Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Methods and Sample Preparation
2.2.1. Preparation of Saturated Ca(OH)2 Solution
2.2.2. Interaction of TiNTs and Fe/TiNTs with Supersaturated Ca(OH)2 Solution
2.2.3. Effect of TiNTs and Fe/TiNTs on the Hydration of C3S
3. Results and Discussion
3.1. Characterization of Educts
3.2. Incorporation of Synthesized TiNTs and Fe/TiNTs in a Cementitious System
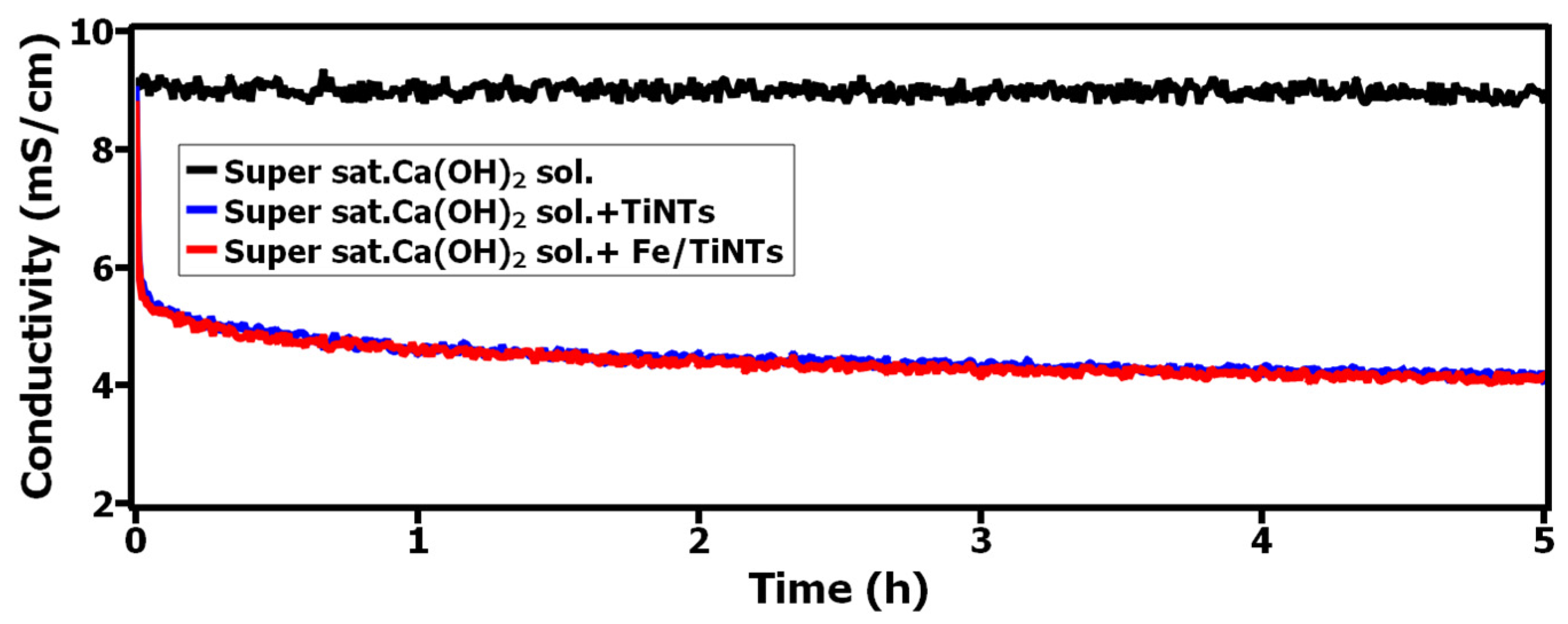
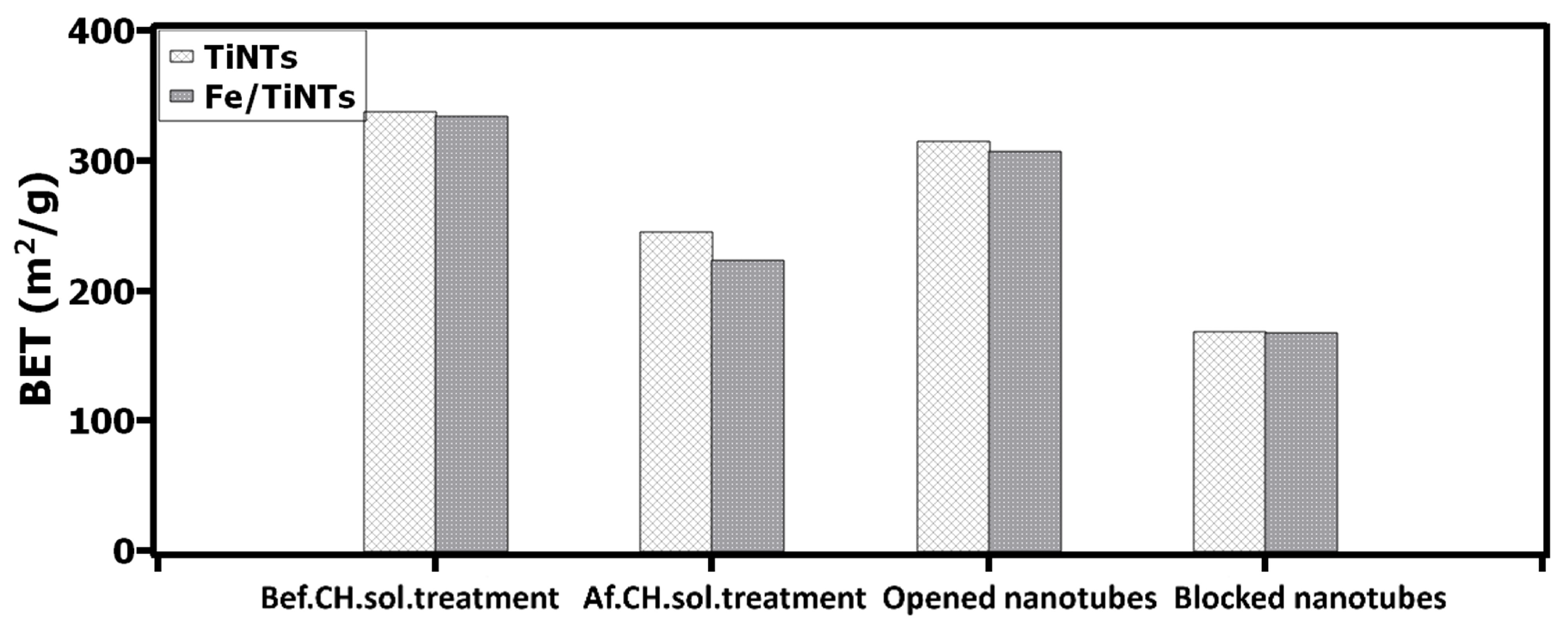
3.2.1. Interaction of TiNTs and Fe/TiNTs with Supersaturated Ca(OH)2 Solution via pH, Conductivity and BET Specific Surface Area Measurement
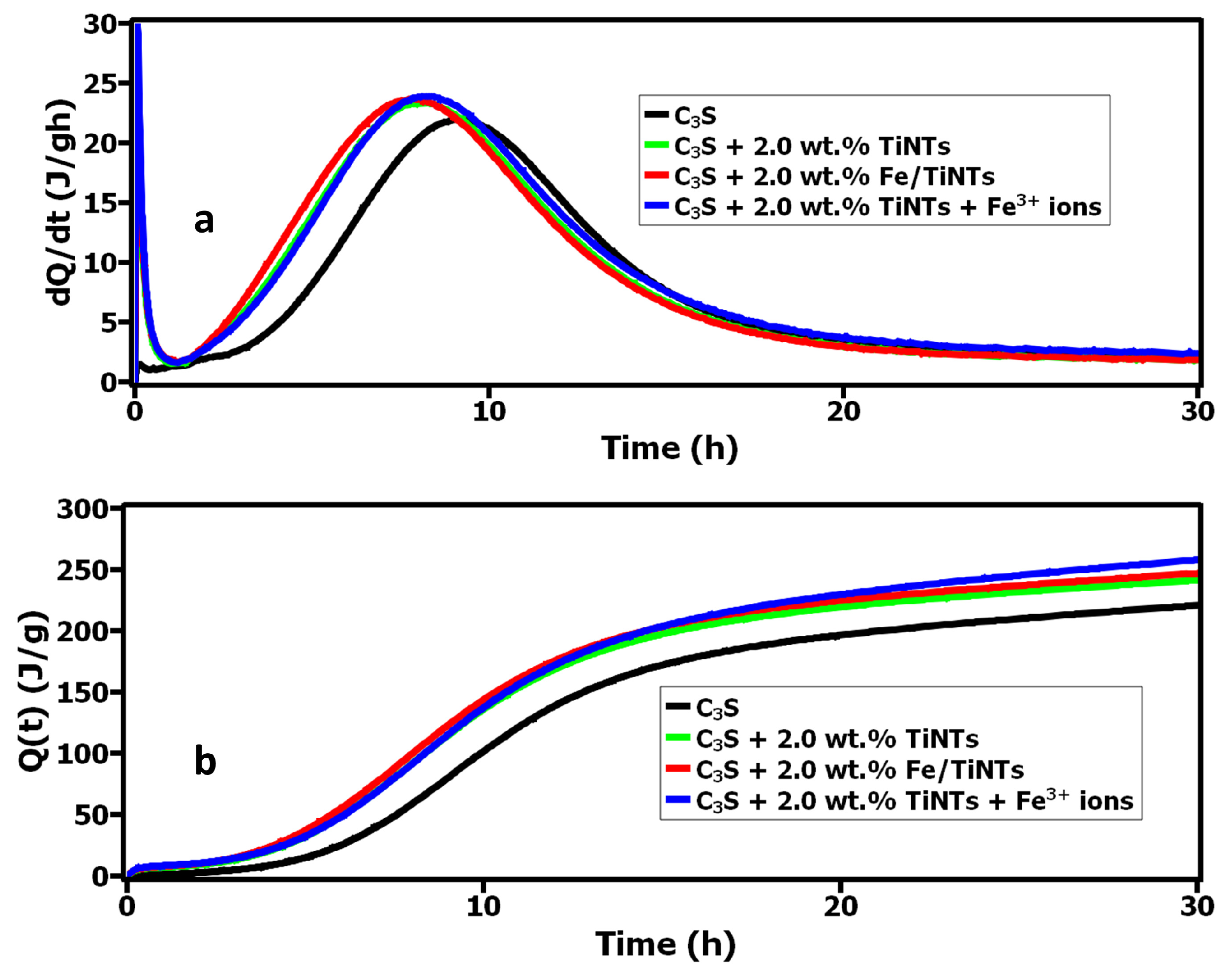
3.2.2. Influence of TiNTs and Fe/TiNTs on the Hydration of C3S
- Course of hydration reaction
- 2.
