Synthesis and Characterization of Iron-Doped TiO2 Nanotubes (Fe/TiNTs) with Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Methods and Sample Preparation
3. Results and Discussion
3.1. Characterization of Synthesized Fe/TiNTs
- Morphology analysis
- Phase analysis
- Specific surface area and pore size distribution
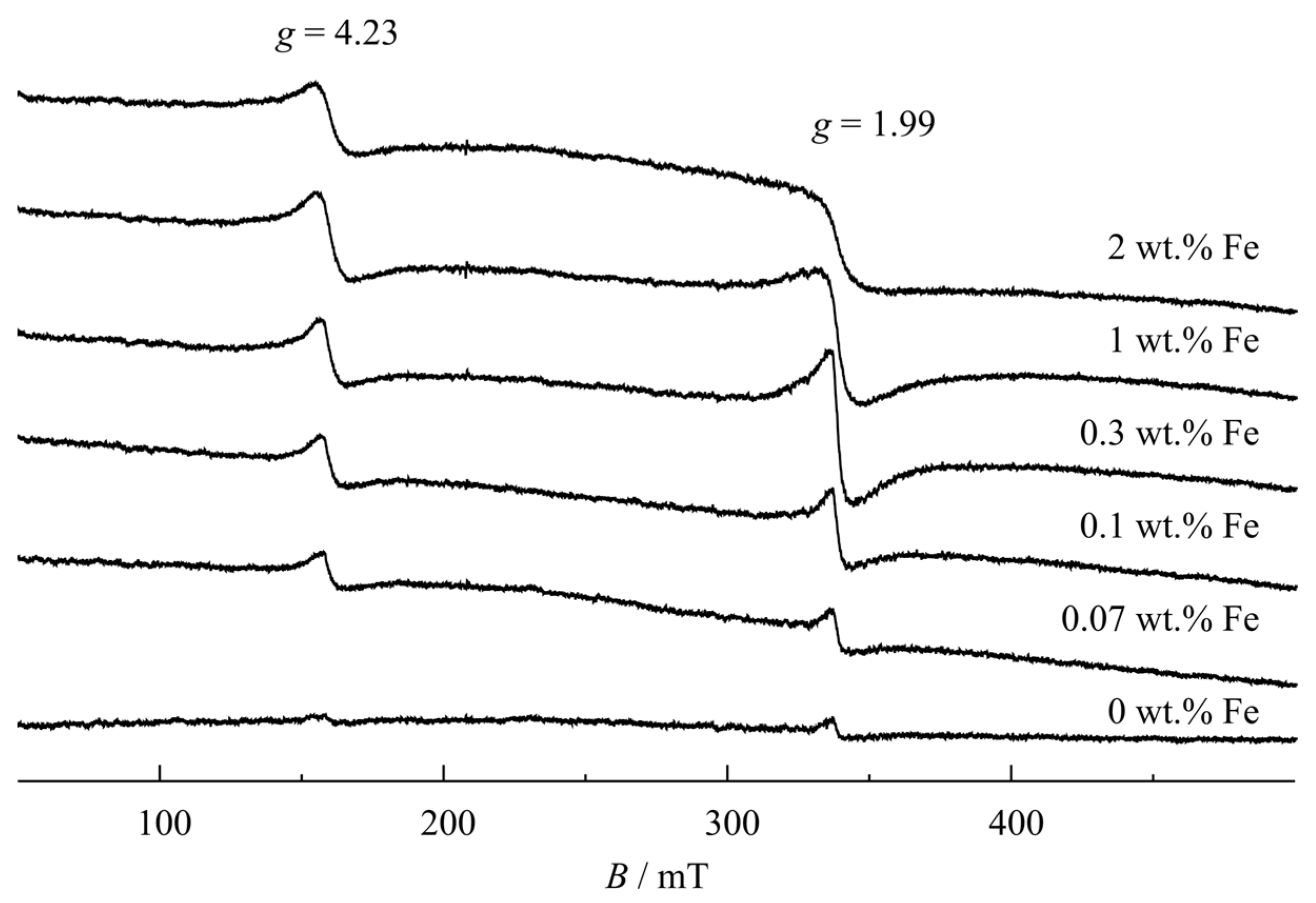
- Incorporation of Fe3+ ions into the crystal structure of TiNTs
- Absorption shift from UV light to visible light and determination of band gap
3.2. The Course of Hydration Reaction
- Evaluation of Photocatalytic Activity
4. Conclusions
- TiNTs were successfully doped with iron and the initial wt% of iron with respect to anatase for the synthesis of Fe/TiNTs were 0.0, 0.07, 0.1, 0.3, 1.0, and 2.00 wt%.
