Abstract
Fatigue-related changes in gait biomechanics, specifically plantar pressures, are well documented in the general population. However, research is generally confined to unilateral measures across a limited range of speeds, while changes in more well-trained populations remain largely unknown. Therefore, we sought to assess the impact of fatigue on bilateral peak plantar pressure (PP) and plantar pressure symmetry angle (SA) in well-trained runners across a range of speeds. Data from 16 (females, n = 9) well-trained runners were collected using in-sole pressure sensors pre- and post-fatigue at the following speeds: walking (1.3 m/s), jogging (2.7 m/s), running (3.3 m/s), and sprinting (4.5 m/s). Pre-fatigue PP significantly increased from walking to jogging (p < 0.001) and from jogging to running (p < 0.005) with no difference between running and sprinting (p > 0.05). Post-fatigue PP for walking was less than jogging (p < 0.002), running (p < 0.001), and sprinting (p < 0.001), with no other significant differences (p > 0.05). Post-fatigue PP was significantly greater when compared to pre-fatigue PP at all speeds (p < 0.001 for all). Though SA was not significantly different pre- to post-fatigue across speeds (p’s > 0.05) at the cohort level, noteworthy changes were observed at the individual level. Overall, fatigue effects are present at all running speeds but isolating these effects to a single side (left or right) may be inadequate.
1. Introduction
Running is a common element amongst sports such as soccer, football, rugby, and basketball, and plays an important role during in-match tactics as well as in physical preparedness for competition [1]. Generally, such sports are characterized by intermittent bouts of running at velocities ranging from walking and jogging to near-maximum velocity sprinting. Throughout the course of a game or training event, fatigue naturally occurs leading to a cascade of physiological and biomechanical phenomena. Fatigue has been shown to alter normal arthrokinematics associated with running which can result in tissue overload and ultimately overuse injury [2,3,4,5]. Accordingly, repeated acute high-fatigue states are considered a major risk factor for overuse injury [5,6,7,8,9] and are therefore important to appropriately manage. Detecting fatigue-related changes earlier to potentially adjust technique or training load, thus mitigating the risk of injury, would be ideal for athletes and coaches [10]; however, accurate objective measures are challenging to perform when considering real-time applications.
In-sole pressure sensors, which work much like force plates by detecting the amount of pressure the foot exerts over the contact area, present as an appealing alternative to more expensive and less portable lab-based technologies for assessing changes in gait biomechanics [11]. Recently, fatigue due to long distance and ramp-style running [12,13,14,15,16] as well as long duration standing [11] has been shown to increase peak plantar pressures (PP) as well as to alter the distribution in novice and trained runners. Additionally non-directional fatigue-induced changes in symmetry angle (SA), a metric derived from pressure sensors used to quantify left–right asymmetries in plantar pressures, have been correlated with increased loads at the hip, knee, and ankle joints in amateur runners [17,18]. Changes in plantar pressure-related variables, such as peak pressure (PP) and SA, are consistently associated with known factors predictive of injury or precursors to running-related injuries [17,19,20,21,22]; however, in healthy athletes, changes in pressure-related variables and symmetry due to fatigue and their effect across a range of speeds are unknown.
Within the existing body of literature, only a few studies such as Eldin and Mattes [13] evaluate fatigue effects at multiple running speeds. Studies reporting both peak pressure and bilateral symmetry across a range of speeds are largely unknown. Fatigue effects on plantar pressure distribution often focus on changes in the left or right foot, without reporting how fatigue may re-distribute loads across the sagittal plane due to changes in symmetry. Thus, the purpose of this study was to determine the effect of fatigue on peak pressure (PP) and symmetry angle (SA) at multiple gait speeds in young well-trained athletes. We hypothesized that PP would increase with increasing speed and fatigue, and that SA would be non-directionally altered. This analysis will lend insight into changes in PP due to accumulated fatigue across gait speeds often attained in athletic competition and training. The results will better inform athletes and coaches making training and competition-related decisions and add to the growing body of research surrounding fatigue and biomechanics.
2. Materials and Methods
2.1. Experimental Approach to Problem
A within-subjects study design was used to assess changes in foot pressure measurements during walking, jogging, running, and near maximum running speed (sprinting) before and after a fatigue-inducing treadmill (FI-TM) running protocol. Following familiarization to all equipment and procedures, subjects reported to the laboratory and were fitted with in-sole foot pressure sensors (3000 Sport-E 125, Tekscan, Boston, MA, USA) and a heart rate (HR) monitor (Polar Electro, Kempele, Finland). Baseline data (pre-fatigue) were collected for 60 s at four TM speeds: (1) walking (W) = 1.3 m/s (2.8 mph); (2) jogging (J) = 2.7 m/s (6 mph); (3) running (R) = 3.3 m/s (7.4 mph); (4) sprinting (S) = 4.5 m/s (10 mph). Immediately following baseline measurements, the in-sole pressure sensors were removed, and subjects completed a ramp-style fatiguing treadmill running protocol followed by a two-minute cool down. Afterwards, the in-sole pressure sensors were placed back in the subject’s shoes and the subject repeated data collection at the four TM speeds (post-fatigue) using identical procedures as baseline measures. Blood lactate was assessed at pre- and post-fatigue. All procedures were approved by Grove City College IRB (#111-2021) prior to implementation and all testing occurred in the Exercise Science Laboratory of Grove City College.