- Study on the hydrated paste of C3S
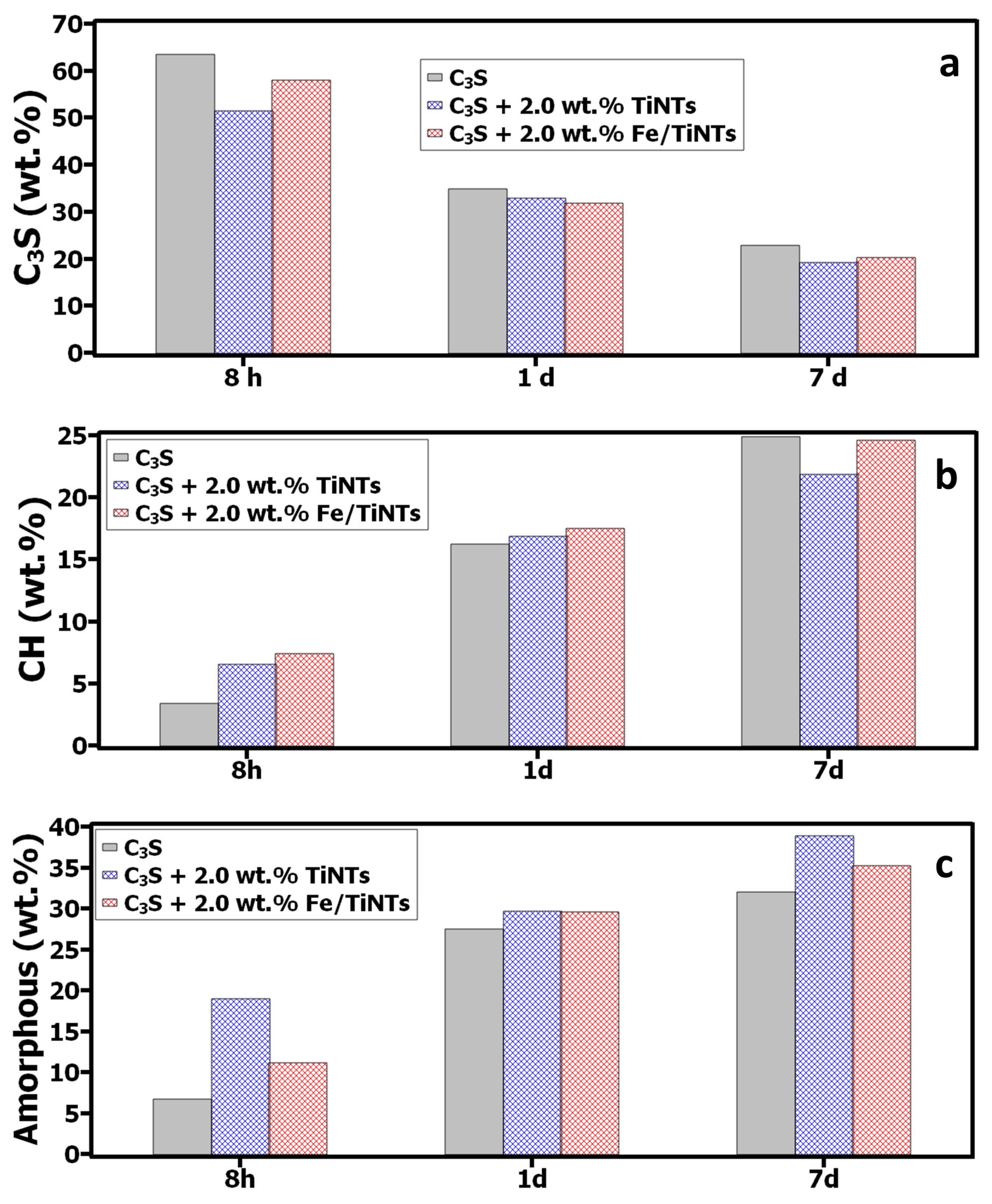
- Characterization of C3S hydration products
- Phase composition and determination of the degree of hydration after 8 h, 1 d and 7 d
- Determination of BET specific surface area of hydration products
- Mechanical study of hydrated C3S prism
4. Conclusions
- Treatment with supersaturated Ca(OH)2 solution.
