Effect of Metakaolin and Lime on Strength Development of Blended Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Calcination Process
2.3. Composite Cement Paste Samples
2.4. Characterization
3. Results
3.1. Phase Analysis of Un-Treated and Calcined Kaolinite Samples with and without Limestone Additions
3.2. Thermal Analysis of Un-Treated and Calcined Kaolinite Samples with and without Limestone Additions
3.3. Amount of Portlandite in Blended Cement Paste Samples
3.4. Hydration Products of Blended Cement Paste Samples
3.5. Evaluating Microstructural Changes Due to Co-Calcined Kaolinite and Limestone in Blended Cement Paste Samples
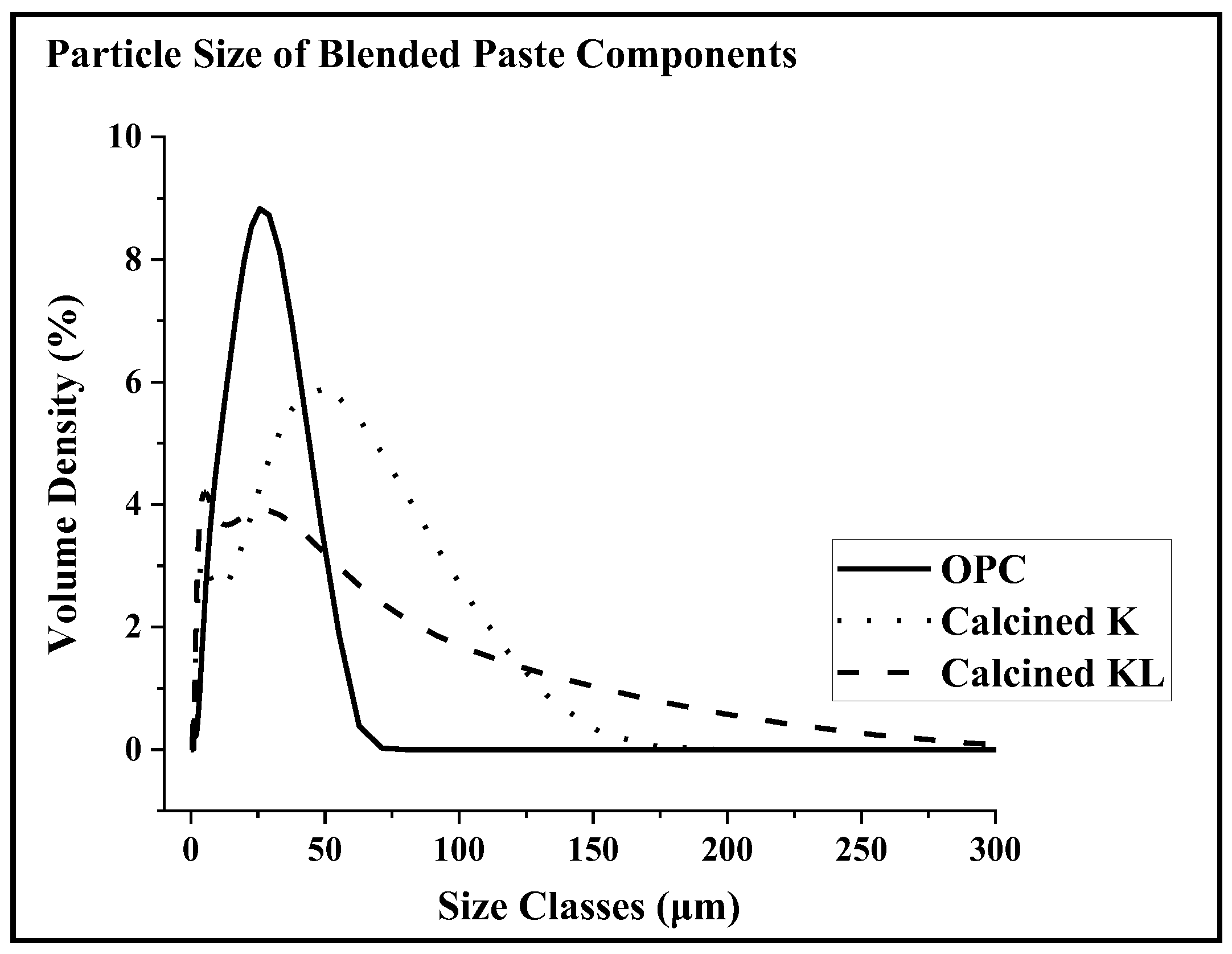
3.6. Particle Size Analysis of Calcined SCM Samples
3.7. Compressive Strength of Blended Cement Paste Samples
4. Discussion
4.1. Using the Full Pozzolanic Potential of Metakaolin
4.2. Increased Compressive Strength in Blended Cement Pastes
- (1)
- the formation of CASH and secondary CSH,
- (2)
- the additional consumption Portlandite,
- (3)
- the increased ettringite conversion to monosulfate and
- (4)
- the reduction in porosity.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delatte, N.J. Lessons from Roman cement and concrete. J. Prof. Issues Eng. Educ. Pract. 2001, 127, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Brandon, C.J.; Hohlfelder, R.L.; Jackson, M.D.; Oleson, J.P. Building for Eternity: The History and Technology of Roman Concrete Engineering in the Sea; Oxbow Books: Oxford, UK, 2014. [Google Scholar]
