A Review of Physicochemical Stabilization for Improved Engineering Properties of Clays
Abstract
:1. Introduction
| Admixture and Reference | Mechanism and Dosage (Dry Mass Basis) | Limitations |
|---|---|---|
| Physical | ||
| Sand [7,8] | Addition of inert and coarse particles (10–90%) |
|
| Shredded tire [19,20] | Addition of inert and fine-to-coarse lightweight particles (≤10%) |
|
| Chemical | ||
| Cement [11,21,22,23,24] | Three stages comprising cation exchange (Na+ and Mg2+ in clay replaced by Ca2+ of cement); cementitious hydration (Ca2+-based compounds in cement react with water to form silicates, aluminates, and hydrated lime); and pozzolanic reaction (Ca2+ on a clay surface react with dissolved silicates and aluminates to form gels) (≤20%) |
|
| Lime [24,25] | Four stages with the first three similar to the above (albeit Ca2+ derived from lime) followed by carbonation cementation where CaO reacts with atmospheric CO2 to precipitate as CaCO3 (≤8%) |
|
| Fly ash [22,23,26,27] | Accelerates cement or lime stabilization (≤20%) |
|
| Cement kiln dust [28,29] | Same as lime due to abundance of free lime (≤8%) |
|
| Steel slag [30,31,32,33,34,35] | Cementitious hydration (albeit Ca2+ derived from lime) similar to cement (≤25%) |
|
| Silica fume [36,37,38] | Silica accelerates the pozzolanic reaction (≤50%) |
|
| Physicochemical | ||
| Nanomaterials [39,40,41,42] | Development of viscous gel due to water adsorption through H-bonding that coats the clay surfaces and reduces the diffuse double-layer thickness (≤3%) |
|
| Biopolymers [14,16,18] | Adsorption through electrostatic attraction and development of hydrogel (≤2%) |
|
| Geopolymers [11,15,43,44,45,46] | Activation of aluminosilicate source by aqueous alkaline solution via geopolymerisation reaction to form geopolymeric gel (source: 10–20%) (activator/source: 0.4–0.8) |
|
2. Background Review
2.1. Composition of Physicochemical Admixtures
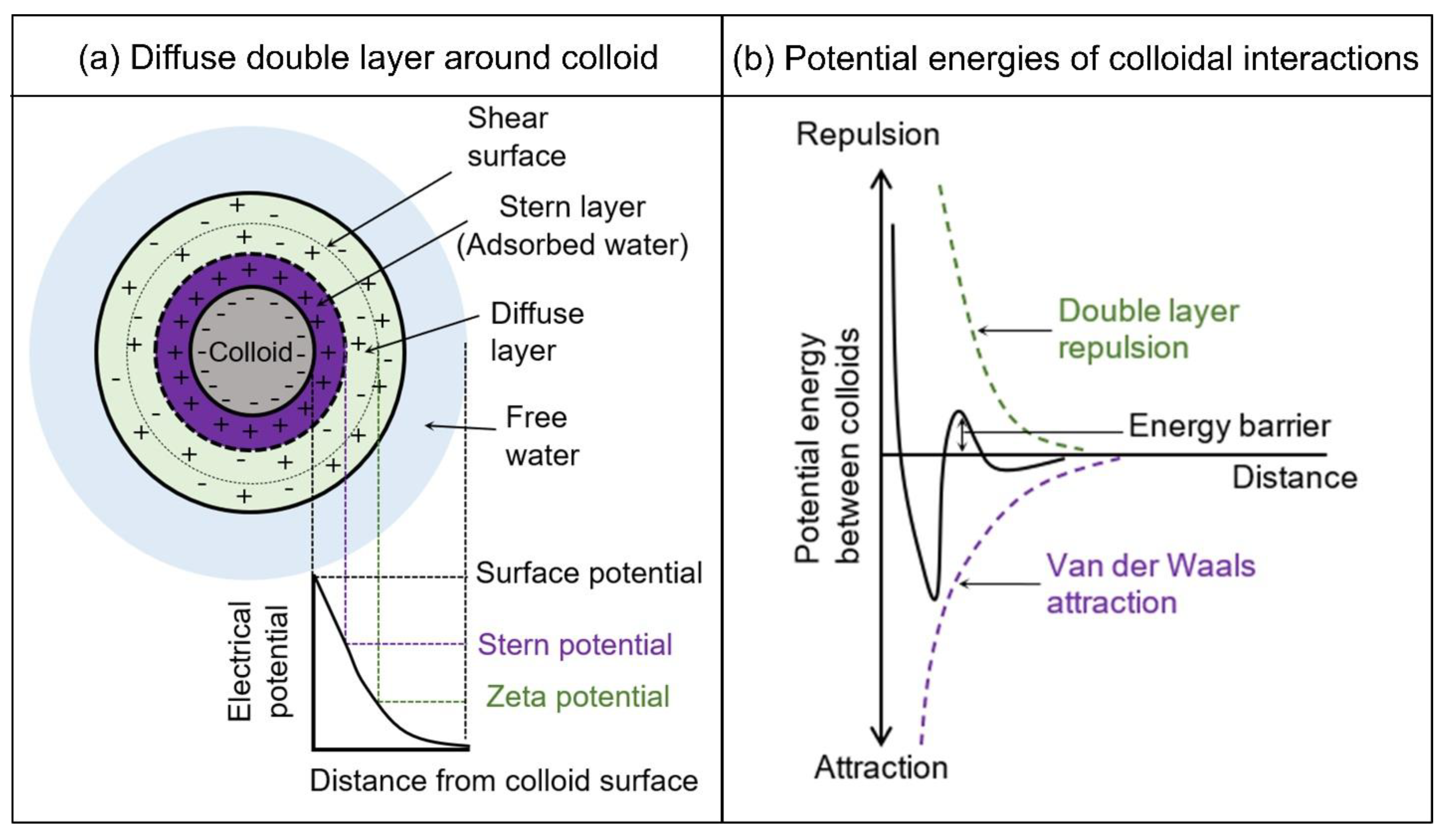
2.2. Interaction of Clays with Water
2.3. Interaction of Admixtures in Clay–Water System
3. Soil Properties

| Soil Property and Reference | Relationships |
|---|---|
| Relationship with dry unit weight | - |
| Hydraulic conductivity [119] | - ln ks = −16.91+15.16 |
| [120] | Best-fit for the reported data |
| Compression index [121] | - |
| [122] | |
| Compressive strength [123] | - |
| [7] | Best-fit for the reported data |
| Relationship with water content | - |
| Hydraulic conductivity [124] | - Best-fit for the reported data |
| [82] | Best-fit for the reported data |
| Compression index [125] | - |
| [126] | (Gs = 2.70) |
| Compressive strength [7] | - Best-fit for the reported data |
| [127] | Best-fit for the reported data |
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Thickness of the diffuse double layer (nm) | |
| Permittivity of vacuum (8.85 × 10−12 C2/J m) | |
| k | Boltzmann’s constant (1.38 × 10−23 J/K) |