- SEM and TEM confirmed the formation of both types of nanotubes, TiNTs and Fe/TiNTs.
- TEM confirmed that the nanotubes were hollow and with a tubular structure. They consist of multilayers with an average outer diameter of 23–48 nm and length of 10–15 µm, respectively.
- XRD proved the doping process of TiNTs with Fe3+- ions, via altering on the XRD pattern. The FWHM of the (101) anatase plane increased from ~1° in the undoped TiNT to ~1.4° in the Fe/TiNT sample with 2.00 wt% Fe. Such an observation is expected to be a direct effect of doping of anatase TiNTs with Fe3+ ions. Furthermore, rutile formation within the anatase structure was observed upon doping higher levels of Fe3+, which is expected to enhance charge separation within the TiNTs and, consequently, enhance the photocatalytic efficiency of the material.
- ESR is in agreement with the interpretation that Fe3+ ions were incorporated into the crystal structure of anatase TiNTs occupying an octahedral site and another site next to an O vacancy .
- DRS showed an enhancement of light absorption in the visible region in the range of 400–600 nm. Particularly with the higher concentration of dopant, the absorption edge becomes more apparent and shifts further into the visible light region.
- A decrease in the optical band gap of Fe/TiNTs samples was revealed by DRS proportionally to the dopant concentration.
- The highest BET-specific surface area for the nanotubes was obtained for the pure TiNTs with 356 m2/g. As the wt% of iron in the samples increased, a decline in the values of BET-specific surface area of Fe/TiNTs was observed. Even though the doped samples showed lower surface areas than their undoped counterparts, their catalytic activity significantly surpassed the TiNTs, which highlights the beneficial nature of Fe3+ doping.
- An improvement in the hydration of Portland cement was observed in the presence of 2.0 wt% Fe/TiNTs compared to the references. The induction period was decreased and an acceleration was observed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, Photocatalytic Activity, and Mechanism of Nano-TiO2 Co-Doped with Nitrogen and Iron (III). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Khan, H.; Swati, I.K. Fe3+-Doped Anatase TiO2 with d-d Transition, Oxygen Vacancies and Ti3+ Centers: Synthesis, Characterization, UV-Vis Photocatalytic and Mechanistic Studies. Ind. Eng. Chem. Res. 2016, 55, 6619–6633. [Google Scholar] [CrossRef]
- Ma, J.; He, H.; Liu, F. Effect of Fe on the Photocatalytic Removal of NOx over Visible Light Responsive Fe/TiO2 Catalysts. Appl. Catal. B Environ. 2015, 179, 21–28. [Google Scholar] [CrossRef]
- Wen, L.; Liu, B.; Zhao, X.; Nakata, K.; Murakami, T.; Fujishima, A. Synthesis, Characterization, and Photocatalysis of Fe-Doped TiO2: A Combined Experimental and Theoretical Study. Int. J. Photoenergy 2012, 2012, 368750. [Google Scholar] [CrossRef]