2.2. Subjects
Participants were recruited through fliers and word of mouth and considered for this study if they met the following inclusion criteria: (1) currently running between 10 and 30 miles per week; (2) between the ages of 18 and 35 years; and (3) running 3–4 times per week (at moderate to high intensity) for at least 1 year. Exclusion criteria included the following: (1) current smoker; (2) presence of any known metabolic or cardiovascular disease; (3) presence of any orthopedic, musculoskeletal, neurological, psychiatric disorder, and/or any medical conditions that prohibit or limit exercise. Participants were instructed to refrain from caffeine and alcohol consumption for 48 h, racing or training for 24 h, and food and drink for 3 h before testing. All participants were made aware of the risks and benefits of participation prior to providing their written informed consent. Participants used their own running shoes including any foot orthosis.
2.3. Procedures
2.3.1. Familiarization Session
During the familiarization session, all experimental procedures were explained and participants were then oriented to the treadmill, heart rate monitors, blood lactate collection procedures, and the Borg 6–20 [23] rating of perceived exertion scale. Following the orientation, anthropometric measures were obtained including height (cm) using a standard physician’s scale (Detecto, Webb City, MO, USA) and mass (kg), fat-free mass (kg), and fat mass (% and kg) using a Tanita bioelectrical impedance analyzer (BIA) (TBF-310GS Tanita Corporation of America, Arlington Heights, IL, USA). Lower limb segment lengths for the right upper thigh, lower leg, and foot were then measured using a flexible tape measure. Lastly, in-sole foot pressure sensors (3000 Sport-E 125, Tekscan, Boston, MA, USA) were trimmed and fitted to the participant’s running shoes.
2.3.2. Experimental Session
Pre-trial. Participants were fitted with the in-sole foot pressure sensors (3000 Sport-E 125, Tekscan, Boston, MA, USA) and associated data logger (F-scan VersaTek Datalogger, Tekscan, Boston, MA, USA). Participants were also fitted with heart rate monitor (Polar Electro, Kempele, Finland). Baseline blood lactate (BL) was then assessed using capillary finger-tip samples and a blood lactate analyzer (Lactate Plus, Nova Biomedical, Waltham, MA, USA). Fingertip blood samples were collected using a lancet following cleaning with alcohol and allowing to air dry. The first droplet was wiped away with a cotton swab and the subsequent droplets were used for analysis.
In-sole pressure sensors. The sensors used in this study are the Tekscan 3000SE with associated data acquisition hardware and software. The system provides a maximum pressure capability of 862 kPa with pressure resolution of 4.8 kPa. The spatial resolution of individual sense elements is 3.9 sensors/cm2. The in-sole sensors were calibrated prior to testing following the vendor protocol. Each sensor was trimmed to the subject shoe, and then calibrated with the subject standing in the shoe. All data were collected at 400 Hz sampling rate. An earlier model Tekscan sensor has been studied for accuracy and reliability [24]. The reliability of the specific sensor system used here was tested in treadmill walking trials by Patrick and Donovan [25]. The authors segmented the foot into different regions and studied peak pressure in each region. In the foot regions where we recorded peak pressure values (heal, fore-foot, toe), Patrick and Donovan report intraclass correlation coefficients (ICCs) from 0.72 to 0.88, but lower (0.50) in the midfoot. None of the peak pressure data reported here come from the mid-foot, meaning we compare data only from the higher ICC values. This repeatability allows a comparison of pre-to-post fatigue changes reported here. During the 60 s of data collection, the in-sole sensors recorded between 50 and 90 steps for walking to sprinting speed. For clarity, we emphasize that the sensors measure the pressure across the plantar surface in real time. We report the average of peak pressure values observed from repeated steps. Because of the large number of steps, the 95% confidence interval for peak pressure was small, typically less than +/−5% of the measured mean value.
The sensors do have limited durability, and some of the hundreds of sensing elements would fail (open circuit) during a test. For every subject test condition, the plantar pressure distribution was inspected after testing to insure that reported peak pressures were not affected by open-circuit elements. As seen later, some subject data were excluded because of this issue.
While the plantar pressure can be related to the vertical ground reaction force [26,27], this is not considered in this study.
Pre-fatigue measurements. Pre-fatigue baseline measurements for foot pressure were collected for 60 s at walk, jog, run, and sprint speeds (W, J, R, S) defined earlier and based on similar work [13,16]. Participants were instructed to straddle the TM after each successful data acquisition while the investigators increased the TM speed to the next testing pace. Participants walked/ran for ~5–10 s at each testing speed prior to data collection to allow their gait pattern to acclimate to the TM speed. Following completion of all four testing speeds, participants stepped off the TM for removal of the in-sole pressure sensors and datalogger to prepare for the fatiguing protocol.
Fatiguing protocol. After baseline measures and removal of pressure sensor technology, participants completed a fatiguing protocol similar to the method used by Hamzavi and Esmaeili [16]. Participants began by running at a speed of 3.0 m/s (6.7 mph) and 0% grade. The speed was increased every 2 min by 0.2 m/s (0.5 mph) and RPE was assessed using the Borg 6–20 scale [23]. Speed increased until participants reached an RPE of 13 and this pace was maintained until an RPE of 17 was reported or 80% of their age-predicted maximum HR was attained. At this point participants continued running for an additional 2 min at this pace. Participants were then instructed to straddle the TM while blood lactate was measured using procedures described earlier. Heart rate at cessation of the fatiguing protocol was recorded. Participants then cooled down on the TM at a self-selected pace for 2 min.