| D | Dielectric constant of the medium |
| T | Temperature (K) |
| Electrolyte concentration (mol/l) | |
| Electronic charge (1.6 × 10−19 C) | |
| Cation valence | |
| ζ | Zeta potential (mV) |
| γdmax | Maximum dry unit weight (kN/m3) |
| wopt | Optimum water content (%) |
| S | Degree of saturation (%) |
| w | Initial water content (%) |
| γd | Initial dry unit weight (kN/m3) |
| ks | Saturated hydraulic conductivity (m/sec) |
| γw | Unit weight of water (9.81 kN/m3) |
| Cc | Compression index |
| qu | Unconfined compressive strength (kPa) |
| Liquid limit (%) | |
| Plasticity index (%) | |
| Gs | Specific gravity (%) |
References
- Basu, S.; Sauchyn, D.J.; Anis, M.R. Hydrological Extremes in the Canadian Prairies in the Last Decade Due to the ENSO Teleconnection—A Comparative Case Study Using WRF. Water 2020, 12, 2970. [Google Scholar] [CrossRef]
- Ito, M.; Azam, S. Relation between Flow through and Volumetric Changes in Natural Expansive Soils. Eng. Geol. 2020, 279, 105885. [Google Scholar] [CrossRef]
- Muricken, D.G.; Jin, Y.C. Groundwater Contamination Modelling Underneath Regina Landfill. In Proceedings of the International Conference “Water, Environment, Energy and Society (WEES)”, New Delhi, India, 12–16 January 2009; pp. 1201–1208. [Google Scholar]
- Pan, C.; Ng, K.T.W.; Richter, A. An Integrated Multivariate Statistical Approach for The Evaluation of Spatial Variations in Groundwater Quality Near an Unlined Landfill. Environ. Sci. Pollut. Res. 2019, 26, 5724–5737. [Google Scholar] [CrossRef]
- Errecalde, I.A. Modelling of the SM-3 DAM, Quebec, Canada. Master’s Thesis, Polytechnic University of Catalonia, Barcelona, Spain, 2012. [Google Scholar]
- Puzrin, A.M.; Alonso, E.E.; Pinyol, N.M. Bearing Capacity Failure: Transcona Grain Elevator, Canada. In Geomechanics of Failures; Springer: Dordrecht, The Netherlands, 2010; pp. 67–84. [Google Scholar]
- Khan, F.S.; Azam, S.; Raghunandan, M.E.; Clark, R. Compressive Strength of Compacted Clay-Sand Mixes. Adv. Mater. Sci. Eng. 2014, 2014, 921815. [Google Scholar] [CrossRef] [Green Version]
- Kollaros, G.; Athanasopoulou, A. Sand as a Soil Stabilizer. Bull. Geol. Soc. Greece 2017, 50, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhao, M.; Zhang, G.; Nowak, P.; Coen, A.; Tao, M. Calcium-Free Geopolymer as a Stabilizer for Sulfate-Rich Soils. Appl. Clay Sci. 2015, 108, 199–207. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D.N. Fly Ash Zeolites for Water Treatment Applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Jeremiah, J.J.; Abbey, S.J.; Booth, C.A.; Kashyap, A. Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review. Geotechnics 2021, 1, 439–459. [Google Scholar] [CrossRef]
- Ramachandran, A.L.; Dubey, A.A.; Dhami, N.K.; Mukherjee, A. Multiscale Study of Soil Stabilization Using Bacterial Biopolymers. J. Geotech. Geoenviron. Eng. 2021, 147, 04021074. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A.; Sarker, P. Use of Fly-Ash Geopolymer Incorporating Ground Granulated Slag for Stabilisation of Kaolin Clay Cured at Ambient Temperature. Geotech. Geol. Eng. 2019, 37, 721–740. [Google Scholar] [CrossRef]