- Elghniji, K.; Atyaoui, A.; Livraghi, S.; Bousselmi, L.; Giamello, E.; Ksibi, M. Synthesis and Characterization of Fe3+ Doped TiO2 Nanoparticles and Films and Their Performance for Photocurrent Response under UV Illumination. J. Alloys Compd. 2012, 541, 421–427. [Google Scholar] [CrossRef]
- Roy, N.; Sohn, Y.; Leung, K.T.; Pradhan, D. Engineered Electronic States of Transition Metal Doped TiO2 Nanocrystals for Low Overpotential Oxygen Evolution Reaction. J. Phys. Chem. C 2014, 118, 29499–29506. [Google Scholar] [CrossRef]
- Shwetharani, R.; Fernando, C.A.N.; Balakrishna, G.R. Excellent Hydrogen Evolution by a Multi Approach via Structure-Property Tailoring of Titania. RSC Adv. 2015, 5, 39122–39130. [Google Scholar] [CrossRef]
- Eadi, S.B.; Kim, S.; Jeong, S.W.; Jeon, H.W. Novel Preparation of Fe Doped TiO2 Nanoparticles and Their Application for Gas Sensor and Photocatalytic Degradation. Adv. Mater. Sci. Eng. 2017, 2017, 2191659. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Ellouzi, I.; El hajjaji, S.; Harir, M.; Schmitt-Kopplin, P.; Laânab, L. Coprecipitation Synthesis of Fe-Doped TiO2 from Various Commercial TiO2 for Photocatalytic Reaction. Int. J. Environ. Res. 2020, 14, 605–613. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, S.; Jeon, E.H.; Baik, J.; Kim, N.; Kim, H.S.; Lee, H. Enhancement of Photo-Oxidation Activities Depending on Structural Distortion of Fe-Doped TiO2 Nanoparticles. Nanoscale Res. Lett. 2016, 11, 41. [Google Scholar] [CrossRef]
- Anatase, K.; Tio, I.; Method, M.S.; Activity, P. ARTICLE ORIGINAL Iron-Doped TiO2 Catalysts with Photocatalytic Activity. J. Water Environ. Nanotechnol. 2019, 4, 60–66. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Zhang, J.D.; Long, Y.F.; Xue, X.; Xu, B.J. Kinetic Study on the Crystal Transformation of Fe-Doped TiO2 via In Situ High-Temperature X-Ray Diffraction and Transmission Electron Microscopy. ACS Omega 2021, 6, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Bonelli, B.; Esposito, S.; Freyria, F.S.; Blangetti, N.; Sannino, D. Fe-Doped TiO2 Prepared by a Three-Block Copolymer Templating Approach. Materials 2021, 14, 3105. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.L.; Pimentel, A.; Reis-Machado, A.S.; Rodrigues, J.; Deuermeier, J.; Fortunato, E.; Martins, R.; Nunes, D. Enhanced Fe-TiO2 Solar Photocatalysts on Porous Platforms for Water Purification. Nanomaterials 2022, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bu, Q.; Zhao, R.; Xu, W.; Jia, N.; Yang, L.; Tang, J. A Novel Preparation of Trace Iron-Doped 3D Urchin-like TiO2 for Efficient Degradation and Mineralization of Trimethoprim under Simulated Sunlight. Ceram. Int. 2024, 50, 11138–11149. [Google Scholar] [CrossRef]
- El Mragui, A.; Aadnan, I.; Zegaoui, O.; Esteves da Silva, J.C.G. Physico-Chemical Characterization and Photocatalytic Activity Assessment under UV-A and Visible-Light Irradiation of Iron-Doped TiO2 Nanoparticles. Arab. J. Chem. 2023, 16, 105331. [Google Scholar] [CrossRef]