Post-fatigue measurements. Following the fatigue protocol, participants were re-fitted with the foot pressure sensors and repeated data collection procedures at each of the four speeds as described in the pre-fatigue baseline measurements.
2.3.3. Data Analysis: Peak Pressure and Symmetry Angle
The Tekscan in-sole sensors measure plantar pressure distribution in real-time as the subject runs. In this study, data were recorded at 400 Hz to ensure fidelity in capturing short peak pressures. Recordings were post-processed to identify the peak pressure in each stance using Tekscan software (F-scan Research version 7.55-02). The software identifies the peak pressure that occurred during each stance, and then averages peak pressures from each stance during the 60 s recordings. The averaging process typically used 50 to 100 recorded stances for the range of speeds: walking, jogging, running, and sprinting. Left and right peak pressures (approximately 50 steps per foot) were averaged for analysis.
A symmetry angle (SA) was calculated for each participant using pre-and post-peak pressure for each foot (approximately 50 steps for each foot) with the following equation (Zifchock, et al. [19]):
In this equation, X is peak pressure from the left foot or the right foot. Noting that peak pressures are positive numbers, the symmetry angle ranges from −50% (left bias, large Xleft/Xright) to +50% (right bias, small Xleft/Xright). Perfect symmetry is 0% SA. It is helpful to visualize the symmetry angle from a vector plot of Xright, Xleft on an x–y graph as discussed by Zifchock et al. [19].
2.4. Statistical Analysis
Statistical analyses were performed using SAS Enterprise Guide 7.15 (SAS Institute Inc, Cary, NC, USA) for comparisons. Normality was assessed for continuous variables; either parametric t tests or nonparametric Wilcoxon signed-rank tests were conducted. Simple and multivariable linear regression on peak pressure (dependent) was used to determine associations with variables such as participant demographic characteristics (e.g., sex), body metrics (e.g., fat%, upper leg length), training metrics (e.g., distance run/week), and clinically relevant fatigue characteristics (e.g., blood lactate). Probability (p) < 0.05 was considered statistically significant in all statistical tests. Variables eligible for inclusion in the multivariable models included those with p < 0.25 in the univariate analysis. Multivariable linear regression analyses were conducted to determine not only which independent covariates are statistically significant with the dependent peak pressure, but also the strength of association between the independent covariates and the dependent peak pressure.
3. Results
3.1. Participant Characteristics