- A strong interaction between Ca2+-ions and both TiNTs and Fe/TiNTs was observed as a result of treatment with super saturated Ca(OH)2 solution. The results were proved by pH, conductivity and gas adsorption measurements.
- The pH and conductivity measurements showed a decrease as a result of Ca2+ ions being sorbed by the nanotubes.
- The amount of sorbed Ca2+-ions by nanotubes were quantitatively determined via EDTA titration and thermal analysis using STA.
- The gas adsorption measurement showed lower BET specific surface area and porosity for both type of nanotubes after treatment with super saturated Ca(OH)2 solution, indicating sorption of Ca2+-ions by nanotubes.
- The effect of Fe/TiNTs on the sorption of Ca2+-ions in supersaturated were superior compared to TiNTs.
- Incorporation of Fe/TiNTs and TiNTs with C3S.
- In terms of hydration course, a shortening of the induction period and earlier acceleration period was observed for the samples containing nanotubes. However, the effect of Fe/TiNTs was found to be superior compared to the control sample (pure C3S) and the sample with TiNTs.
- Concerning the studies on hydration products, more hydration products were formed in the presence of Fe/TiNTs and TiNTs, particularly for the samples with Fe/TiNTs content.
- The degree of hydration increased, especially in the presence of Fe/TiNTs. The BET specific surface area of hydration products was quantitatively determined via combing XRD and gas adsorption data.
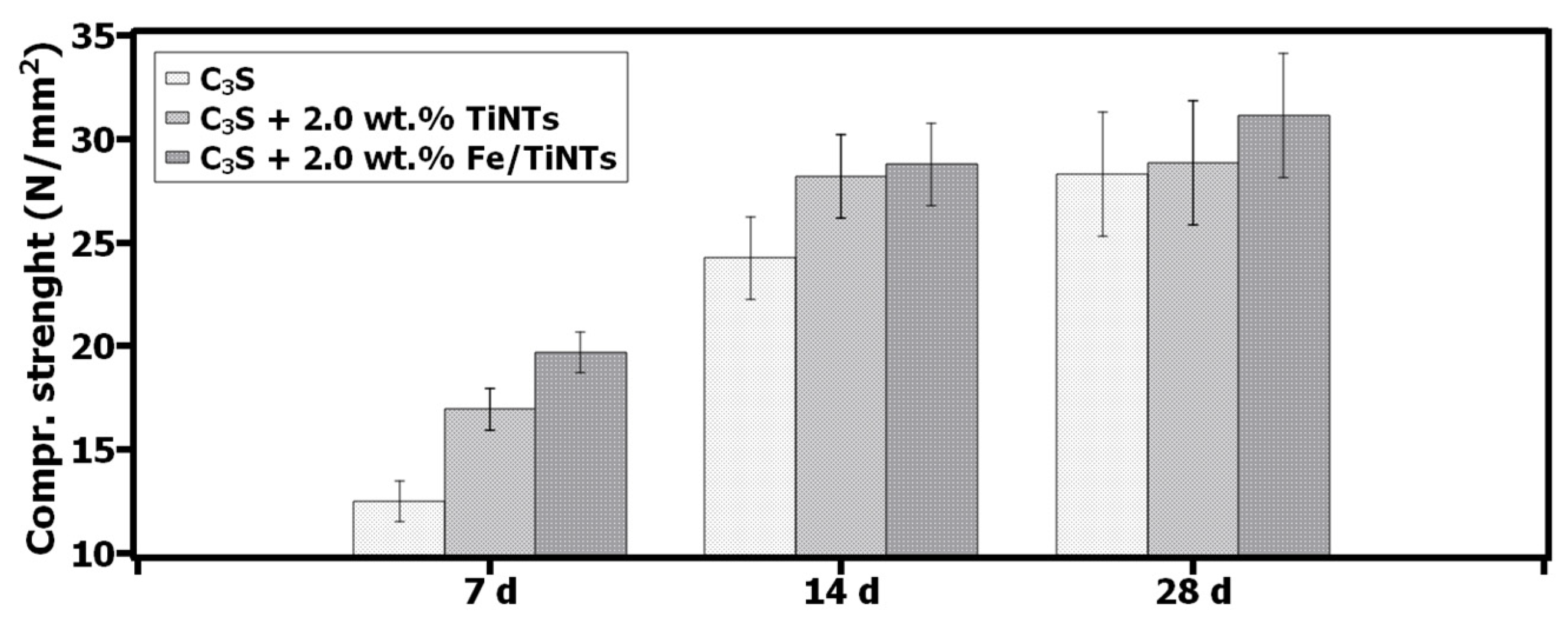
- A 36%, 16% and 9% increase in compressive strength of Fe/TiNTs samples for 7 d, 14 d and 28 d hydrated C3S paste was obtained.