- Soundarya, T.L.; Harini, R.; Manjunath, K.; Udayabhanu; Nirmala, B.; Nagaraju, G. Pt-Doped TiO2 Nanotubes as Photocatalysts and Electrocatalysts for Enhanced Photocatalytic H2 Generation, Electrochemical Sensing, and Supercapacitor Applications. Int. J. Hydrogen Energy 2023, 48, 31855–31874. [Google Scholar] [CrossRef]
- Eroğlu, Ö.; Kizil, H. The Effect of the Iron Doping on Anatase TiO2 Anode for Electrochemical Performance of Sodium-Ion Batteries. Solid State Ionics 2023, 393, 116168. [Google Scholar] [CrossRef]
- Lai, C.W.; Juan, J.C.; Ko, W.B.; Bee Abd Hamid, S. An Overview: Recent Development of Titanium Oxide Nanotubes as Photocatalyst for Dye Degradation. Int. J. Photoenergy 2014, 2014, 524135. [Google Scholar] [CrossRef]
- Pozio, A. Effect of Tantalum Doping on TiO2 Nanotube Arrays for Water-Splitting. Mod. Res. Catal. 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Lu, D.; Yang, J. Enhanced Visible Light Photocatalytic Activity for TiO2 Nanotube Array Films by Codoping with Tungsten and Nitrogen. Int. J. Photoenergy 2013, 2013, 471674. [Google Scholar] [CrossRef]
- Lu, N.; Quan, X.; Li, J.Y.; Chen, S.; Yu, H.T.; Chen, G. Fabrication of Boron-Doped TiO2 Nanotube Array Electrode and Investigation of Its Photoelectrochemical Capability. J. Phys. Chem. C 2007, 111, 11836–11842. [Google Scholar] [CrossRef]
- Sreekantan, S.; Zaki, S.M.; Lai, C.W.; Tzu, T.W. Copper-Incorporated Titania Nanotubes for Effective Lead Ion Removal. Mater. Sci. Semicond. Process. 2014, 26, 620–631. [Google Scholar] [CrossRef]
- Syamsul, S.; Harun, Z.; Norhayati, W.; Salleh, W.; Nur, M.; Abd, H.; Sazali, N.; Hussin, R.; Basri, H. The Influence of Fe Doped TiO2 as Inorganic Additive on the Properties of Polysulfone Ultrafitration Membrane. Malaysian J. Anal. Sci. 2019, 15, 725–730. [Google Scholar]
- Transactions, E.C.S.; Society, T.E. Investigations on the Fe-Doped TiO2 Nanotube Arrays as a Photoanode for Cathodic Protection of Stainless Steel. Society 2008, 3, 1. [Google Scholar]
- Eder, D.; Motta, M.; Windle, A.H. Iron-Doped Pt-TiO2 Nanotubes for Photo-Catalytic Water Splitting. Nanotechnology 2009, 20, 055602. [Google Scholar] [CrossRef] [PubMed]
- Szirmai, P.; Horváth, E.; Náfrádi, B.; Micković, Z.; Smajda, R.; Djokić, D.M.; Schenk, K.; Forró, L.; Magrez, A. Synthesis of Homogeneous Manganese-Doped Titanium Oxide Nanotubes from Titanate Precursors. J. Phys. Chem. C 2013, 117, 697–702. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmidt, B.; Kunze, J.; Schmuki, P. Photoresponse in the Visible Range from Cr Doped TiO2 Nanotubes. Chem. Phys. Lett. 2007, 433, 323–326. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Liu, D.; Zhu, B.; Huang, W.; Wu, S.; Zhang, S. Synthesis, Characterization of Fe-Doped TiO2 Nanotubes with High Photocatalytic Activity. Catal. Lett. 2009, 129, 513–518. [Google Scholar] [CrossRef]