- Weise, K.; Ukrainczyk, N.; Duncan, A.; Koenders, E. Enhanced Metakaolin Reactivity in Blended Cement with Additional Calcium Hydroxide. Materials 2022, 15, 367. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Murat, M.; Comel, C. Hydration reaction and hardening of calcined clays and related minerals III. Influence of calcination process of kaolinite on mechanical strengths of hardened metakaolinite. Cem. Concr. Res. 1983, 13, 631–637. [Google Scholar] [CrossRef]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Güneyisi, E.; Gesoğlu, M.; Mermerdaş, K. Improving strength, drying shrinkage, and pore structure of concrete using metakaolin. Mater. Struct. 2008, 41, 937–949. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Jovein, H.B. Influence of metakaolin as supplementary cementing material on strength and durability of concretes. Constr. Build. Mater. 2012, 30, 470–479. [Google Scholar] [CrossRef]
- El-Diadamony, H.; Amer, A.A.; Sokkary, T.M.; El-Hoseny, S. Hydration and characteristics of metakaolin pozzolanic cement pastes. HBRC J. 2018, 14, 150–158. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Mujedu, K.A.; Ab-Kadir, M.A.; Sarbini, N.N.; Ismail, M. Microstructure and compressive strength of self-compacting concrete incorporating palm oil fuel ash exposed to elevated temperatures. Constr. Build. Mater. 2021, 274, 122025. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent progress of utilization of activated kaolinitic clay in cementitious construction materials. Compos. Part B Eng. 2021, 211, 108636. [Google Scholar] [CrossRef]
- Dung, T.N.; Chang, T.-P.; Chen, C.-T. Hydration process and compressive strength of slag-CFBC fly ash materials without portland cement. J. Mater. Civ. Eng. 2015, 27, 04014213. [Google Scholar] [CrossRef]
- Menchaca-Ballinas, L.; Martinez-Lopez, R.; Escalante-Garcia, J. Compressive strength and microstructure of alkali-activated waste glass-slag cements. J. Sustain. Cem.-Based Mater. 2022, 11, 1–12. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Hassan Nezhad, K. Activation of Aluminosilicate Materials. Master’s Thesis, Sabancı University, Istanbul, Turkey, 2020. [Google Scholar]
- Mokhtari, P. New Low Cost and Green Composite Binder for Construction. Ph.D. Thesis, Sabancı University, Istanbul, Turkey, 2019. [Google Scholar]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132, 552–560. [Google Scholar] [CrossRef]