- On the basis of above obtained results, the following conclusions were achieved:
- Iron-doped TiNTs (Fe/TiNTs) can serve as a promising reinforcement nanomaterial for cement-based construction materials.
- Lowering the values of pH and conductivity, decreasing the concentration of Ca2+-ions in supersaturated Ca(OH)2 solution and decreasing the specific surface area of nanotubes after the treatment with supersaturated Ca(OH)2 are indications of improvement in hydration reactions.
- The main hydration products of cement, such as C-S-H and CH phases, are formed in the media of saturated Ca(OH)2 solution. Finding a strong interaction between Fe/TiNTs and Ca2+-ions from the Ca(OH)2 solution treatment is an indication of improvement of C3S hydration, resulting in a higher amount of hydration products.
- One of the remarkable features of Fe/TiNTs is their enhanced photocatalytic activity at higher wavelengths. This makes them a viable option for the production of self-cleaning concrete in indoor environments where the intensity of UV radiation is low.
- The fabricated nanotube can directly incorporate into concrete when there is a need for this material in a particular case, such as the surface of concrete, but not within the bulk of concrete. The fabricated Fe/TiNTs have high specific surface area of Fe/TiNTs; even fewer percentages (1–2% or even less) of the nanotube is sufficient to use in the construction industry compared to other additives, which can stabilize the cost-benefit. If we take this into consideration, we only need less material and only the top layer of concrete should be modified; then, the overall cost will be not that high.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pustovgar, E.; Sangodkar, R.P.; Andreev, A.S.; Palacios, M.; Chmelka, B.F. Understanding Silicate Hydration from Quantitative Analyses of Hydrating Tricalcium Silicates. Nat. Commun. 2016, 10952, 10952. [Google Scholar] [CrossRef] [Green Version]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Alizadeh, R.; Raki, L.; Makar, J.M.; Beaudoin, J.J.; Moudrakovskid, I. Hydration of Tricalcium Silicate in the Presence of Synthetic Calcium–Silicate–Hydrate. J. Mater. Chem. 2009, 19, 7937–7946. [Google Scholar] [CrossRef] [Green Version]
- Jong, B.J.G.M.d.; Stein, H.N.; Stevels, J.M. Hydration of Tricalcium Silicate. J. Mater. Chem. 1967, 17. [Google Scholar] [CrossRef]
- Sakalli, Y.; Trettin, R. Investigation of C3S Hydration by Environmental Scanning Electron Microscope. J. Microsc. 2015, 259, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Trettin, R. Reactivity and Mechanism of Hydration of Cement Phases. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997. [Google Scholar]
- Trettin, R.; Wieker, W. Zur Hydratation von Tricalciumsilicat I. Ursachen Der Induktionsperiod, Silikattechnik. Silikattechnik 1986, 37, 75–78. [Google Scholar]
- Korpa, A.; Trettin, R. The Influence of Different Drying Methods on Cement Paste Microstructures as Reflected by Gas Adsorption-Comparison between Freeze-Drying (F-Drying), D-Drying, P-Drying and Oven-Drying Methods. Cem. Concr. Res. 2006, 36, 634–649. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Hydration Behaviour, Structure and Morphology of Hydration Phases in Advanced Cement-Based Systems Containing Micro and Nanoscale Pozzolanic Additives. Cem. Concr. Res. 2008, 38, 955–962. [Google Scholar] [CrossRef]
- Korpa, A.; Trettin, R.; Böttger, K.-G.; Thieme, J.; Schmidt, C. Pozzolanic Reactivity of Nanoscale Pyrogene Oxides and Their Strength Contribution in Cement-Based Systems. Adv. Cem. Res. 2008, 20, 35–46. [Google Scholar] [CrossRef]
- Stötzel, T.; Ali, M.; Trettin, R. Neue Nanopartikel Zur Verbesserung Der Eigenschaften von Zementären Bindemitteln, Posterpräsentation. In GDCh Tagungsband: Tagung Bauchemie, Hamburg; GDCh Tagungsband: Hamburg, Germany, 2011; pp. 344–349. [Google Scholar]