- Nyankson, E.; Agyei-Tuffour, B.; Adjasoo, J.; Ebenezer, A.; Dodoo-Arhin, D.; Yaya, A.; Mensah, B.; Efavi, J.K. Synthesis and Application of Fe-Doped TiO2-Halloysite Nanotubes Composite and Their Potential Application in Water Treatment. Adv. Mater. Sci. Eng. 2019, 2019, 4270310. [Google Scholar] [CrossRef]
- Teng, H.; Xu, S.; Sun, D.; Zhang, Y. Preparation of Fe-Doped TiO2 Nanotubes and Their Photocatalytic Activities under Visible Light. Int. J. Photoenergy 2013, 2013, 981753. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Cui, X.; Yao, W.; Duan, T. One-Step Hydrothermal Synthesis of Iron and Nitrogen Co-Doped TiO2 Nanotubes with Enhanced Visible-Light Photocatalytic Activity. CrystEngComm 2015, 17, 8368–8376. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, K.W.; Perng, T.P. Electron Field Emission from Fe-Doped TiO2 Nanotubes. Appl. Phys. Lett. 2010, 96, 143102. [Google Scholar] [CrossRef]
- Hussain, S.T.; Siddiqa, A. Iron and Chromium Doped Titanium Dioxide Nanotubes for the Degradation of Environmental and Industrial Pollutants. Iran. J. Environ. Health Sci. Eng. 2011, 8, 351–362. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Yap, H.C.; Abdullah, A.Z. Sonocatalytic Degradation of Rhodamine B in the Presence of Iron-Doped TiO2 Nanotubes: Characterizations and Reaction Kinetic Studies. AIP Conf. Proc. 2017, 1828, 020010. [Google Scholar] [CrossRef]
- Qattali, S.M.Y.; Pritzel, C.; Kowald, T.; Moni, S.M.F.K.; Killian, M.S.; Trettin, R. Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Constr. Mater. 2023, 3, 259–275. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, J.J.; Yoo, D.Y. Benefits of TiO2 Photocatalyst on Mechanical Properties and Nitrogen Oxide Removal of Ultra-High-Performance Concrete. Constr. Build. Mater. 2021, 285, 122921. [Google Scholar] [CrossRef]
- Wu, L.; Mei, M.; Li, Z.; Liu, S.; Wang, X. Study on Photocatalytic and Mechanical Properties of TiO2 Modified Pervious Concrete. Case Stud. Constr. Mater. 2022, 17, e01606. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; He, T. SiO2/TiO2 and PDMS Modified Self-Cleaning Coating and Its Application in Decorative UHPC Surface. Ceram. Int. 2024, 50, 6194–6206. [Google Scholar] [CrossRef]
- Machala, L.; Zboril, R.; Gedanken, A. Amorphous Iron(III) Oxide—A Review. J. Phys. Chem. B 2007, 111, 4003–4018. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Schwerdtfeger, C.F. Reinterpretation and Temperature Dependence of EPR in TiO2: Fe3+ (Anatase). Solid State Commun. 1970, 8, 1741–1743. [Google Scholar] [CrossRef]
- Horn, M.; Schwerdtfeger, C.F. EPR of Substitutional and Charge Compensated Fe3+ in Anatase (TiO2). J. Phys. Chem. Solids 1971, 32, 2529–2538. [Google Scholar] [CrossRef]
- Moser, J.; Grätzel, M.; Gallay, R. Inhibition of Electron-Hole Recombination in Substitutionally Doped Colloidal Semiconductor Crystallites. Helv. Chim. Acta 1987, 70, 1596–1604. [Google Scholar] [CrossRef]