- He, C.; Osbaeck, B.; Makovicky, E. Pozzolanic reactions of six principal clay minerals: Activation, reactivity assessments and technological effects. Cem. Concr. Res. 1995, 25, 1691–1702. [Google Scholar] [CrossRef]
- Sperinck, S.; Raiteri, P.; Marks, N.; Wright, K. Dehydroxylation of kaolinite to metakaolin—A molecular dynamics study. J. Mater. Chem. 2011, 21, 2118–2125. [Google Scholar] [CrossRef]
- Ambroise, J.; Martin-Calle, S.; Pera, J. Pozzolanic behavior of thermally activated kaolin. Spec. Publ. 1992, 132, 731–748. [Google Scholar]
- Bredy, P.; Chabannet, M.; Pera, J. Microstructure and porosity of metakaolin blended cements. MRS Online Proc. Libr. 1988, 136, 275–280. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Carrasco, M.F.; Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength optimization of “tailor-made cement” with limestone filler and blast furnace slag. Cem. Concr. Res. 2005, 35, 1324–1331. [Google Scholar] [CrossRef]
- Güneyisi, E.; Gesoğlu, M. Properties of self-compacting portland pozzolana and limestone blended cement concretes containing different replacement levels of slag. Mater. Struct. 2011, 44, 1399–1410. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Mounanga, P.; Khokhar, M.I.A.; El Hachem, R.; Loukili, A. Improvement of the early-age reactivity of fly ash and blast furnace slag cementitious systems using limestone filler. Mater. Struct. 2011, 44, 437–453. [Google Scholar] [CrossRef]