- Trettin, R.; Satava, V.; Wieker, W. Studies on Physico-Chemical Changes in Time of Initial Setting of C3S. In First International Workshop on Hydration and Setting, Dijon 1991; RILEM, Ed.; E&FN Spon: London, UK, 1992; pp. 289–297. [Google Scholar]
- Korpa, A.; Trettin, R. Nanoscale Pozzolans for Improving Ultra High Performance Cementitious Binders. Cem. Int. 2007, 1, 74–83. [Google Scholar]
- Wang, J. Steady-State Chloride Diffusion Coefficient and Chloride Migration Coefficient of Cracks in Concrete. J. Mater. Civ. Eng. 2017, 29, 4017117. [Google Scholar] [CrossRef]
- Qinfei, L.; Wang, Y.; Heng, C.; Pengkun, H.; Xin, C. Effect of Calcium Chloride on Hydration Kinetics and Pore Structure of Hydrated Tricalcium Silicate. In International Conference on Optoelectronic Science and Materials 2019; Materials Science and Engineering; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2020; p. 12055. [Google Scholar]
- Dorn, T.; Blask, O.; Stephan, D. Acceleration of Cement Hydration–A Review of the Working Mechanisms, Effects on Setting Time, and Compressive Strength Development of Accelerating Admixtures. Constr. Build. Mater. 2022, 323, 126554. [Google Scholar] [CrossRef]
- Mingli, C.; Xing, M.; Kaiyu; Li, L.; Shirley, S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites—A Review. Materials 2019, 12, 781. [Google Scholar]
- Birgisson, B.; Mukhopadhyay, A.K.; Geary, G.; Khan, M. Nanotechnology in Concrete Materials; Transportation Research Board: Washington, DC, USA, 2012. [Google Scholar]
- Dorn, T.; Hirsch, T.; Stephan, D. Working Mechanism of Calcium Nitrate as an Accelerator for Portland Cement Hydration. J. Am. Ceram. Soc. 2022, 106, 752–766. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E.; Li, L. Characterization on the Recycling of Waste Seashells with Portland Cement towards Sustainable Cementitious Materials. J. Clean. Prod. 2019, 220, 235–252. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Wang, J. Effects of Aggregate Micro Fines (AMF), Aluminum Sulfate and Polypropylene Fiber (PPF) on Properties of Machine-Made Sand Concrete. Appl. Sci. 2019, 9, 2250. [Google Scholar] [CrossRef] [Green Version]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and Concrete Nanoscience and Nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef] [Green Version]
- Sahin, R.; Oltulu, M. New Materials For Concrete Technology: Nano Powders. In Proceedings of the 33rd Conference on OUR World in Concrete & Structures, Singapore, 25–27 August 2008; CI-Premier PTE LTD: Singapore, 2008. [Google Scholar]
- Hunashyal, A.M.; Tippa, S.V.; Quadri, S.S.; Banapurmath, N.R. Experimental Investigation on Effect of Carbon Nanotubes and Carbon Fibres on the Behavior of Plain Cement Mortar Composite Round Bars under Direct Tension. Int. Sch. Res. Netw. 2011, 2011, 856849. [Google Scholar] [CrossRef] [Green Version]
- Chena, J.; Akono, A.-T. Influence of Multi-Walled Carbon Nanotubes on the Hydration Products of Ordinary Portland Cement Paste. Cem. Concr. Res. 2020, 137, 106197. [Google Scholar] [CrossRef]
- Ge, Z.; Gao, Z. Applications of Nanotechnology and Nanomaterials in Construction. In Proceedings of the First International Conference on Construction in Developing Countries (ICCIDC–I)“Advancing and Integrating Construction Education, Research & Practice”, Karachi, Pakistan, 4–5 August 2008. [Google Scholar]
- Kowald, T.; Trettin, R. Improvement of Cementitious Binders by Multi-Walled Carbon Nanotubes. Nanotechnol. Constr. 2009, 3, 261–266. [Google Scholar]