- Armistead, S.J.; Smith, C.C.; Staniland, S.S. Sustainable Biopolymer Soil Stabilization in Saline Rich, Arid Conditions: A ‘Micro to Macro’ Approach. Sci. Rep. 2022, 12, 2880. [Google Scholar] [CrossRef] [PubMed]
- Sargent, P.; Hughes, P.N.; Rouainia, M.; White, M.L. The Use of Alkali Activated Waste Binders in Enhancing the Mechanical Properties and Durability of Soft Alluvial Soils. Eng. Geol. 2013, 152, 96–108. [Google Scholar] [CrossRef]
- Agbovi, H.K. Biopolymer Flocculant Systems and Their Chemically Modified Forms for Aqueous Phosphate and Kaolinite Removal. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2020. [Google Scholar]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and Solubility. In Solubility of Polysaccharides; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar]
- Akbarimehr, D.; Eslami, A.; Aflaki, E. Geotechnical Behaviour of Clay Soil Mixed with Rubber Waste. J. Clean. Prod. 2020, 271, 122632. [Google Scholar] [CrossRef]
- Bekhiti, M.; Trouzine, H.; Rabehi, M. Influence of Waste Tire Rubber Fibers on Swelling Behavior, Unconfined Compressive Strength and Ductility of Cement Stabilized Bentonite Clay Soil. Constr. Build. Mater. 2019, 208, 304–313. [Google Scholar] [CrossRef]
- Puppala, A.; Congress, S.; Banerjee, A. Research Advancements in Expansive Soil Characterization, Stabilization and Geoinfrastructure Monitoring. In Frontiers in Geotechnical Engineering; Latha, G.M., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 15–29. [Google Scholar]
- Kaniraj, S.R.; Havanagi, V.G. Behaviour of Cement-Stabilized Fiber-Reinforced Fly Ash-Soil Mixtures. J. Geotech. Geoenviron. Eng. 2001, 127, 574–584. [Google Scholar] [CrossRef]
- Firoozi, A.A.; Guney Olgun, C.; Firoozi, A.A.; Baghini, M.S. Fundamentals of Soil Stabilization. Int. J. Geo-Eng. 2017, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Prusinski, J.R.; Bhattacharja, S. Effectiveness of Portland Cement and Lime in Stabilizing Clay Soils. Transp. Res. Rec. J. Transp. Res. Board 1999, 1652, 215–227. [Google Scholar] [CrossRef]
- Barman, D.; Dash, S.K. Stabilization of Expansive Soils Using Chemical Additives: A Review. J. Rock. Mech. Geotech. Eng. 2022, 14, 1319–1342. [Google Scholar] [CrossRef]
- Hassett, D.J.; Heebink, L.V. Environmental Evaluation for Utilization of Ash in Soil Stabilization; Final Report; Energy & Environmental Research Center, University of Minnesota: Grand Forks, ND, USA, 2001. [Google Scholar]
- Khare, A.; Gupta, S.K.; Sah, S.; Mukesh, K.; Toppo, A.; Jain, A.; Jaiswal, S.K. Implication of Fly Ash in Stabilizing Expansive Soil. Intersect 2023, 16. Available online: https://ojs.stanford.edu/ojs/index.php/intersect/article/download/2294/1553/9206 (accessed on 29 June 2023).