- Pera, J.; Husson, S.; Guilhot, B. Influence of finely ground limestone on cement hydration. Cem. Concr. Compos. 1999, 21, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.D.; Chae, S.R.; Mulcahy, S.R.; Meral, C.; Taylor, R.; Li, P.; Emwas, A.H.; Moon, J.; Yoon, S.; Vola, G.; et al. Unlocking the secrets of Al-tobermorite in Roman seawater concrete. Am. Mineral. 2013, 98, 1669–1687. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Kanaujia, S.K.; Mithia, S.; Bishnoi, S. Hydration kinetics and mechanisms of carbonates from stone wastes in ternary blends with calcined clay. Constr. Build. Mater. 2018, 164, 265–274. [Google Scholar] [CrossRef]
- Krishnan, S.; Emmanuel, A.C.; Bishnoi, S. Hydration and phase assemblage of ternary cements with calcined clay and limestone. Constr. Build. Mater. 2019, 222, 64–72. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K.L. The Effect of Calcite and Gibbsite Impurities in Calcined Clay on Its Reactivity. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2020; pp. 357–362. [Google Scholar]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Karunadasa, K.S.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Portland Cement. In Science and Technology of Concrete Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–51. [Google Scholar]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Yudenfreund, M.; Hanna, K.M.; Skalny, J.; Older, I.; Brunauer, S. Hardened Portland cement pastes of low porosity V. Compressive strength. Cem. Concr. Res. 1972, 2, 731–743. [Google Scholar] [CrossRef]
- Auskern, A.; Horn, W. Capillary porosity in hardened cement paste. J. Test. Eval. 1973, 1, 74–79. [Google Scholar]
- Pantazopoulou, S.J.; Mills, R. Microstructural aspects of the mechanical response of plain concrete. Mater. J. 1995, 92, 605–616. [Google Scholar]
- Chindaprasirt, P.; Hatanaka, S.; Chareerat, T.; Mishima, N.; Yuasa, Y. Cement paste characteristics and porous concrete properties. Constr. Build. Mater. 2008, 22, 894–901. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvarzman, A.; Kovler, K.; Grader, G.S.; Shter, G.E. The effect of dehydroxylation/amorphization degree on pozzolanic activity of kaolinite. Cem. Concr. Res. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Andrade, C. Calcium carbonate decomposition. J. Therm. Anal. Calorim. 2013, 111, 1197–1202. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016; Volume 540. [Google Scholar]
- Chen, X.; Wu, S. Influence of water-to-cement ratio and curing period on pore structure of cement mortar. Constr. Build. Mater. 2013, 38, 804–812. [Google Scholar] [CrossRef]
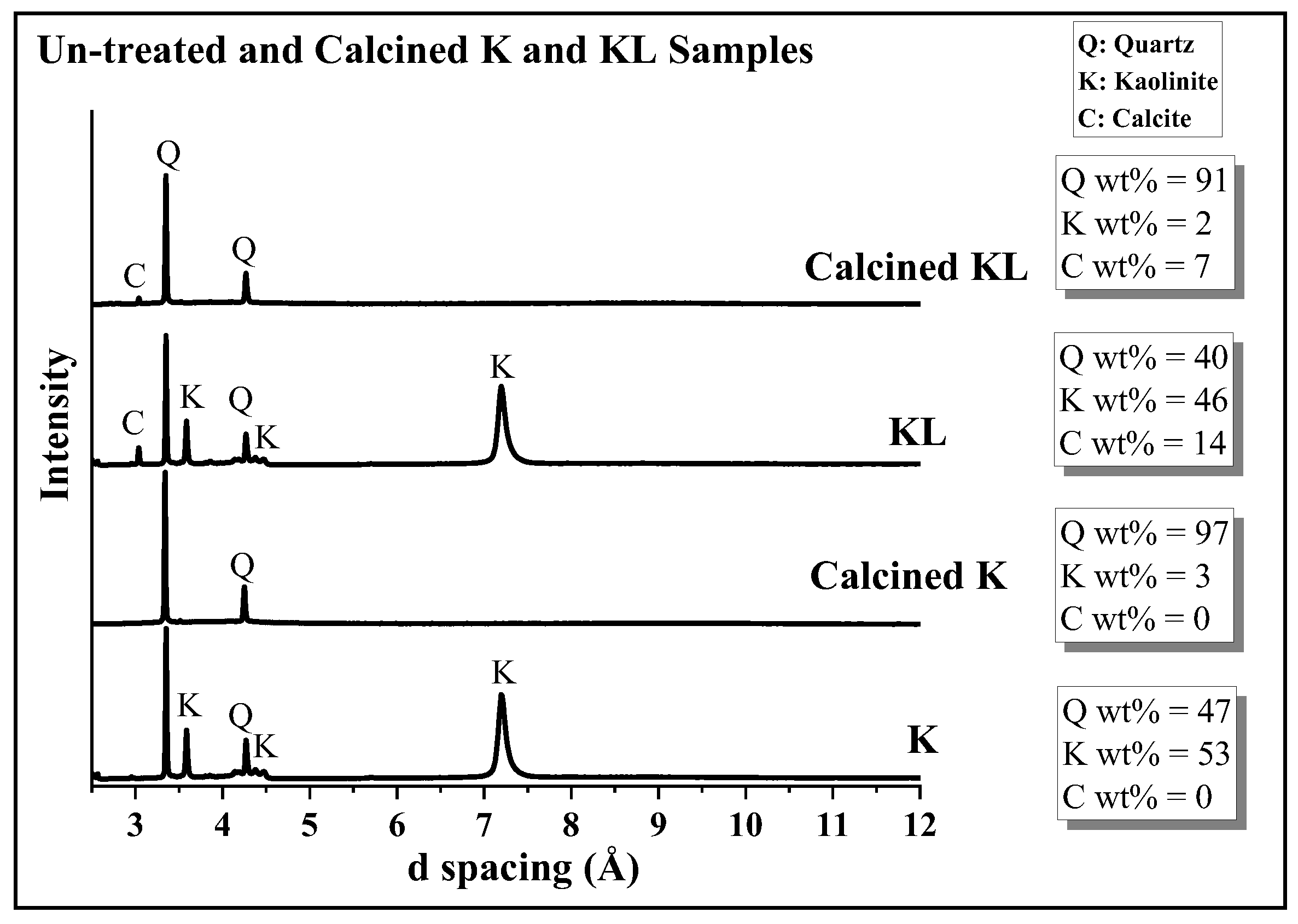

