočí, K. Photocatalytic CO2 reduction over mesoporous TiO2 photocatalysts. Mater. Sci. Semicond. Process. 2024, 169, 107927. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Formal, F.L.; Moon, S.J.; Cevey-Ha, L.; Humphry-Baker, R.; Grätzel, C.; Zakeeruddin, S.M.; Grätzel, M. Solid-State Dye-Sensitized Solar Cells using Ordered TiO2 Nanorods on Transparent Conductive Oxide as Photoanodes. J. Phys. Chem. C 2012, 116, 3266–3273. [Google Scholar] [CrossRef]

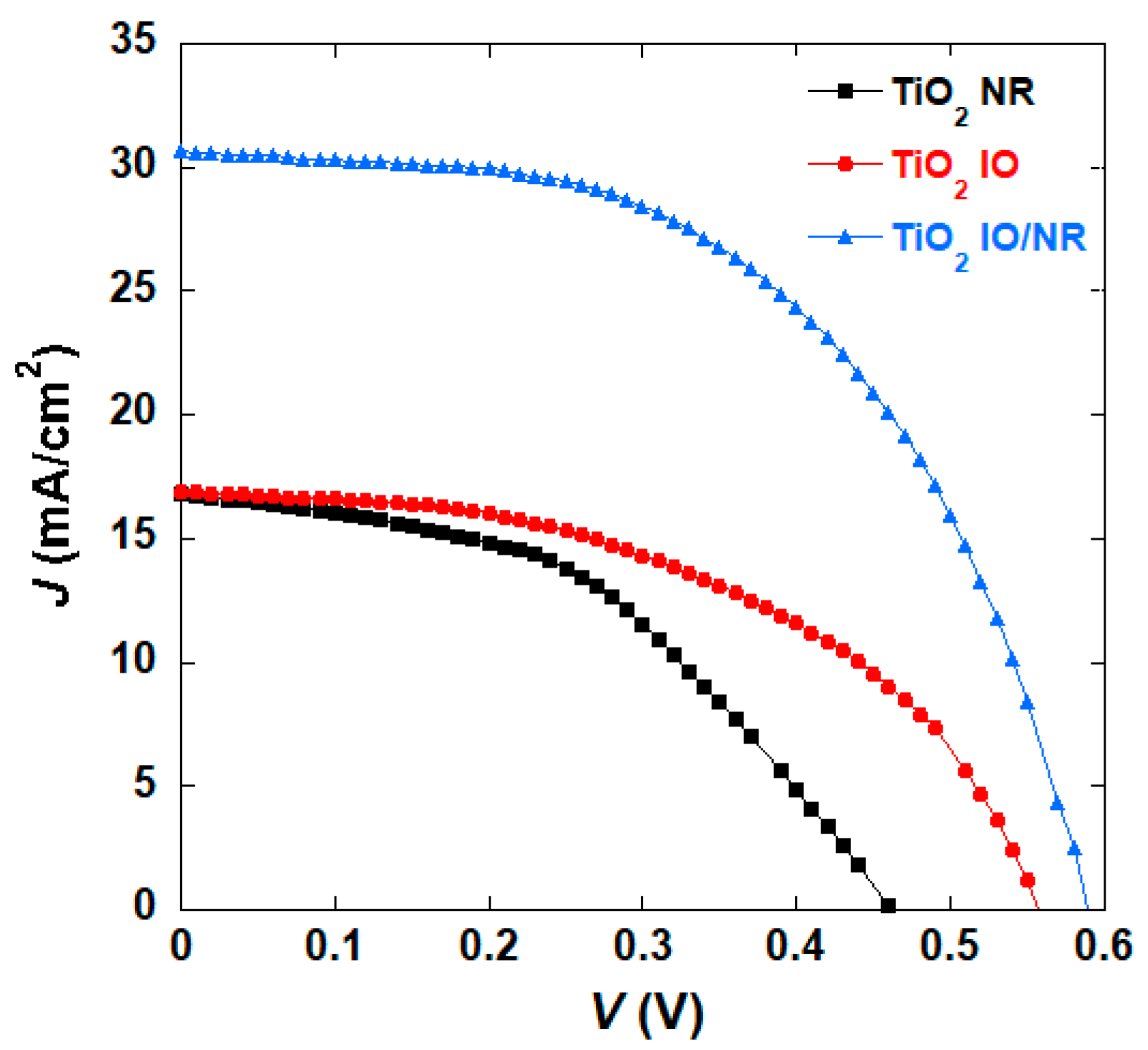
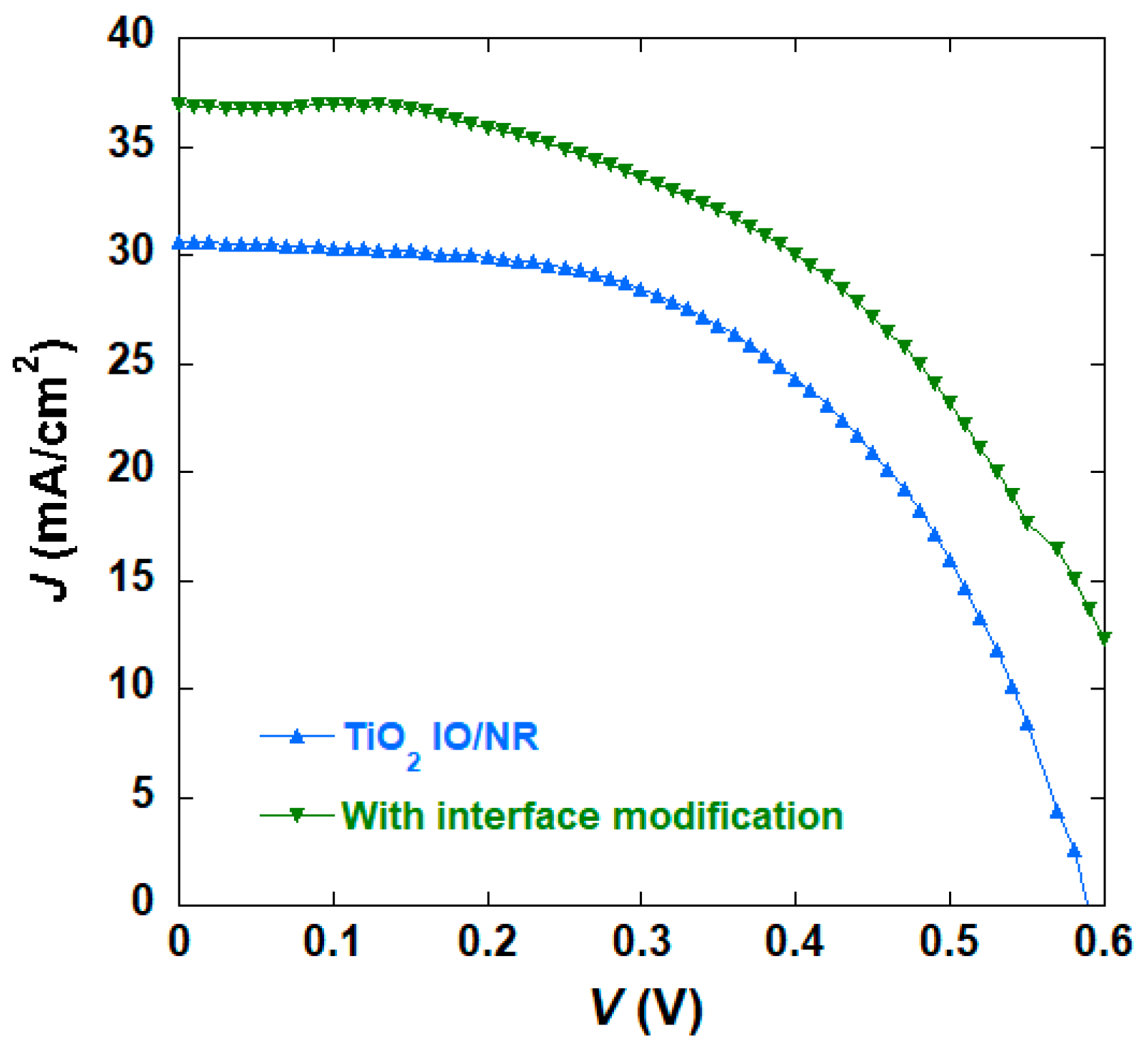


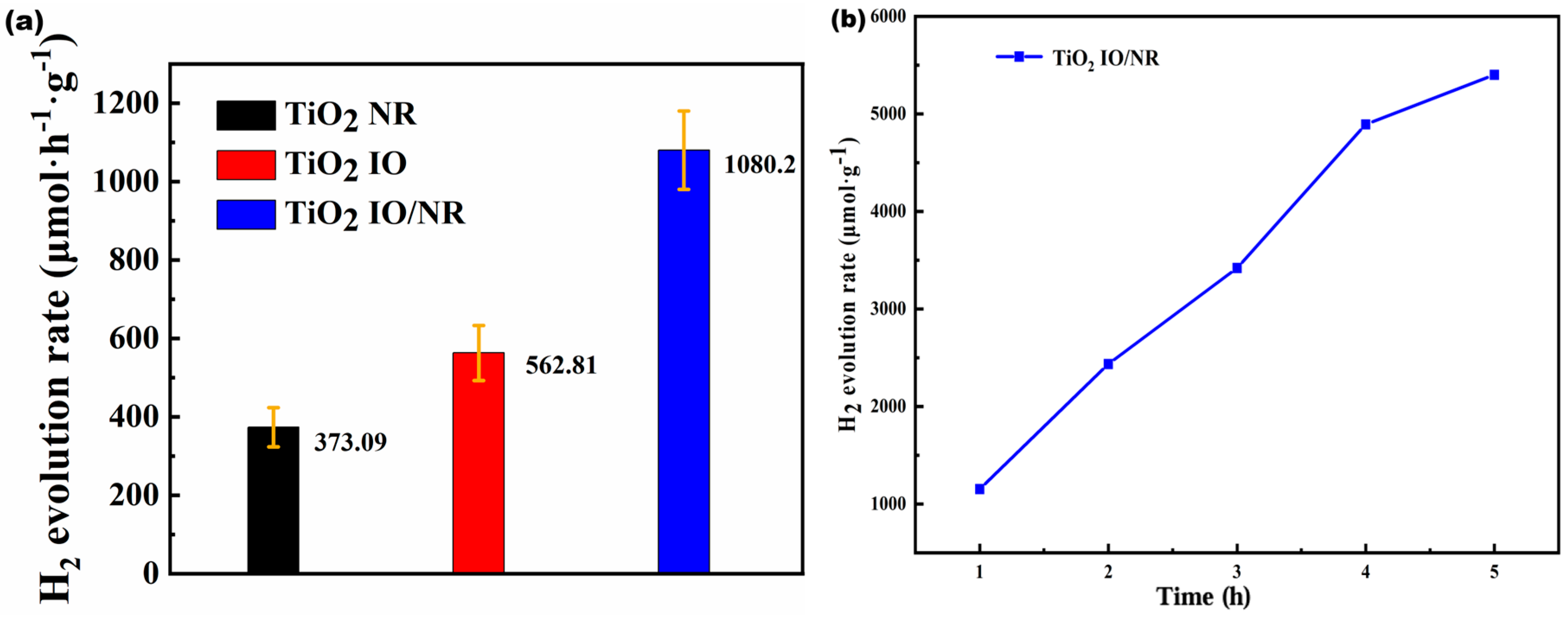
| TiO2 Morphology | Voc (V) | Jsc (mA/cm2) | η (%) | FF |