A total of 19 participants were recruited to participate in the study between February 2022 and April 2022. Of the 19 participants, data from 3 participants were excluded due to issues with F-Scan in-sole sensors. The median age of the cohort was 19 years and 9 out of 16 participants were female (56%) (Table 1). Overall BMI, fat free mass, fat mass, and fat % along with body measurements are outlined in Table 1. The participants were all experienced runners having participated in the sport a median of 7 years and running a median of 24.14 km over 5 days per week.

Table 1.
Cohort (N = 19) characteristics including demographics, body metrics, training metrics, and clinically relevant measures. Values are median (IQR) or frequency (%). BMI = body mass index; IQR = interquartile range (25–75%).
3.2. Peak Pressure
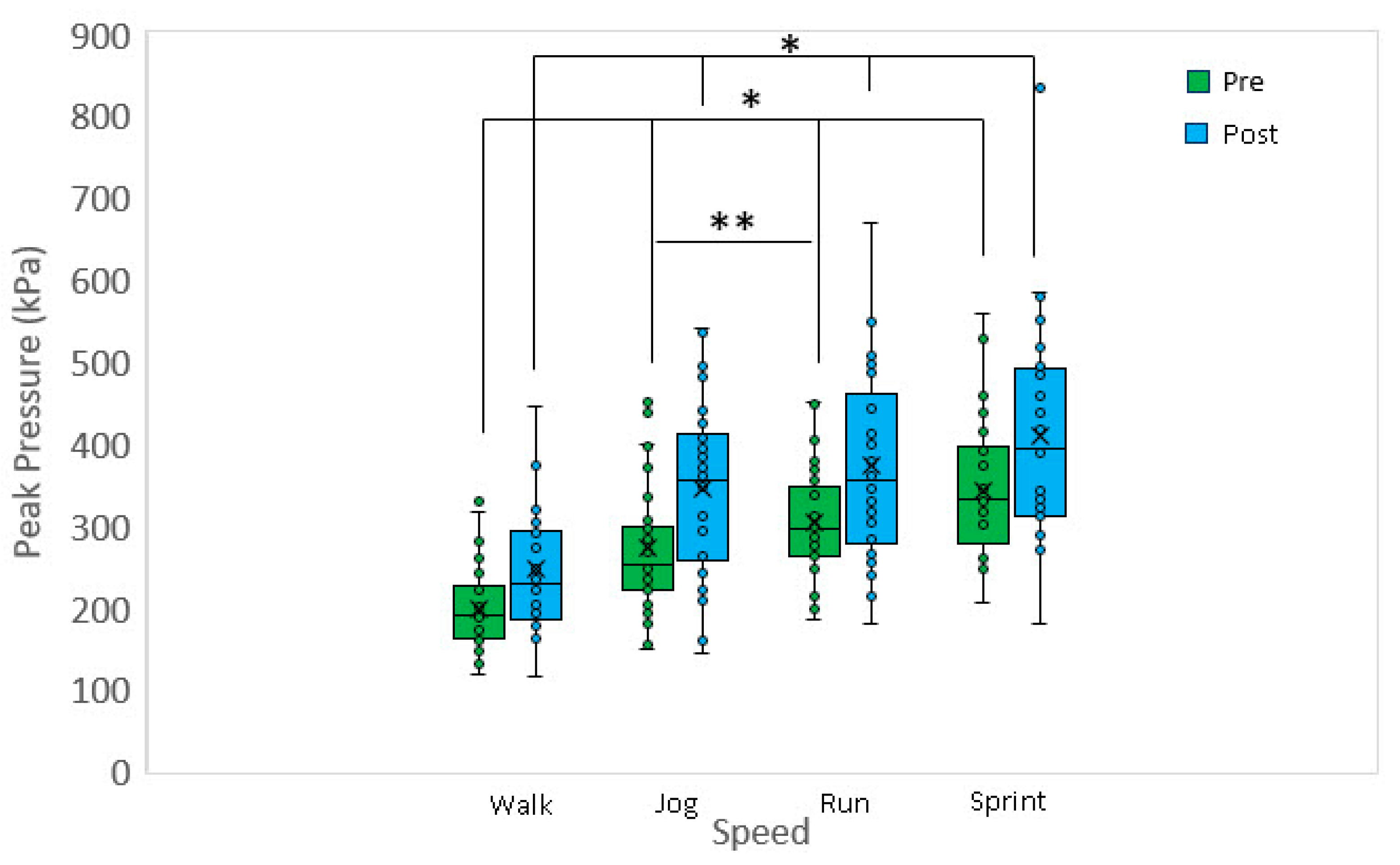
Approximately 50 to 100 recorded stances for the range of speeds: walking, jogging, running, and sprinting for both the left and right feet were averaged for calculating PP. Figure 1 shows a boxplot of the peak pressure data for different speeds and pre-/post-fatigue. At each speed, the post-fatigue peak pressure was greater when compared to the pre-fatigue peak pressure (p < 0.001 for all). Considering the pre-fatigue data, walking peak pressure was significantly less when compared to jogging (p < 0.001), running (p < 0.001) and sprinting (p < 0.001). A significant difference was also detected between jogging and running (p < 0.005), but no differences were detected between running and sprinting. Considering the post-fatigue data, walking peak pressure was significantly less when compared to jogging (p < 0.002), running (p < 0.001), and sprinting (p < 0.001). However, no other significant differences in post-fatigue peak pressures were detected versus speed (p > 0.5 for all). Fatigue did not lead to a substantial change in cadence and appears not to be a covariate. Post-fatigue cadence was typically within +/−2% of the pre-fatigue condition.
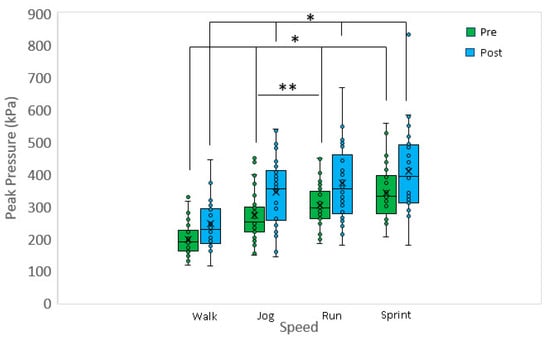
Figure 1.
Pre-fatigue peak pressures at jog, run, and sprint are significantly greater when compared to walk. Additionally, pre-fatigue peak pressure at run was significantly greater when compared to jog. Post-fatigue peak pressures at jog, run and sprint are significantly greater when compared to walk. Walk: 1.3 m/s, jog: 2.7 m/s, run: 3.3 m/s, sprint: 4.5 m/s. * p < 0.001, ** p < 0.005.
3.3. Symmetry Angle %
Table 2 shows the pre- and post-fatigue peak pressure symmetry angles (SA%) compared at each speed. The results showed no statistically significant difference in pre- versus post-SA% at all speeds (p > 0.05). Additionally, we evaluated both SA% pre- and SA% post-fatigue between speeds and found no significant differences between speeds (e.g., SA% walk pre-fatigue vs. SA% jog pre-fatigue) (p > 0.5 for both pre- and post-fatigue speed comparisons).

Table 2.
SA% in pre- vs. post-fatigue at four different speeds (walking, jogging, running, sprinting). Values are median (IQR). N = 16. a = p value by Wilcoxon signed-rank test. IQR = interquartile range.