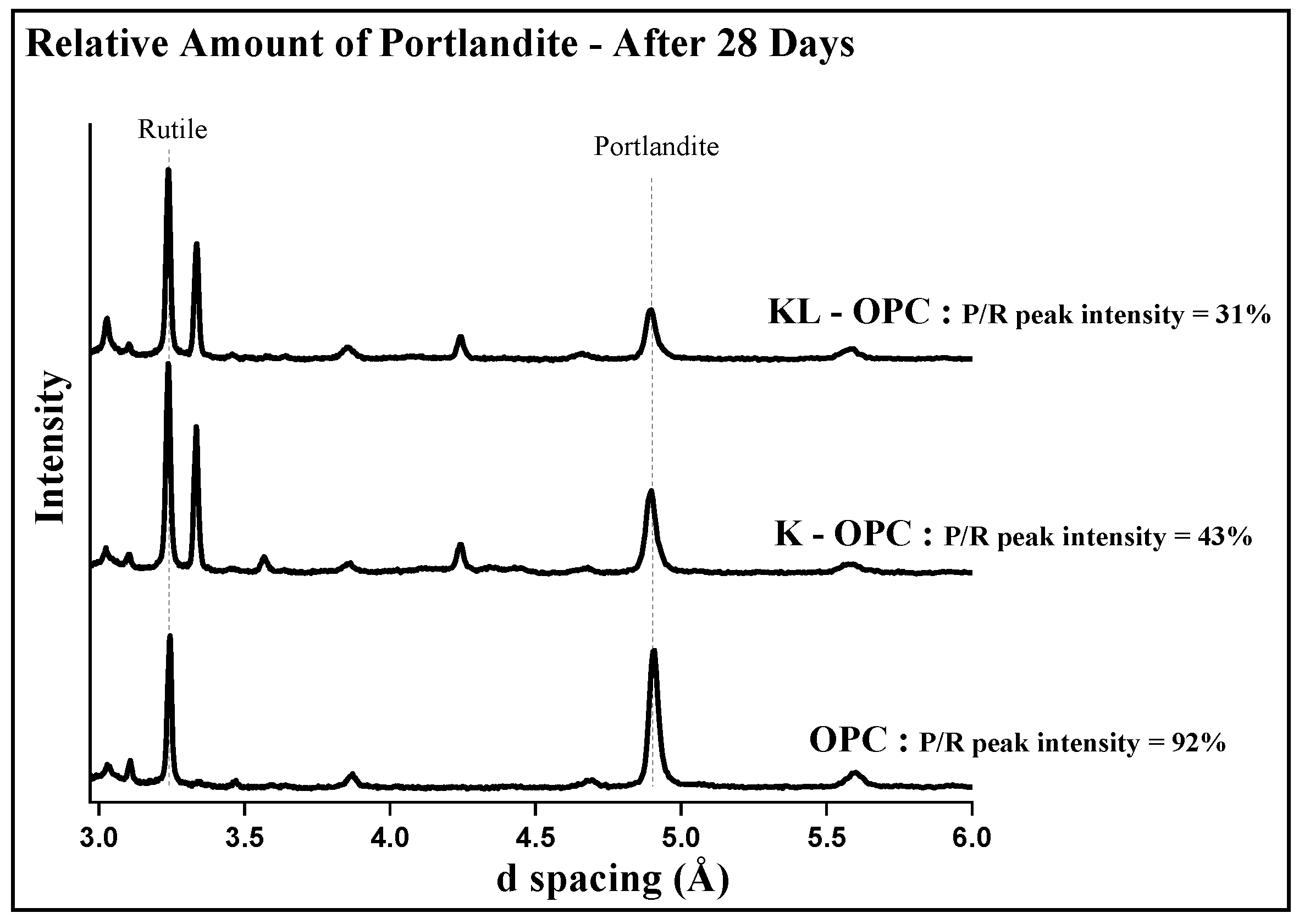



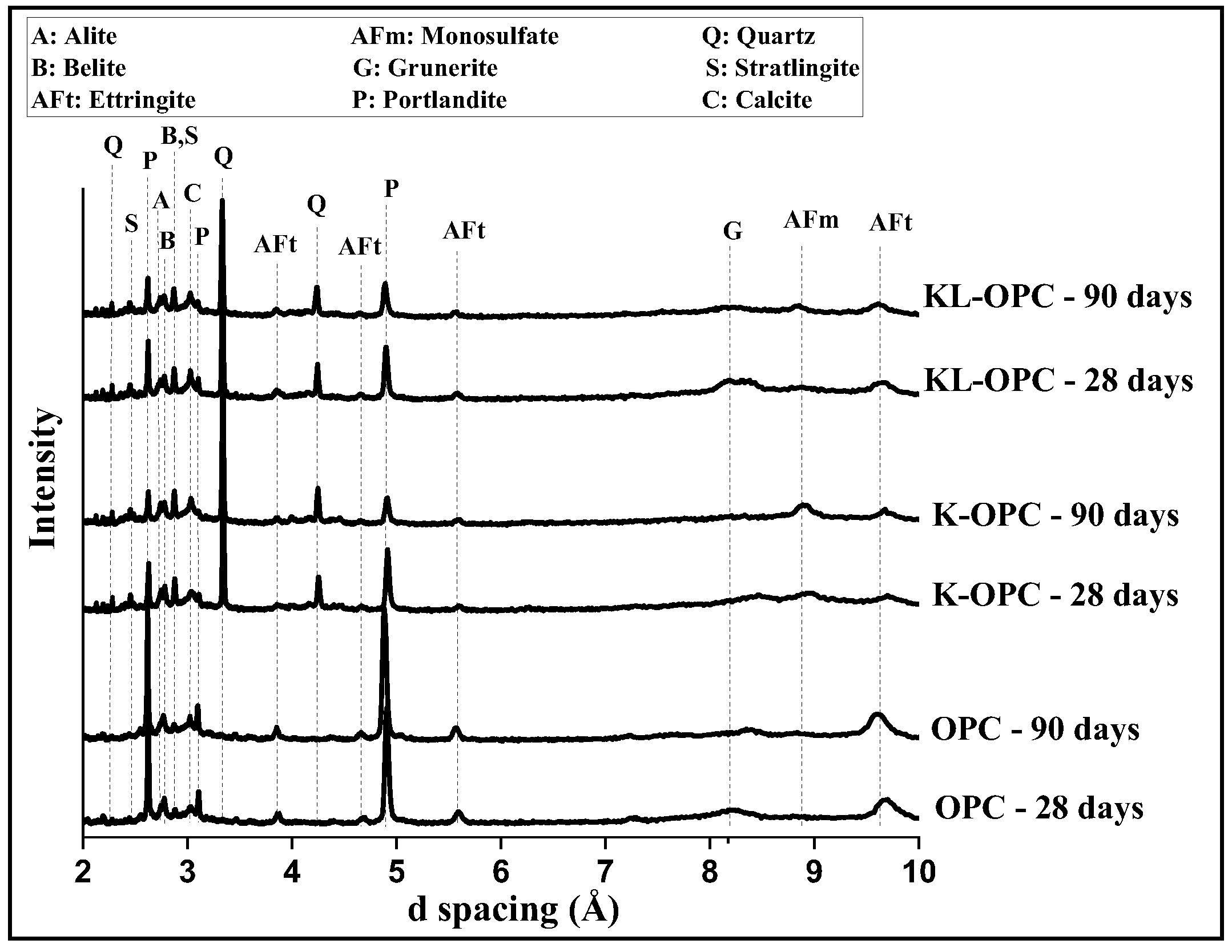


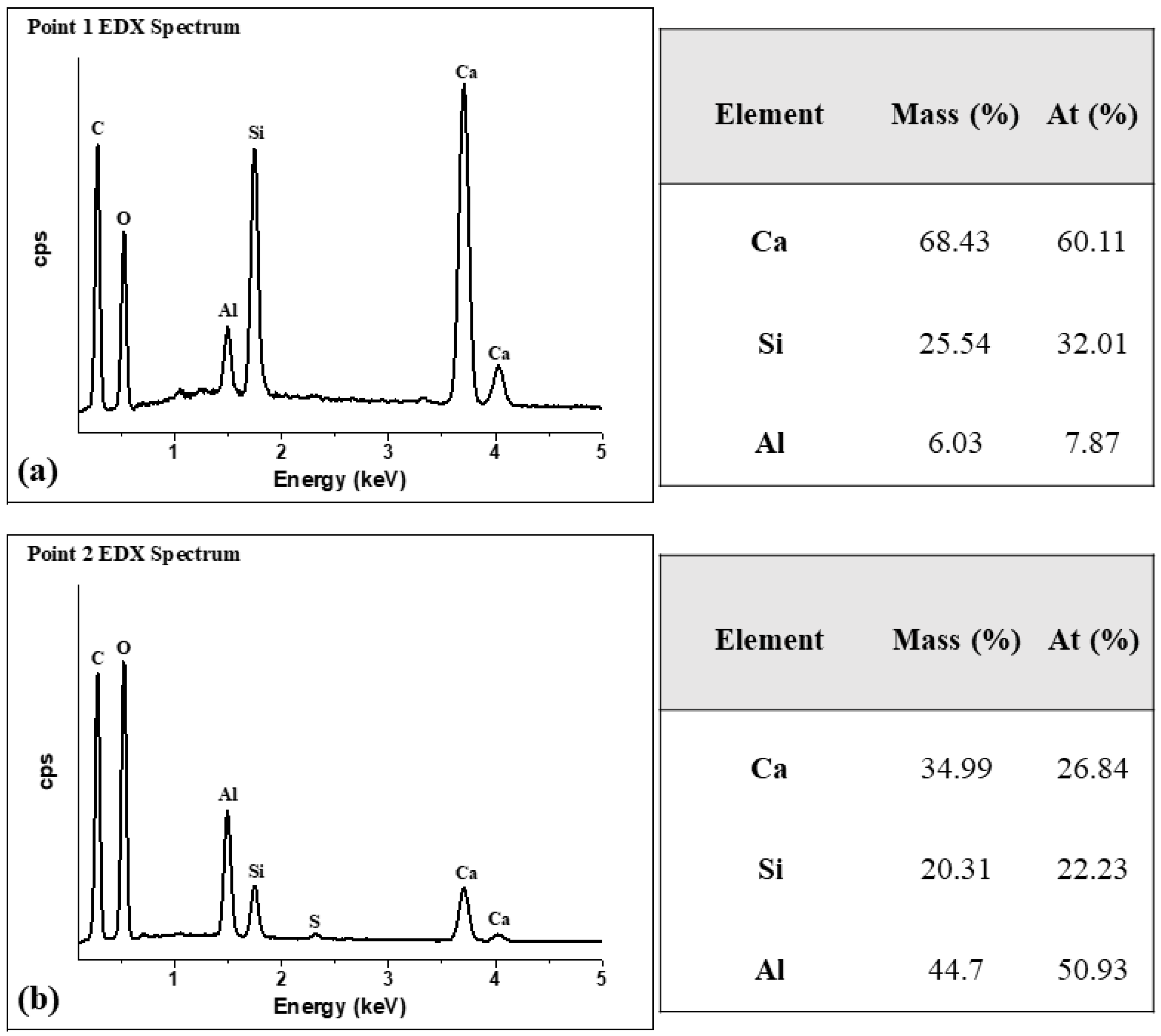

| Sample | Phase Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C3S | C2S | C3A | C4AF | Gypsum | Kaolinite | Quartz | Calcite | |
| OPC | 71 | 14 | 2 | 8 | 5 | - | - | - |
| Kaolinite | - | - | - | - | - | 53 | 47 | - |
| Limestone | - | - | - | - | - | - | 1 | 99 |
| Cement Paste Sample | Cement (wt%) | Calcined Kaolinite (Metakaolin) (wt%) | Co-Calcined Limestone (wt%) |
|---|---|---|---|
| OPC | 100 | - | - |
| K-OPC | 70 | 30 | - |
| KL-OPC | 70 | 25.5 | 4.5 |
| Sample | Weight Loss (300–650 °C) | Weight Loss (650–800 °C) |
|---|---|---|
| K | 8% | 0% |
| KL | 6.8% | 6.6% |
| Calcined K | 0.8% | 0% |
| Calcined KL | 0.4% | 3.5% |
| Sample | Portlandite Amount after 28 Days | Portlandite Amount after 90 Days |
|---|---|---|
| OPC | 9.8% | 16.3% |
| K-OPC | 9.1% | 6.8% |
| KL-OPC | 7.7% | 5% |
| Chemical Phases | OPC 28 Days | K-OPC 28 Days | KL-OPC 28 Days | OPC 90 Days | K-OPC 90 Days | KL-OPC 90 Days |
|---|---|---|---|---|---|---|
| Amorphous | 50.7% | 52.3% | 50% | 57.4% | 55.5% | 61.8% |
| Ettringite | 11.8% | 3.5% | 4.4% | 10% | 2.2% | 3.1% |
| Grunerite | 2.8% | 3.1% | 2.2% | 1.3% | 3.3% | 2.5% |
| Monosulfate | 1.2% | 5.6% | 3.2% | 1.6% | 5.2% | 3.8% |