- Almuaythir, S.; Abbas, M.F. Expansive Soil Remediation Using Cement Kiln Dust as Stabilizer. Case Stud. Constr. Mater. 2023, 18, e01983. [Google Scholar] [CrossRef]
- Mahmoud, M.; Rimes, B. Leaching Characteristics of Cement Kiln Dust from Alberta. In Proceedings of the Annual Conference—Canadian Society for Civil Engineering, Edmonton, AB, Canada, 6–9 June 2012; pp. 899–907. [Google Scholar]
- Kabeta, W.F.; Lemma, H. Modeling the Application of Steel Slag in Stabilizing Expansive Soil. Model. Earth Syst. Environ. 2023. [Google Scholar] [CrossRef]
- Maghool, F.; Arulrajah, A.; Du, Y.J.; Horpibulsuk, S.; Chinkulkijniwat, A. Environmental Impacts of Utilizing Waste Steel Slag Aggregates as Recycled Road Construction Materials. Clean. Technol. Environ. Policy 2017, 19, 949–958. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, T.; Li, S.; Wang, W. Engineering Properties and Environmental Impact of Soil Mixing with Steel Slag Applied in Subgrade. Appl. Sci. 2023, 13, 1574. [Google Scholar] [CrossRef]
- Yu, C.; Cui, C.; Wang, Y.; Zhao, J.; Wu, Y. Strength Performance and Microstructural Evolution of Carbonated Steel Slag Stabilized Soils in the Laboratory Scale. Eng. Geol. 2021, 295, 106410. [Google Scholar] [CrossRef]
- O’Connor, J.; Nguyen, T.B.T.; Honeyands, T.; Monaghan, B.; O’Dea, D.; Rinklebe, J.; Vinu, A.; Hoang, S.A.; Singh, G.; Kirkham, M.B.; et al. Production, Characterisation, Utilisation, and Beneficial Soil Application of Steel Slag: A Review. J. Hazard. Mater. 2021, 419, 126478. [Google Scholar] [CrossRef]
- Montenegro-Cooper, J.M.; Celemín-Matachana, M.; Cañizal, J.; González, J.J. Study of the Expansive Behavior of Ladle Furnace Slag and Its Mixture with Low Quality Natural Soils. Constr. Build. Mater. 2019, 203, 201–209. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Akbari, H.R.; Salimi, M. Enhanced Stabilization of Highly Expansive Clays by Mixing Cement and Silica Fume. Appl. Clay Sci. 2016, 132–133, 675–684. [Google Scholar] [CrossRef]
- Singh, P.; Dash, H.K.; Samantaray, S. Effect of Silica Fume on Engineering Properties of Expansive Soil. Mater. Today Proc. 2020, 33, 5035–5040. [Google Scholar] [CrossRef]
- Mansour, E.; Kinuthia, J.; Oti, J.; Al-Waked, Q. Sulfate Soil Stabilization with Binary Blends of Lime–Silica Fume and Lime–Ground Granulated Blast Furnace Slag. Transp. Geotech. 2022, 37, 100888. [Google Scholar] [CrossRef]
- Tomar, A.; Sharma, T.; Singh, S. Strength Properties and Durability of Clay Soil Treated with Mixture of Nano Silica and Polypropylene Fiber. Mater. Today Proc. 2019, 26, 3449–3457. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic-Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Omurlu, C.; Pham, H.; Nguyen, Q.P. Interaction of Surface-Modified Silica Nanoparticles with Clay Minerals. Appl. Nanosci. 2016, 6, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Metin, C.O.; Nguyen, Q.P. Interaction of Surface Modified Silica Nanoparticles with Clay Minerals. In Proceedings of the CTSI Cleantech, Energy, Renewables, Environment & Materials; CRC Press: Boca Raton, FL, USA, 2014; pp. 186–189. [Google Scholar]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L.; Karrech, A. Cyclic Behaviour of Clay Stabilised with Fly-Ash Based Geopolymer Incorporating Ground Granulated Slag. Transp. Geotech. 2021, 26, 100430. [Google Scholar] [CrossRef]
- Disu, A.A.; Kolay, P.K. A Critical Appraisal of Soil Stabilization Using Geopolymers: The Past, Present and Future. Int. J. Geosynth. Ground Eng. 2021, 7, 23. [Google Scholar] [CrossRef]
- Noolu, V.; Mallikarjuna Rao, G.; Sudheer Kumar Reddy, B.; Chavali, R.V.P. Strength and Durability Characteristics of GGBS Geopolymer Stabilized Black Cotton Soil. Mater. Today Proc. 2021, 43, 2373–2376. [Google Scholar] [CrossRef]
- Odeh, N.A.; Al-Rkaby, A.H.J. Strength, Durability, and Microstructures Characterization of Sustainable Geopolymer Improved Clayey Soil. Case Stud. Constr. Mater. 2022, 16, e00988. [Google Scholar] [CrossRef]