|---|---|---|---|---|
| NR | 0.47 | 16.77 | 3.54 (±0.5) * | 0.45 |
| IO | 0.56 | 16.89 | 4.64 (±0.7) | 0.49 |
| IO/NR | 0.59 | 30.64 | 9.73 (±1.0) | 0.54 |
| TiO2 Morphology | Rs (Ω) | Rct1 (Ω) | Rct2 (Ω) | τ (ms) |
|---|---|---|---|---|
| NR | 3.81 | 5.86 | 12.75 | 23.69 |
| IO | 1.70 | 2.83 | 7.80 | 60.13 |
| IO/NR | 1.26 | 0.75 | 5.03 | 47.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Chen, J.; Du, S.; Xiong, Y. Enhanced Performance of TiO2 Composites for Solar Cells and Photocatalytic Hydrogen Production. Nanoenergy Adv. 2025, 5, 14. https://doi.org/10.3390/nanoenergyadv5040014
Bai X, Chen J, Du S, Xiong Y. Enhanced Performance of TiO2 Composites for Solar Cells and Photocatalytic Hydrogen Production. Nanoenergy Advances. 2025; 5(4):14. https://doi.org/10.3390/nanoenergyadv5040014
Chicago/Turabian StyleBai, Xue, Jian Chen, Shengxi Du, and Yan Xiong. 2025. "Enhanced Performance of TiO2 Composites for Solar Cells and Photocatalytic Hydrogen Production" Nanoenergy Advances 5, no. 4: 14. https://doi.org/10.3390/nanoenergyadv5040014
APA StyleBai, X., Chen, J., Du, S., & Xiong, Y. (2025). Enhanced Performance of TiO2 Composites for Solar Cells and Photocatalytic Hydrogen Production. Nanoenergy Advances, 5(4), 14. https://doi.org/10.3390/nanoenergyadv5040014