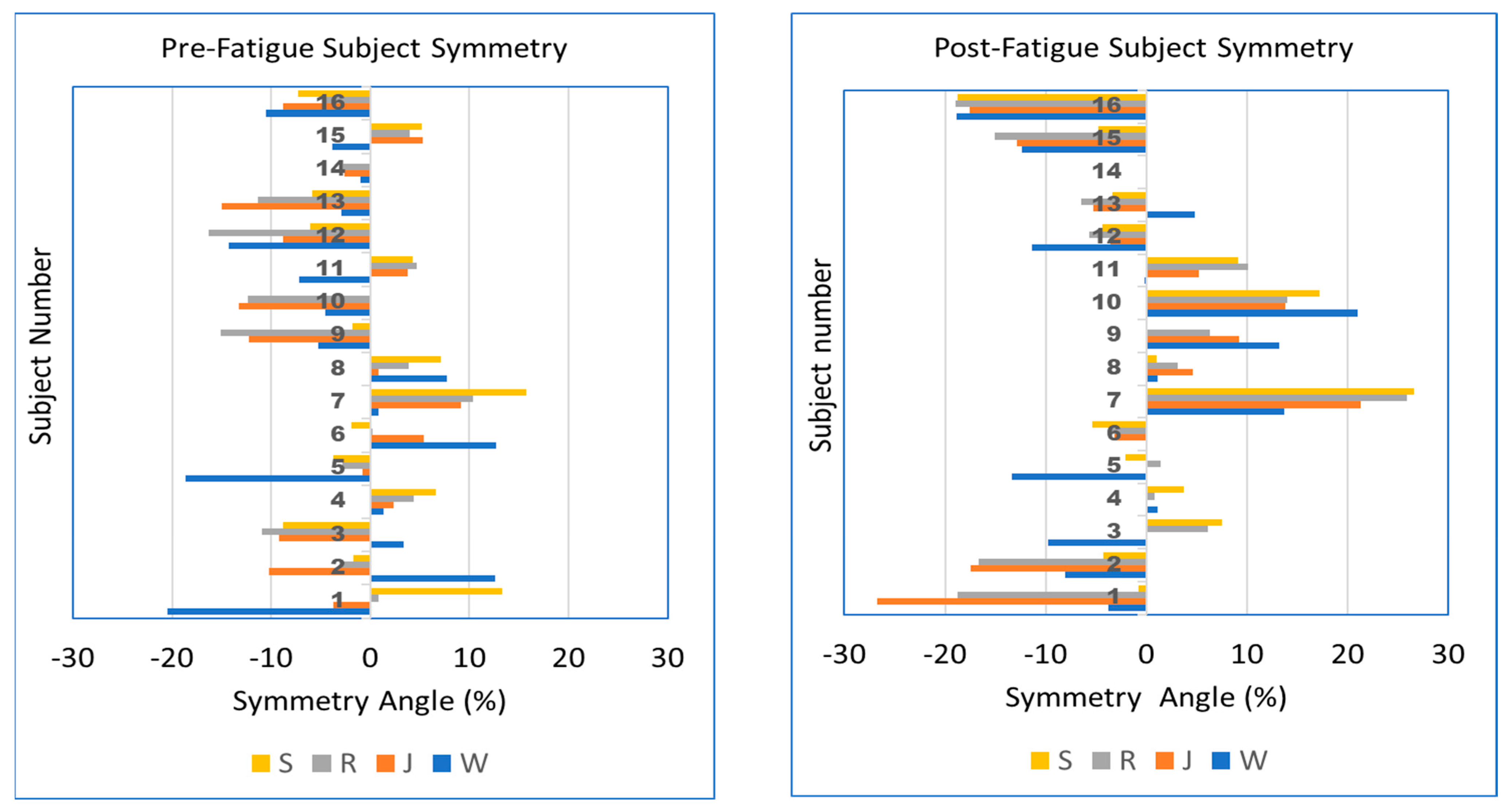
Although our results show little effect of either speed or fatigue on the median peak pressure SA% for the cohort, a graphical representation of SA% for individual participants allows a better understanding of right- vs. left-sided bias and how individual symmetry changes with speed and fatigue. This comparison is shown in Figure 2. The plots show the individual nature of symmetry response to speed and fatigue. For example, participant 1 pre-fatigue shows a left bias when walking and jogging (W, J), but transitions to a right bias at higher speeds (R, S). After fatigue, the same participant shows a left bias at all speeds. Other participants such as 7 and 16 show an increase in the magnitude of left or right asymmetry with fatigue. Fatigue causes participants 9 and 10 to transition from a left bias to right bias at all speeds. These results are further considered in the discussion below.
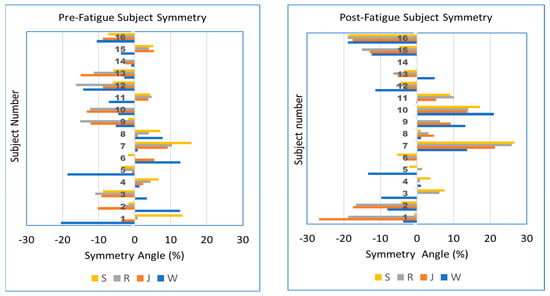
Figure 2.
Peak pressure SA% for participants (n = 16) arranged in vertical groups. Walk, jog, run, and sprint (W, J, R, S) speeds are shown in different colors. Left: pre-fatigue, Right: post-fatigue. Cases not shown due to faulty sensing cases 9—post-sprint, 10—pre-sprint, 14—pre- and post-sprint.
3.4. Linear Regression Analysis Evaluating Associations of Fatigue and Speed with Peak Pressure
In order to determine if the fatigue protocol had an effect and to control for the various independent variables (demographics, speed, pre-fatigue peak pressure, body composition, body dimensions, blood lactate, and heart rate), we first evaluated the associations using a univariable regression analyses on the dependent variable (post-fatigue peak pressure) (Table 3).

Table 3.
Univariable linear regression evaluating the association of independent covariates with peak pressure (N = 16).
Age and sex had a significantly negative association with post-fatigue peak pressure (p = 0.039 and p = 0.010 respectively). Though BMI had no significant association, fat mass, and fat% both had a significantly negative association (p = 0.012 and p = 0.001 respectively), which may be attributed to a higher number of females within the study having an increased FM when compared to males while, overall, and also having lower peak pressures. Additionally, heart rate post-fatigue (p = 0.017) had a significant positive association with post-fatigue peak pressure.
Of great interest, each speed, with walking as the reference, was significantly associated with post-fatigue peak pressure (p ≤ 0.001 for all) and pre-fatigue peak pressure was also significantly associated with post-fatigue peak pressure (p < 0.001).