- Míguez, H.; Meseguer, F.; López, C.; Mifsud, A.; Moya, J.S.; Vá Zquez, L. Evidence of FCC Crystallization of SiO2 Nanospheres. Langmuir 1997, 13, 6009–6011. [Google Scholar] [CrossRef]
- Srdić, V.V.; Cvejić, Z.; Milanović, M.; Stojanović, G.; Rakić, S. Metal Oxide Structure, Crystal Chemistry, and Magnetic Properties. In Magnetic, Ferroelectric, and Multiferroic Metal Oxides; Stojanovic, B.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 313–332. [Google Scholar]
- Wong, C.; Pedrotti, M.; El Mountassir, G.; Lunn, R.J. A Study on The Mechanical Interaction Between Soil and Colloidal Silica Gel for Ground Improvement. Eng. Geol. 2018, 243, 84–100. [Google Scholar] [CrossRef] [Green Version]
- Coo, J.L.; So, Z.P.S.; Ng, C.W.W. Effect of Nanoparticles on the Shrinkage Properties of Clay. Eng. Geol. 2016, 213, 84–88. [Google Scholar] [CrossRef]
- Krishnan, J.; Shukla, S. The Behaviour of Soil Stabilised with Nanoparticles: An Extensive Review of The Present Status and Its Applications. Arab. J. Geosci. 2019, 12, 436. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y. Basic Building Blocks: Biopolymers. In Biological Materials Science; Cambridge University Press: Cambridge, UK, 2014; pp. 53–101. [Google Scholar]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as Green Binders for Soil Improvement in Geotechnical Applications: A Review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials 2022, 15, 1725. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al Ratio on the Structure and Properties of Metakaolin Based Geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization Reaction, Microstructure and Simulation of Metakaolin-Based Geopolymers at Extended Si/Al Ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Ayoola, H.O.; House, S.D.; Bonifacio, C.S.; Kisslinger, K.; Saidi, W.A.; Yang, J.C. Evaluating the Accuracy of Common γ-Al2O3 Structure Models by Selected Area Electron Diffraction from High-Quality Crystalline γ-Al2O3. Acta Mater. 2020, 182, 257–266. [Google Scholar] [CrossRef]
- Schmahl, W.W.; Swainson, I.P.; Dove, M.T.; Graeme-Barber, A. Landau Free Energy and Order Parameter Behaviour of the α/ß Phase Transition in Cristobalite. Z. Für Krist. 1992, 201, 125–145. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behaviour, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; New York, NY, USA, 2005. [Google Scholar]
- Liu, C.; Meng, Y. Tribology at Charged Solid-Liquid Interfaces. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Derjaguin, B.; Landau, L. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Prog. Surf. Sci. 1941, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids. J. Colloid. Sci. 1955, 10, 224–225. [Google Scholar] [CrossRef] [Green Version]
- Chibowski, E. Flocculation and Dispersion Phenomena in Soils. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2011; pp. 301–304. [Google Scholar]
- Mohamed, A.-M.O.; Paleologos, E.K. Soil-Water Interaction. In Fundamentals of Geoenvironmental Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 161–203. [Google Scholar]
- Schmitz, R.M. Can the Diffuse Double Layer Theory Describe Changes in Hydraulic Conductivity of Compacted Clays? Geotech. Geol. Eng. 2006, 24, 1835–1844. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The Nanosilica Hazard: Another Variable Entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changizi, F.; Haddad, A. Effect of Nano-SiO2 on the Geotechnical Properties of Cohesive Soil. Geotech. Geol. Eng. 2016, 34, 725–733. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine Wastes Based Geopolymers: A Critical Review. Clean. Eng. Technol. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Azam, S. Solid-Liquid Separation of Laterite Slurries. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2003. [Google Scholar]
- Rima, U.S.; Azam, S. Centrifuge Dewatering of Polymer-Amended Oil Sand Tailings. Environ. Geotech. 2015, 2, 175–180. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.B.; White, G.N. Bonding Mechanisms and Conformation of Poly(Ethylene Oxide)-Based Surfactants in Interlayer of Smectite. Colloid. Polym. Sci. 2006, 284, 347–356. [Google Scholar] [CrossRef]