- Amorelli, A.; Evans, J.C.; Rowlands, C.C.; Egerton, T.A. An Electron Spin Resonance Study of Rutile and Anatase Titanium Dioxide Polycrystalline Powders Treated with Transition-Metal Ions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 3541. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, G.; Zeng, G.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Zeng, J.; Wu, J.; Liu, W. Hydrothermal Synthesis of Graphene Wrapped Fe-Doped TiO2 Nanospheres with High Photocatalysis Performance. Ceram. Int. 2018, 44, 7473–7480. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, Í.; Fernández, J.M.; Alvarez, J.I. The Effect of TiO2 Doped Photocatalytic Nano-Additives on the Hydration and Microstructure of Portland and High Alumina Cements. Nanomaterials 2017, 7, 329. [Google Scholar] [CrossRef]
- Moalej, N.S.; Ahadi, S.; Sheibani, S. Photocatalytic Degradation of Methylene Blue by 2 Wt.% Fe Doped TiO2 Nanopowder under Visible Light Irradiation. J. Ultrafine Grained Nanostructured Mater. 2019, 52, 133–141. [Google Scholar] [CrossRef]
- Mishra, S.; Chakinala, N.; Chakinala, A.G.; Surolia, P.K. Photocatalytic Degradation of Methylene Blue Using Monometallic and Bimetallic Bi-Fe Doped TiO2. Catal. Commun. 2022, 171, 106518. [Google Scholar] [CrossRef]



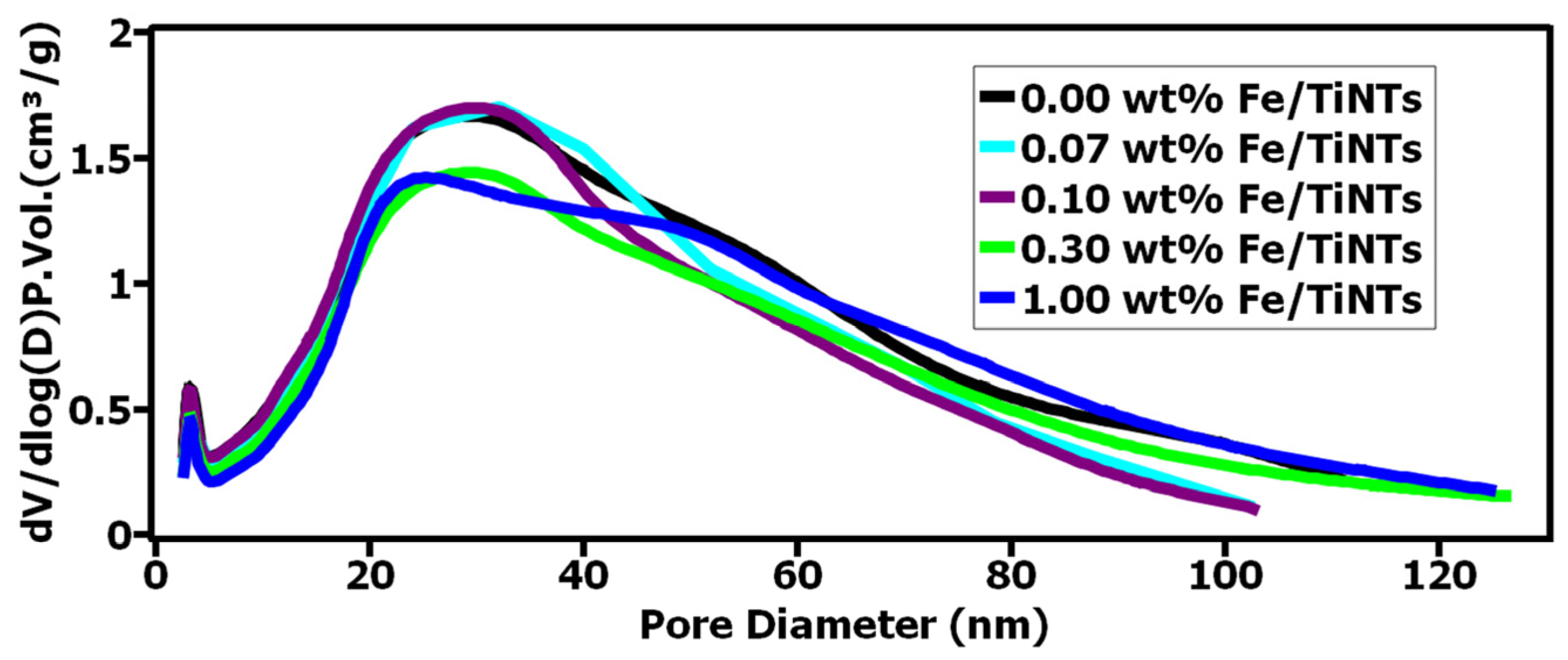





| Name of the Sample | BET Surface Area (m2/g) | BJH Surface Area of Pores (m2/g) |
|---|---|---|
| TiNTs | 356 | 384 |
| 0.07 wt% Fe/TiNTs | 335 | 365 |
| 0.10 wt% Fe/TiNTs | 334 | 375 |
| 0.30 wt% Fe/TiNTs | 301 | 316 |
| 1.00 wt% Fe/TiNTs | 270 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qattali, S.M.Y.; Nasir, J.; Pritzel, C.; Kowald, T.; Sakalli, Y.; Moni, S.M.F.K.; Schmedt auf der Günne, J.; Wickleder, C.; Trettin, R.H.F.; Killian, M.S. Synthesis and Characterization of Iron-Doped TiO2 Nanotubes (Fe/TiNTs) with Photocatalytic Activity. Constr. Mater. 2024, 4, 315-328. https://doi.org/10.3390/constrmater4020017
Qattali SMY, Nasir J, Pritzel C, Kowald T, Sakalli Y, Moni SMFK, Schmedt auf der Günne J, Wickleder C, Trettin RHF, Killian MS. Synthesis and Characterization of Iron-Doped TiO2 Nanotubes (Fe/TiNTs) with Photocatalytic Activity. Construction Materials. 2024; 4(2):315-328. https://doi.org/10.3390/constrmater4020017
Chicago/Turabian StyleQattali, S. Mohd. Yonos, Jamal Nasir, Christian Pritzel, Torsten Kowald, Yilmaz Sakalli, S. M. Fuad Kabir Moni, Jörn Schmedt auf der Günne, Claudia Wickleder, Reinhard H. F. Trettin, and Manuela S. Killian. 2024. "Synthesis and Characterization of Iron-Doped TiO2 Nanotubes (Fe/TiNTs) with Photocatalytic Activity" Construction Materials 4, no. 2: 315-328. https://doi.org/10.3390/constrmater4020017
APA StyleQattali, S. M. Y., Nasir, J., Pritzel, C., Kowald, T., Sakalli, Y., Moni, S. M. F. K., Schmedt auf der Günne, J., Wickleder, C., Trettin, R. H. F., & Killian, M. S. (2024). Synthesis and Characterization of Iron-Doped TiO2 Nanotubes (Fe/TiNTs) with Photocatalytic Activity. Construction Materials, 4(2), 315-328. https://doi.org/10.3390/constrmater4020017