A multivariable regression analysis was performed to determine the influence on post-fatigue peak pressure while controlling for multiple variables in demographics, body composition, body metrics, and sport conditioning covariates (inclusion p < 0.25 in univariable analysis) (Table 4). Age is attenuated in each multivariable model while sex remains significantly associated with post-fatigue peak pressure. Specifically, being female is associated with a decrease in post-fatigue peak pressure throughout each model. Body composition metrics were highly correlated, and FM was chosen to represent body composition in the multivariable models. Though significant in the univariable analysis, FM is attenuated to non-significance in the multivariable models.

Table 4.
Multivariable regression analysis of covariates evaluating association with post-fatigue peak pressure (N = 16). Multivariable regression models were created from the univariable analysis with variables having p < 0.25 association. Collinearity was considered using variance of inflation (VIF < 10 were considered).
In Model 2, upper and lower leg length along with heart rate post-fatigue are added into the model. The upper leg length continues to be significantly associated with post-fatigue peak pressure while the other variables are attenuated. In Model 3, pre-fatigue peak pressure and upper leg length are added to the base model (age, sex, FM) to determine the association with post-peak pressure while adjusting for other factors. From the results, although pre-fatigue peak pressure is significantly associated, the beta-coefficient is near 1. This result suggests that while significant, the impact on post-fatigue peak pressure is not as impactful when considering other covariates such as sex (beta (SE) = −72.1(19), p < 0.001). Model 4 is similar to Model 3, but evaluates speed (walk, jog, run, sprint) while adjusting for the base model, FM, and upper leg length. Walking is the reference speed with which each subsequent speed is benchmarked and significantly associated with an increasing beta coefficient as we have found previously with increasing speed. In addition, upper leg length and sex also remain significantly associated.
Lastly, in Model 5, we evaluate the base model (age, sex, FM) with body dimension (upper leg length), pre-fatigue peak pressure, and speed. The results reveal that while pre-fatigue peak pressure (p < 0.001) does remain significantly associated, other factors also remain associated with post-fatigue peak pressure including upper leg length (p = 0.027) and sprinting (p = 0.009). We found no significant interactions between speed and pre-fatigue peak pressure in the multivariable models (Model 5: p = 0.780, not shown).
4. Discussion
The current study provided novel data collection and results on the effect of fatigue on plantar pressure and gait asymmetries in young, well-trained male and female participants. Our data collection involved four different speeds while collecting between 50- and 100-foot strikes in both right and left feet. We report significant differences in peak pressure and asymmetry for pre- vs. post-fatigue at the cohort and individual levels, respectively, along with significant associations between speed and fatigue with post-fatigue pressure.
Prior studies reporting plantar pressure show mixed results of fatigue with some studies showing increases in peak pressure and peak force when analyzing one side [28] and others showing an increase or decrease in peak pressure dependent on foot region being analyzed [29] or the type of runner foot strike (forefoot vs. rearfoot) [13,16,29]. Many studies consider barefoot runners [13,16,30], or just a single running speed [9,27,28,29,30,31]. Hazzaa Walaa Eldin et al. [13] evaluated a range of speeds at or above our jogging speed and found different changes in foot pressure due to fatigue depending on the region of the foot, speed, and foot strike type, but focused on barefoot runners fatigued by local ankle dynamometer only.
The present study focuses on shod runners, with a running fatigue protocol, and a range of speeds including walking. As expected, the walking peak pressures were lower than the running cases. Referring to Figure 1, walking pressures increased with fatigue, with median pressure values rising from 195 to 228 kPa due to fatigue. Significant pressure increases with fatigue occurred with the running cases as well. Among the other studies noted above, the Weist et al. [28] approach was most similar to this study, using shod runners, in-sole sensors, and a running fatigue protocol. Their analysis was limited to just the left side, and a single running speed, but showed that fatigue caused an increase in peak plantar pressure of 12%. Figure 1 shows a comparable effect, extended to walking and multiple running speeds, when reporting an average of left/right pressures. Although our study did not evaluate different regions of the foot, we found statistically significant differences in pre- vs. post-peak pressure for all walking/running speeds. Our subsequent linear regression analyses also confirmed the significant association of a fatigue state and speed (all speeds) with peak pressure even after adjusting for participant-specific features.
One might argue that above the evaluation of singular plantar pressures, detecting gait asymmetry at any speed would be beneficial in deterring fatigue, preventing injury, and increasing overall fitness. In Table 1, we reported the peak pressure symmetry angle [19] and associated statistics before and after fatigue. The results showed no statistically significant change in SA at the cohort level. This is consistent with other studies of the effect of fatigue on symmetry across a group of subjects [31,32].
However, we also evaluated the SA at the individual level, presenting subject-specific results in Figure 2. The figure shows that for some individuals, the SA bias moves from left to right (or vice versa) as speed increases, while in other individuals, the bias remains on the same side, both of which also are pre- versus post-fatigue dependent. These observations are consistent with Zandbergen et al.’s review finding large inter-individual responses to fatigue [33]. Given that Figure 2 shows individual subjects with asymmetric response to fatigue, future studies of fatigue may benefit by considering individual foot response (left and right) rather than focusing exclusively on one foot.