- Azam, S. Parametric Modeling of Polymer-Assisted Slurry Sedimentation for Nickel Laterite Mining. J. Environ. Inform. 2008, 11, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Czemierska, M.; Szcześ, A.; Jarosz-Wilkołazka, A. Purification of Wastewater by Natural Flocculants. Biotechnologia 2015, 96, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; Patel, D.; Hickson, B.; Desrochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Zhou, C.; So, P.S.; Chen, X.W. A Water Retention Model Considering Biopolymer-Soil Interactions. J. Hydrol. 2020, 586, 124874. [Google Scholar] [CrossRef]
- Gu, B.; Doner, H. The Interaction of Polysaccharides with Silver Hill Illite. Clays Clay Miner. 1992, 40, 151–156. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A State-of-the-Art Review of Polymers Used in Soil Stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Ahmad, Z.; Ahmad, M.S.B.; Ariffin, A. Treatment of Pulp and Paper Mill Wastewater with Various Molecular Weight of PolyDADMAC Induced Flocculation. Chem. Eng. J. 2011, 166, 529–535. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Samuel, R.; Puppala, A.J.; Banerjee, A.; Huang, O.; Radovic, M.; Chakraborty, S. Improvement of Strength and Volume-Change Properties of Expansive Clays with Geopolymer Treatment. Transp. Res. Rec. 2021, 2675, 308–320. [Google Scholar] [CrossRef]
- Casagrande, A. Classification and Identification of Soils. Trans. Am. Soc. Civ. Eng. 1948, 113, 901–930. [Google Scholar] [CrossRef]
- Holtz, R.D.; Kovacs, W.D.; Sheahan, T.C. An Introduction to Geotechnical Engineering; Pearson: London, UK, 2011; ISBN 9780130317216. [Google Scholar]
- Leroueil, S.; Hight, D.W. Compacted Soils: From Physics to Hydraulic and Mechanical Behaviour. In Advances in Unsaturated Soils; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9780203771075. [Google Scholar]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L.; Karrech, A. Systematic Approach to Assessing the Applicability of Fly-Ash-Based Geopolymer for Clay Stabilization. Can. Geotech. J. 2020, 57, 1356–1368. [Google Scholar] [CrossRef]
- Changizi, F.; Haddad, A. Improving the Geotechnical Properties of Soft Clay with Nano-Silica Particles. Proc. Inst. Civ. Eng. Ground Improv. 2017, 170, 62–71. [Google Scholar] [CrossRef]
- Choobbasti, A.J.; Samakoosh, M.A.; Kutanaei, S.S. Mechanical Properties Soil Stabilized with Nano Calcium Carbonate and Reinforced with Carpet Waste Fibers. Constr. Build. Mater. 2019, 211, 1094–1104. [Google Scholar] [CrossRef]
- Fakhrabadi, A.; Ghadakpour, M.; Choobbasti, A.J.; Kutanaei, S.S. Evaluating the Durability, Microstructure and Mechanical Properties of a Clayey-Sandy Soil Stabilized with Copper Slag-Based Geopolymer against Wetting-Drying Cycles. Bull. Eng. Geol. Environ. 2021, 80, 5031–5051. [Google Scholar] [CrossRef]
- Hamza, M.; Nie, Z.; Aziz, M.; Ijaz, N.; Ameer, M.F.; Ijaz, Z. Geotechnical Properties of Problematic Expansive Subgrade Stabilized with Xanthan Gum Biopolymer. Road Mater. Pavement Des. 2022, 24, 1869–1883. [Google Scholar] [CrossRef]
- Luo, H.-L.; Hsiao, D.-H.; Lin, D.-F.; Lin, C.-K. Cohesive Soil Stabilized Using Sewage Sludge Ash/Cement and Nano Aluminum Oxide. Int. J. Transp. Sci. Technol. 2012, 1, 83–100. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Das, R.; Seth, D. Plasticity and Strength Characteristics of Expansive Soil Treated with Xanthan Gum Biopolymer. In Problematic Soils and Geoenvironmental Concerns; Gali, M.L., Rao, P.R., Eds.; Lecture Notes in Civil Engineering 88; Springer: Singapore, 2021; Volume 88, pp. 649–663. [Google Scholar]
- Sujatha, E.R.; Saisree, S. Geotechnical Behaviour of Guar Gum-Treated Soil. Soils Found. 2019, 59, 2155–2166. [Google Scholar] [CrossRef]
- Thomas, G.; Rangaswamy, K. Strengthening of Cement Blended Soft Clay with Nano-Silica Particles. Geomech. Eng. 2020, 20, 505–516. [Google Scholar] [CrossRef]