- Gammampila, R.; Mendis, P.; Ngo, T.; Aye, L.; Jayalath, A.S.; Rupasinghe, R.A.M. Application Of Nanomaterials In The Sustainable Built Environment. In Proceedings of the International Conference on Sustainable Built Environment (ICSBE-2010), Kandy, Sri Lanka, 13–14 December 2010. [Google Scholar]
- Abd Elrahman, M.; Sikora, P.; Chung, S.; Stephan, D. The Performance of Ultra-Lightweight Foamed Concrete Incorporating Nanosilica. Arch. Civ. Mech. Eng. 2021, 21, 79. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kurtis, K.E. Influence of TiO2 Nanoparticles on Early C3S Hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar] [CrossRef]
- Katya, N.K.; Ahluwalia, S.C.; Parkash, R. Effect of TiO2 on the Hydration of Tricalcium Silicate. Cem. Concr. Res. 1999, 29, 1851–1855. [Google Scholar] [CrossRef]
- Cook, R.; Ma, H.; Kumar, A. Influence of size-classified and slightly soluble mineral additives on hydration of tricalcium silicate. J. Am. Ceram. Soc. 2019, 103, 2764–2779. [Google Scholar] [CrossRef]
- Wu, L.; Mei, M.; Li, Z.; Liu, S.; Wang, X. Study on Photocatalytic and Mechanical Properties of TiO2 Modified Pervious Concrete. Case Stud. Constr. Mater. 2022, 17, e01606. [Google Scholar] [CrossRef]
- Sadeghi Nik, A.; Bahari, A.; Amiri, B. Nanostructural Properties of Cement–Matrix Composite. J. Basic Appl. Sci. Res. 2011, 1, 2167–2173. [Google Scholar]
- Yu, X.; Kang, S.; Long, X. Compressive Strength of Concrete Reinforced by TiO2 Nanoparticles. In 3rd International Conference on Materials Science, Resource and Environmental Engineering (MSREE 2018); AIP Publishing: Melville, NY, USA, 2018; p. 030006. [Google Scholar]
- Li, Z.; Wang, J.; Li, Y.; Yu, X.; Baoguo, H. Investigating Size Effect of Anatase Phase Nano TiO2 on the Property of Cement-Based Composites. Mater. Res. Express 2018, 5, 85034. [Google Scholar] [CrossRef]
- Jee, H.; Park, J.; Zalnezhad, E.; Jeong, K.; Woo, S.M.; Seok, S.; Sungchul, B. Characterization of Titanium Nanotube Reinforced Cementitious Composites: Mechanical Properties, Microstructure, and Hydration. Materials 2019, 12, 1617. [Google Scholar] [CrossRef] [Green Version]
- Siang, N.D.; Chandra, P.S.; Anggraini, V.; Kong, S.Y.; Shams, Q.T.; Romero, R.C.; Liu, Q.; Šavija, B. Influence of SiO2, TiO2 and Fe2O3 Nanoparticles on the Properties of Fly Ash Blended Cement Mortars. Constr. Build. Mater. 2020, 258, 119627. [Google Scholar] [CrossRef]
- Stötzel, T. Influence of Titanate Nanotubes on the Hydration of Tricalciumsilicate; Siegen University: Siegen, Germany, 2014. [Google Scholar]
- Liu, J.; Suh, H.; Jee, H.; Xu, J.; Zal Nezhad, E.; Choi, C.-S.; Bae, S. Synergistic Effect of Carbon Nanotube/TiO2 Nanotube Multi-Scale Reinforcement on Mechanical Properties and Hydration Process of Portland Cement Paste. Constr. Build. Mater. 2021, 293, 123447. [Google Scholar] [CrossRef]
- Mohd, S.; Qattali, Y. Modification of TiO2 Nanotubes via Doping with Fe3+-Ion Dopant and Its Application in Cementitious Systems. Ph.D. Thesis, University of Siegen, Siegen, Germany, 2020. [Google Scholar]
- Moni, S.M.; Oatalli SM, Y.; Pritzel, C.; Trettin, R. Interaction of Titania Nanotubes with Ca(OH)2 and C3S via Hydrothermal Approach. ACI Mater. J. 2020, 117, 233–239. [Google Scholar]
- Pérez-Nicolás, M.; Navarro-Blasco, Í.; Fernández, J.M.; Alvarez, J.I. The Effect of TiO2 Doped Photocatalytic Nano-Additives on the Hydration and Microstructure of Portland and High Alumina Cements. Nanomaterials 2017, 7, 329. [Google Scholar] [CrossRef] [Green Version]
- Trettin, R.; Ali, M.; Stötzel, T. The Effect of Titanate Nanotubes on Cementitious Systems for UHPC Application. In NICOM5; Springer International Publishing: Chicago, IL, USA, 2015. [Google Scholar]
- Korpa, A.; Kowald, T.; Treein, R. Principles of development, phase composition and nanostructural features of multiscale Ultra High Performance Concrete modified with pyrogenic nanoparticles–A review article. Am. J. Mater. Sci. Appl 2014, 2, 17–30. [Google Scholar]