As with any study, ours also has some limitations. Although we initially had 19 participants, some of the insole pressure sensors became prematurely corrupted and we were unable to use full data on all 19 participants, reducing the statistical power to 70%. The Tekscan in-sole sensors do not provide a true vertical ground reaction force (vGRF) due to the curvature of the foot and are unable to account for the vertical component of any shearing load. Hebert-Copley et al. reported that maximum forces recorded by in-sole sensors are 94 to 98% of a true vGRF recorded by a force-plate measure [27]. We also chose to investigate the peak pressure across the entire foot and not segmented by different regions of the foot. Although interesting, our goal for this analysis was not to assess the biomechanical degradation with respect to the heel or metatarsal. We may determine to reach this level of granularity for future analyses. Lastly, due to safety concerns for our athletes, we did not randomize the speeds for running, but allowed a progressive increase in speed. We also believe that a progression of increasing speed would be more in line with how an athlete would accelerate from walking or jogging to running or sprinting.
5. Conclusions
Our results demonstrate that peak plantar pressures increase as running speed increases and following fatigue. Fatigue produced PPs of similar magnitude at jogging, running, and sprinting speeds. Given the association between PP and running-related injuries, these results suggest that caution should be taken when jogging, running, or walking in a fatigued state. The results of this study also show substantial speed and fatigue effects on individual bilateral symmetry including increasing the magnitude of a left–right bias or switching the bias from one foot to the other. Our data suggest that isolating fatigue effects to a single side (left or right) may be inadequate and hence the pressure under both feet should be considered when studying fatigue effects. After controlling for age, sex, and body metrics (e.g., FM), our multivariate regression showed that pre-fatigue PP and speed remained significantly associated with post-fatigue PP. Consequently, identifying athletes who demonstrate high peak pressures (possibly due to asymmetries) in non-fatigue states may be a strategy for reducing fatigue-related running-related injuries. Confirming the observed fatigue effects on PP in larger cohorts is warranted.
Author Contributions
K.J.S.—formal analysis, writing—original document, writing—review and editing; G.A.R.—conceptualization, formal analysis, methodology, supervision, writing—original document, writing—review and editing; J.B.—conceptualization, methodology, writing—original document, writing—review and editing; H.N.—formal analysis; C.A.—methodology; J.R.—supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Some equipment used in this research was funded by Highmark Health.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Grove City College (protocol code 111-2021 20 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Research data are available upon request.
Acknowledgments
Data reported in this study were collected with the help of a team of undergraduate research students under the directions of authors Richards and Buxton. The dedication and commitment of these students is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gamble, P. Strength and Conditioning for Team Sports: Sport-Specific Physical Preparation for High Performance, 2nd ed.; Routledge: London, UK, 2012; ISBN 978-0-203-08425-0. [Google Scholar]
- Apte, S.; Prigent, G.; Stöggl, T.; Martínez, A.; Snyder, C.; Gremeaux-Bader, V.; Aminian, K. Biomechanical Response of the Lower Extremity to Running-Induced Acute Fatigue: A Systematic Review. Front. Physiol. 2021, 12, 646042. [Google Scholar] [CrossRef]
- Orejel Bustos, A.; Belluscio, V.; Camomilla, V.; Lucangeli, L.; Rizzo, F.; Sciarra, T.; Martelli, F.; Giacomozzi, C. Overuse-Related Injuries of the Musculoskeletal System: Systematic Review and Quantitative Synthesis of Injuries, Locations, Risk Factors and Assessment Techniques. Sensors 2021, 21, 2438. [Google Scholar] [CrossRef]
- Winter, S.; Gordon, S.J.; Watt, K. Effects of Fatigue on Kinematics and Kinetics during Overground Running: A Systematic Review. J. Sports Med. Phys. Fitness 2017, 57, 887–899. [Google Scholar] [CrossRef]
- van Gent, R.N.; Siem, D.; van Middelkoop, M.; van Os, A.G.; Bierma-Zeinstra, S.M.A.; Koes, B.W. Incidence and Determinants of Lower Extremity Running Injuries in Long Distance Runners: A Systematic Review. Br. J. Sports Med. 2007, 41, 469–480. [Google Scholar] [CrossRef]
- Warden, S.J.; Davis, I.S.; Fredericson, M. Management and Prevention of Bone Stress Injuries in Long-Distance Runners. J. Orthop. Sports Phys. Ther. 2014, 44, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Dempster, J.; Dutheil, F.; Ugbolue, U.C. The Prevalence of Lower Extremity Injuries in Running and Associated Risk Factors: A Systematic Review. Phys. Act. Health 2021, 5, 133–145. [Google Scholar] [CrossRef]
- Mizrahi, J.; Verbitsky, O.; Isakov, E. Fatigue-Related Loading Imbalance on the Shank in Running: A Possible Factor in Stress Fractures. Ann. Biomed. Eng. 2000, 28, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Clansey, A.C.; Hanlon, M.; Wallace, E.S.; Lake, M.J. Effects of Fatigue on Running Mechanics Associated with Tibial Stress Fracture Risk. Med. Sci. Sports Exerc. 2012, 44, 1917–1923. [Google Scholar] [CrossRef]
- Buckley, C.; O’Reilly, M.A.; Whelan, D.; Farrell, A.V.; Clark, L.; Longo, V.; Gilchrist, M.D.; Caulfield, B. Binary Classification of Running Fatigue Using a Single Inertial Measurement Unit. In Proceedings of the 2017 IEEE 14th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Eindhoven, The Netherlands, 9–12 May 2017; pp. 197–201. [Google Scholar]
- Yang, D. Assessing the Feasibility of Using Pressure Sensing Insoles to Measure Physical Fatigue Due to Prolonged Standing—ProQuest. Available online: https://www.proquest.com/openview/0907782df7c009bea44542ecfc33516d/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 10 August 2022).