- Ugwu, O.O.; Arop, J.B.; Nwoji, C.U.; Osadebe, N.N. Nanotechnology as a Preventive Engineering Solution to Highway Infrastructure Failures. J. Constr. Eng. Manag. 2013, 139, 987–993. [Google Scholar] [CrossRef]
- Vydehi, K.V.; Moghal, A.A.B. Effect of Biopolymeric Stabilization on the Strength and Compressibility Characteristics of Cohesive Soil. J. Mater. Civ. Eng. 2022, 34, 04021428. [Google Scholar] [CrossRef]
- Huang, O.D.; Samuel, R.; Banerjee, A.; Puppala, A.J.; Radovic, M. Development of Alternative Stabilization Methods for Transportation Infrastructure Based on Geopolymers. MATEC Web Conf. 2019, 271, 02008. [Google Scholar] [CrossRef] [Green Version]
- Majeed, Z.H.; Taha, R.; Jawad, I.T. Stabilization of Soft Soil Using Nanomaterials. Res. J. Appl. Sci. Eng. Technol. 2014, 8, 503–509. [Google Scholar] [CrossRef]
- Onyia, M.E.; Agunwamba, J.C.; Nwonu, D.C. Hydraulic Conductivity Behaviour of Expansive Soil Geopolymer Binders. Arab. J. Geosci. 2021, 14, 503. [Google Scholar] [CrossRef]
- Samuel, R.; Huang, O.; Banerjee, A.; Puppala, A.; Das, J.; Radovic, M. Case Study: Use of Geopolymers to Evaluate the Swell-Shrink Behavior of Native Clay in North Texas. In Proceedings of the Geo-Congress 2019 GSP 309, Philadelphia, PA, USA, 24–27 March 2019; ASCE: Reston, VA, USA, 2019; pp. 167–178. [Google Scholar]
- Taha, M.R.; Taha, O.M.E. Influence of Nano-Material on the Expansive and Shrinkage Soil Behavior. J. Nanoparticle Res. 2012, 14, 1190. [Google Scholar] [CrossRef]
- Vydehi, K.V.; Moghal, A.A.B. Compressibility Characteristics of Guar Gum-Treated Expansive Soil. In Proceedings of Indian Geotechnical Conference: Ground Improvement and Reinforced Soil Structures; Reddy, S., Saride, S., Krishna, M., Eds.; Springer: Singapore, 2022; pp. 339–345. [Google Scholar]
- Das, B.M.; Sobhan, K. Principles of Geotechnical Engineering, 9th ed.; Cengage Learning: Boston, MA, USA, 2018. [Google Scholar]
- Chowdhury, R.H. Shear Strength Properties of Compacted Expansive Soils. Master’s Thesis, University of Regina, Regina, SK, Canada, 2013. [Google Scholar]
- Yin, P.; Vanapalli, S.K. Model for Predicting Evolution of Microstructural Void Ratio in Compacted Clayey Soils. Can. Geotech. J. 2022, 59, 1602–1621. [Google Scholar] [CrossRef]
- Chowdhury, R.H.; Azam, S. Unsaturated Shear Strength Properties of a Compacted Expansive Soil from Regina, Canada. Innov. Infrastruct. Solut. 2016, 1, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Z.; Wang, F.; Zhang, X. Comparison of Soil Tortuosity Calculated by Different Methods. Geoderma 2021, 402, 115358. [Google Scholar] [CrossRef]
- Khalid, N.; Mukri, M.; Kamarudin, F.; Abdul Ghani, A.H. Effect of Compaction Characteristics on Hydraulic Conductivity Performance for Sedimentary Residual Soil Mixed Bentonite as Compacted Liners. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Johor, Malaysia, 19–20 December 2019; Institute of Physics Publishing: Bristol, UK, 2020; Volume 498. [Google Scholar]
- Azam, S.; Chowdhury, R.H. Swell-Shrink-Consolidation Behavior of Compacted Expansive Clays. Int. J. Geotech. Eng. 2013, 7, 424–430. [Google Scholar] [CrossRef]
- Biju, M.S.; Arnepalli, D.N. Effect of Biopolymers on Permeability of Sand-Bentonite Mixtures. J. Rock. Mech. Geotech. Eng. 2020, 12, 1093–1102. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Experimental Investigation of Using a Recycled Glass Powder-Based Geopolymer to Improve the Mechanical Behavior of Clay Soils. Constr. Build. Mater. 2018, 170, 302–313. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Awraheem, M.H.; Khalaf, M.M. Geotechnical Properties of a Low-Plasticity Clay with Biopolymer. J. Mater. Civ. Eng. 2018, 30, 04018170. [Google Scholar] [CrossRef]
- Emmanuel, E.; Lau, C.C.; Anggraini, V.; Pasbakhsh, P. Stabilization of a Soft Marine Clay Using Halloysite Nanotubes: A Multi-Scale Approach. Appl. Clay Sci. 2019, 173, 65–78. [Google Scholar] [CrossRef]
- Joga, J.R.; Varaprasad, B.J.S. Sustainable Improvement of Expansive Clays Using Xanthan Gum as a Biopolymer. Civ. Eng. J. 2019, 5, 1893–1903. [Google Scholar] [CrossRef]