- Feng, D.; Xie, N.; Gong, C.; Leng, Z.; Xiao, H.; Li, H.; Shi, X. Portland Cement Paste Modified by TiO2 Nanoparticles: A Microstructure Perspective. Ind. Eng. Chem. Res. 2013, 52, 11575–11582. [Google Scholar] [CrossRef]
- Liu, J.; Jee, H.; Lim, M.; Kim, J.H.; Kwon, S.J.; Lee, K.M.; Nezhad, E.Z.; Bae, S. Photocatalytic Performance Evaluation of Titanium Dioxide Nanotube-Reinforced Cement Paste. Materials 2020, 13, 5423. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Gao, Y. Effect of TiO2 Nanoparticles on Physical and Mechanical Properties of Cement at Low Temperatures. Adv. Mater. Sci. Eng. 2018, 2018, 8934689. [Google Scholar] [CrossRef] [Green Version]






| Free Lime Franke Method (wt.%) | Mineralogical Composition, XRD (wt.%) | Mean Particle Size. LG (µm) | ||
|---|---|---|---|---|
| C | C | C3S | C2S | d50 |
| 0.2 | 0.0 | 100 | 0 | 10.5 |
| Sample | End of Induction Period (h) | Duration of Acceleration Period (h) | Duration of Main Peak (h) |
|---|---|---|---|
| C3S | 4.5 | 6.8 | 15.3 |
| C3S + 1.0% TiNTs | 3.5 | 6.7 | 15.1 |
| C3S + 1.0% Fe/TiNTs | 3.0 | 6.8 | 15.4 |
| C3S + 2.0% TiNTs | 2.3 | 6.8 | 15.2 |
| C3S + 2.0% Fe/TiNTs | 2.1 | 7.0 | 15.9 |
| Age | Sample | C3S 4331-ICSD (%) | CH 61185-ICSD (%) | Cc 40107-ICSD (%) | Amorphous (tot. (%)) | Tot. Phy. Water (%) |
|---|---|---|---|---|---|---|
| 8 h | C3S | 63.47 | 0.75 | 0.50 | 11.30 | 23.97 |
| C3S + 2.0 wt.% TiNTs | 51.38 | 1.94 | 0.00 | 23.49 | 23.18 | |
| C3S + 2.0 wt.% Fe/TiNTs | 57.93 | 2.37 | 0.42 | 16.15 | 23.12 | |
| 1 d | C3S | 34.81 | 3.74 | 0.52 | 39.94 | 20.98 |
| C3S + 2.0 wt.% TiNTs | 32.87 | 6.80 | 0.48 | 39.75 | 20.10 | |
| C3S + 2.0 wt.% Fe/TiNTs | 31.85 | 6.82 | 0.61 | 40.25 | 20.46 | |
| 7 d | C3S | 22.90 | 12.67 | 1.38 | 44.18 | 18.86 |
| C3S + 2.0 wt.% TiNTs | 19.18 | 11.39 | 1.97 | 49.33 | 18.13 | |
| C3S + 2.0 wt.% Fe/TiNTs | 20.24 | 13.74 | 1.22 | 46.08 | 18.72 |
| Age | Sample | Weight Loss <105 °C | Chemically Bound Water (105–550 °C) | Tot. C3S | Excess Water | Ca(OH)2 (400–550 °C) | CaCO3 (550–700 °C) |
|---|---|---|---|---|---|---|---|
| 8 h | C3S | 0.44 | 2.27 | 66.67 | 31.53 | 3.37 | 0.84 |
| C3S + 2.0 wt.% TiNTs | 0.86 | 3.89 | 66.67 | 30.17 | 6.45 | 0.86 | |
| C3S + 2.0 wt.% Fe/TiNTs | 0.69 | 4.19 | 66.67 | 30.07 | 7.40 | 0.00 | |
| 1 d | C3S | 1.30 | 8.88 | 66.67 | 26.55 | 16.20 | 1.95 |
| C3S + 2.0 wt.% TiNTs | 1.21 | 10.25 | 66.67 | 25.69 | 16.86 | 0.95 | |
| C3S + 2.0 wt.% Fe/TiNTs | 1.34 | 10.07 | 66.67 | 25.73 | 17.51 | 0.00 | |
| 7 d | C3S | 1.08 | 14.03 | 66.67 | 23.26 | 24.87 | 3.45 |
| C3S + 2.0 wt.% TiNTs | 2.69 | 14.12 | 66.67 | 22.13 | 21.87 | 4.68 | |
| C3S + 2.0 wt.% Fe/TiNTs | 1.13 | 14.31 | 66.67 | 23.03 | 24.58 | 3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qattali, S.M.Y.; Pritzel, C.; Kowald, T.; Moni, S.M.F.K.; Killian, M.S.; Trettin, R. Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Constr. Mater. 2023, 3, 259-275. https://doi.org/10.3390/constrmater3020017
Qattali SMY, Pritzel C, Kowald T, Moni SMFK, Killian MS, Trettin R. Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Construction Materials. 2023; 3(2):259-275. https://doi.org/10.3390/constrmater3020017
Chicago/Turabian StyleQattali, S. Mohd. Yonos, Christian Pritzel, Torsten Kowald, S. M. Fuad Kabir Moni, Manuela S. Killian, and Reinhard Trettin. 2023. "Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate" Construction Materials 3, no. 2: 259-275. https://doi.org/10.3390/constrmater3020017
APA StyleQattali, S. M. Y., Pritzel, C., Kowald, T., Moni, S. M. F. K., Killian, M. S., & Trettin, R. (2023). Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Construction Materials, 3(2), 259-275. https://doi.org/10.3390/constrmater3020017