- Anbarian, M.; Esmaeili, H. Effects of Running-Induced Fatigue on Plantar Pressure Distribution in Novice Runners with Different Foot Types. Gait Posture 2016, 48, 52–56. [Google Scholar] [CrossRef]
- Hazzaa Walaa Eldin, E.; Mattes, K. Influence of Foot Strike Pattern and Local Fatigue of Plantar Flexors and Dorsiflexors on Plantar Pressure during Running. Dtsch. Z. Für Sportmed. 2018, 2018, 19–26. [Google Scholar] [CrossRef]
- Willems, T.M.; De Ridder, R.; Roosen, P. The Effect of a Long-Distance Run on Plantar Pressure Distribution during Running. Gait Posture 2012, 35, 405–409. [Google Scholar] [CrossRef]
- Nagel, A.; Fernholz, F.; Kibele, C.; Rosenbaum, D. Long Distance Running Increases Plantar Pressures beneath the Metatarsal Heads: A Barefoot Walking Investigation of 200 Marathon Runners. Gait Posture 2008, 27, 152–155. [Google Scholar] [CrossRef]
- Hamzavi, B.; Esmaeili, H. Effects of Running-Induced Fatigue on Plantar Pressure Distribution in Runners with Different Strike Types. Gait Posture 2021, 88, 132–137. [Google Scholar] [CrossRef]
- Gao, Z.; Fekete, G.; Baker, J.S.; Liang, M.; Xuan, R.; Gu, Y. Effects of Running Fatigue on Lower Extremity Symmetry among Amateur Runners: From a Biomechanical Perspective. Front. Physiol. 2022, 13, 899818. [Google Scholar] [CrossRef]
- Zifchock, R.A.; Davis, I.; Higginson, J.; McCaw, S.; Royer, T. Side-to-Side Differences in Overuse Running Injury Susceptibility: A Retrospective Study. Hum. Mov. Sci. 2008, 27, 888–902. [Google Scholar] [CrossRef]
- Zifchock, R.A.; Davis, I.; Higginson, J.; Royer, T. The Symmetry Angle: A Novel, Robust Method of Quantifying Asymmetry. Gait Posture 2008, 27, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Zifchock, R.A.; Davis, I.; Hamill, J. Kinetic Asymmetry in Female Runners with and without Retrospective Tibial Stress Fractures. J. Biomech. 2006, 39, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Mei, Q.; Fekete, G.; Baker, J.S.; Gu, Y. The Effect of Prolonged Running on the Symmetry of Biomechanical Variables of the Lower Limb Joints. Symmetry 2020, 12, 720. [Google Scholar] [CrossRef]
- Ménard, A.-L.; Begon, M.; Barrette, J.; Green, B.; Ballaz, L.; Nault, M.-L. Plantar Pressure Analysis: Identifying Risk of Foot and Ankle Injury in Soccer Players. Transl. SPORTS Med. 2021, 4, 684–690. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Price, C.; Parker, D.; Nester, C. Validity and Repeatability of Three In-Shoe Pressure Measurement Systems. Gait Posture 2016, 46, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Patrick, K.; Donovan, L. Test–Retest Reliability of the Tekscan® F-Scan® 7 in-Shoe Plantar Pressure System during Treadmill Walking in Healthy Recreationally Active Individuals. Sports Biomech. 2018, 17, 83–97. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, J.; Trabia, M.B.; Dufek, J.S.; Le Gall, Y.; Da Silva Sacoto, N. Enhancing the Accuracy of Vertical Ground Reaction Force Measurement During Walking Using Pressure-Measuring Insoles. J. Biomech. Eng. 2020, 143, 011010. [Google Scholar] [CrossRef]
- Herbert-Copley, A.G.; Sinitski, E.H.; Lemaire, E.D.; Baddour, N. Temperature and Measurement Changes over Time for F-Scan Sensors. In Proceedings of the 2013 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Gatineau, QC, Canada, 4–5 May 2013; pp. 265–267. [Google Scholar]
- Weist, R.; Eils, E.; Rosenbaum, D. The Influence of Muscle Fatigue on Electromyogram and Plantar Pressure Patterns as an Explanation for the Incidence of Metatarsal Stress Fractures. Am. J. Sports Med. 2004, 32, 1893–1898. [Google Scholar] [CrossRef]
- Bisiaux, M.; Moretto, P. The Effects of Fatigue on Plantar Pressure Distribution in Walking. Gait Posture 2008, 28, 693–698. [Google Scholar] [CrossRef]
- Askari, Z.; Esmaeili, H. Effect of Trunk Muscles Fatigue on Plantar Pressure Distribution in Novice Runners. J. Biomech. 2021, 122, 110487. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Tucker, C.B. Gait Variability and Symmetry Remain Consistent during High-Intensity 10,000 m Treadmill Running. J. Biomech. 2018, 79, 129–134. [Google Scholar] [CrossRef]
- Girard, O.; Brocherie, F.; Morin, J.-B.; Millet, G.P. Lower Limb Mechanical Asymmetry during Repeated Treadmill Sprints. Hum. Mov. Sci. 2017, 52, 203–214. [Google Scholar] [CrossRef]
- Zandbergen, M.A.; Marotta, L.; Bulthuis, R.; Buurke, J.H.; Veltink, P.H.; Reenalda, J. Effects of Level Running-Induced Fatigue on Running Kinematics: A Systematic Review and Meta-Analysis. Gait Posture 2023, 99, 60–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).