- Murmu, A.L.; Jain, A.; Patel, A. Mechanical Properties of Alkali Activated Fly Ash Geopolymer Stabilized Expansive Clay. KSCE J. Civ. Eng. 2019, 23, 3875–3888. [Google Scholar] [CrossRef]
- Nagaraju, T.V.; Mounika, K.N. Swelling Characteristics of Fly Ash Based Geopolymer Expansive Clay Blends. In Proceedings of Indian Geotechnical Conference 2020: Ground Improvement and Reinforced Soil Structures; Reddy, S., Saride, S., Krishna, M., Eds.; Springer: Singapore, 2022; pp. 233–240. [Google Scholar]
- Ndayambaje, R. Engineering Geopolymer Soil Material Using Fine Dredged Material (FDM) and Alkali-Activated Fly Ash Cement (AAFA). Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2020. [Google Scholar]
- Ng, C.W.W.; Coo, J.L. Hydraulic Conductivity of Clay Mixed with Nanomaterials. Can. Geotech. J. 2015, 52, 808–811. [Google Scholar] [CrossRef]
- Syed, M.; GuhaRay, A.; Agarwal, S.; Kar, A. Stabilization of Expansive Clays by Combined Effects of Geopolymerization and Fiber Reinforcement. J. Inst. Eng. Ser. A 2020, 101, 163–178. [Google Scholar] [CrossRef]
- Taha, M.R.; Alsharef, J.M.A. Performance of Soil Stabilized with Carbon Nanomaterials. Chem. Eng. Trans. 2018, 63, 757–762. [Google Scholar] [CrossRef]
- Ren, J.; Shen, Z.-z.; Yang, J.; Zhao, J.; Yin, J.-n. Effects of Temperature and Dry Density on Hydraulic Conductivity of Silty Clay under Infiltration of Low-Temperature Water. Arab. J. Sci. Eng. 2014, 39, 461–466. [Google Scholar] [CrossRef]
- Widomski, M.K.; Stępniewski, W.; Horn, R.; Bieganowski, A.; Gazda, L.; Franus, M.; Pawłowska, M. Shrink-Swell Potential, Hydraulic Conductivity and Geotechnical Properties of Clay Materials for Landfill Liner Construction. Int. Agrophysics 2015, 29, 365–375. [Google Scholar] [CrossRef]
- Shimobe, S.; Spagnoli, G. A General Overview on the Correlation of Compression of Clays with Some Geotechnical Index Properties. Geotech. Geol. Eng. 2021, 40, 311–324. [Google Scholar] [CrossRef]
- Akbarimehr, D.; Eslami, A.; Imam, R. Correlations between Compression Index and Index Properties of Undisturbed and Disturbed Tehran Clay. Geotech. Geol. Eng. 2021, 39, 5387–5393. [Google Scholar] [CrossRef]
- Alshkane, Y.M.; Rashed, K.A.; Daoud, H.S. Unconfined Compressive Strength (UCS) and Compressibility Indices Predictions from Dynamic Cone Penetrometer Index (DCP) for Cohesive Soil in Kurdistan Region/Iraq. Geotech. Geol. Eng. 2020, 38, 3683–3695. [Google Scholar] [CrossRef]
- Bello, A.A. Hydraulic Conductivity of Three Compacted Reddish Brown Tropical Soils. KSCE J. Civ. Eng. 2013, 17, 939–948. [Google Scholar] [CrossRef]
- Skempton, A.W. Notes on the Compressibility of Clays. Q. J. Geol. Soc. Lond. 1944, 100, 119–136. [Google Scholar] [CrossRef]
- Wroth, C.P.; Wood, D.M. The Correlation of Index Properties with Some Basic Engineering Properties of Soils. Can. Geotech. J. 1978, 15, 137–145. [Google Scholar] [CrossRef]
- Salem, T.; Hussain, M. Effect of Water on the Strength of El-Arish Clay (An Arid Calcareous Deposit in North Sinai). In Proceedings of the Al-Azhar Engineering 6th International Conference, Cairo, Egypt, 1–4 September 2000. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhary, A.; Azam, S. A Review of Physicochemical Stabilization for Improved Engineering Properties of Clays. Geotechnics 2023, 3, 744-759. https://doi.org/10.3390/geotechnics3030041
Bukhary A, Azam S. A Review of Physicochemical Stabilization for Improved Engineering Properties of Clays. Geotechnics. 2023; 3(3):744-759. https://doi.org/10.3390/geotechnics3030041
Chicago/Turabian StyleBukhary, Ahmed, and Shahid Azam. 2023. "A Review of Physicochemical Stabilization for Improved Engineering Properties of Clays" Geotechnics 3, no. 3: 744-759. https://doi.org/10.3390/geotechnics3030041
APA StyleBukhary, A., & Azam, S. (2023). A Review of Physicochemical Stabilization for Improved Engineering Properties of Clays. Geotechnics, 3(3), 744-759. https://doi.org/10.3390/geotechnics3030041