| Portlandite | 22% | 6.8% | 6.8 % | 20.6% | 4.7% | 3.7% |
| Alite | 4.2% | 3.5% | 7.9% | 8% | 4.4% | 3.1% |
| Belite | 7.3% | 8.1% | 7.8% | 4.9% | 7.2% | 6.2% |
| Stratlingite | - | 1.1% | 1% | - | 2.5% | 2.9% |
| Quartz | - | 16% | 14% | - | 15% | 10.6% |
| Calcite | - | - | 2.7% | - | - | 2.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassannezhad, K.; Akyol, Y.; Dursun, M.C.; Ow-Yang, C.W.; Gulgun, M.A. Effect of Metakaolin and Lime on Strength Development of Blended Cement Paste. Constr. Mater. 2022, 2, 297-313. https://doi.org/10.3390/constrmater2040019
Hassannezhad K, Akyol Y, Dursun MC, Ow-Yang CW, Gulgun MA. Effect of Metakaolin and Lime on Strength Development of Blended Cement Paste. Construction Materials. 2022; 2(4):297-313. https://doi.org/10.3390/constrmater2040019
Chicago/Turabian StyleHassannezhad, Kosar, Yasemin Akyol, Mehmet Can Dursun, Cleva W. Ow-Yang, and Mehmet Ali Gulgun. 2022. "Effect of Metakaolin and Lime on Strength Development of Blended Cement Paste" Construction Materials 2, no. 4: 297-313. https://doi.org/10.3390/constrmater2040019
APA StyleHassannezhad, K., Akyol, Y., Dursun, M. C., Ow-Yang, C. W., & Gulgun, M. A. (2022). Effect of Metakaolin and Lime on Strength Development of Blended Cement Paste. Construction Materials, 2(4), 297-313. https://doi.org/10.3390/constrmater2040019





