The Impact of Chronic Stress and Eating Concern on Acylated Ghrelin Following Acute Psychological Stress in Healthy Men
Abstract
:1. Introduction
2. Results
2.1. Demographic Parameters
2.2. Baseline Eating Behavior Parameters
2.3. Baseline Psychological Parameters
2.4. Change of Eating Behavior Parameters
2.5. Change of Psychological Parameters
2.6. Change of Physiological Parameters
2.7. Effect of the TSST-G on Acylated Ghrelin
3. Discussion
4. Methods
4.1. Subjects
4.2. Trier Social Stress Test (TSST)
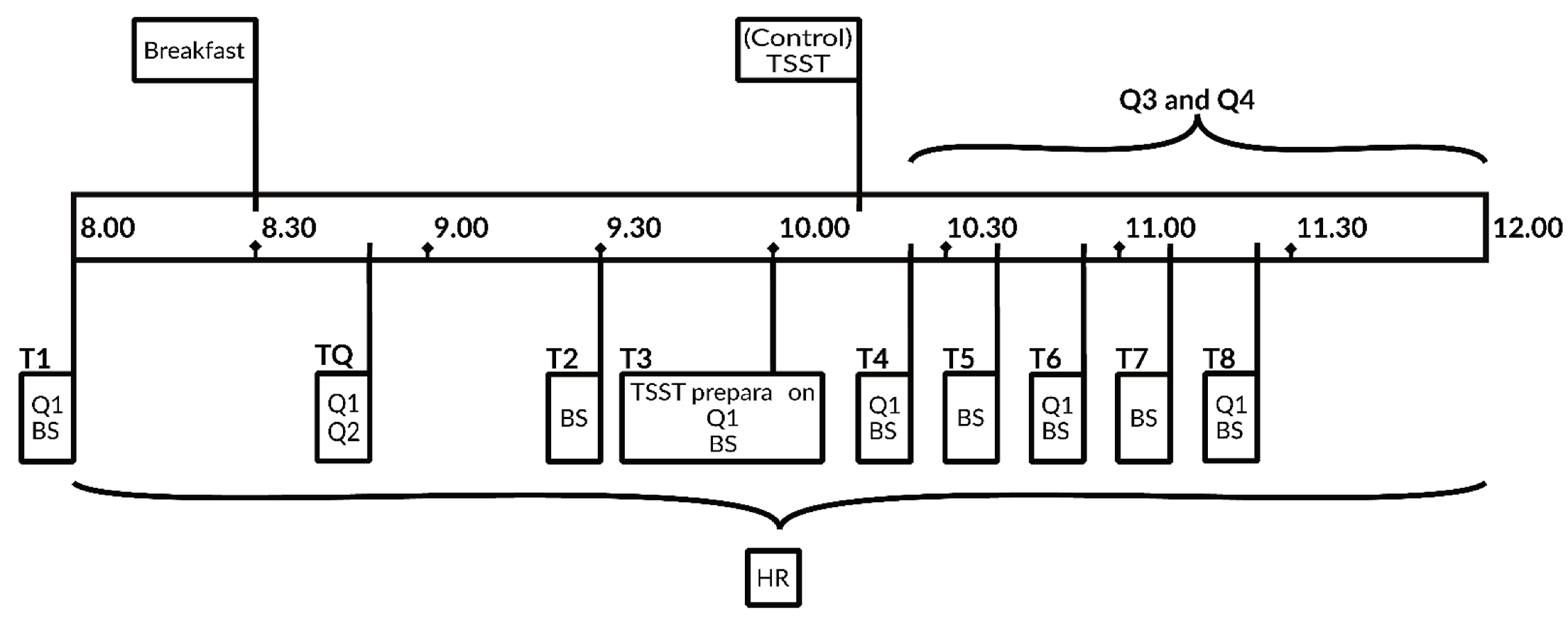
4.3. Study Design
4.4. Breakfast Composition
4.5. 24 h Recalls
4.6. Trier Inventory of Chronic Stress (TICS)
4.7. Dutch Eating Behavior Questionnaire (DEBQ)
4.8. Positive and Negative Affect Scale (PANAS)
4.9. Heart Rate Measurement
4.10. Blood Sampling and Analysis
4.11. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida, D.M.; Charles, S.T.; Mogle, J.; Drewelies, J.; Aldwin, C.M.; Spiro, A.; Gerstorf, D. Charting adult development through (historically changing) daily stress processes. Am. Psychol. 2020, 75, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Fulton, S.; Wilson, M.; Petrovich, G.; Rinaman, L. Stress exposure, food intake and emotional state. Stress 2015, 18, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R. Role of addiction and stress neurobiology on food intake and obesity. Biol. Psychol. 2018, 131, 5–13. [Google Scholar] [CrossRef]
- Sinha, R.; Jastreboff, A.M. Stress as a Common Risk Factor for Obesity and Addiction. Biol. Psychiatry 2013, 73, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, A.; Bimonte, S.; De Palma, G.; Luciano, A.; Rea, D.; Giudice, A.; Scognamiglio, G.; La Mantia, E.; Franco, R.; Perdonà, S.; et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int. J. Oncol. 2015, 47, 527–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, A.; Palma, G.; Rosati, A.; Giudice, A.; Falco, A.; Petrillo, A.; Petrillo, M.; Bimonte, S.; Di Benedetto, M.; Esposito, G.; et al. Role of endothelial nitric oxide synthase (eNOS) in chronic stress-promoted tumour growth. J. Cell. Mol. Med. 2011, 16, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, A.G.; Rutters, F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008, 94, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and Function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nat. Cell Biol. 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Kristenssson, E.; Sundqvist, M.; Astin, M.; Kjerling, M.; Mattsson, H.; de la Cour, C.D.; Håkanson, R.; Lindström, E. Acute psychological stress raises plasma ghrelin in the rat. Regul. Pept. 2006, 134, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Rouach, V.; Bloch, M.; Rosenberg, N.; Gilad, S.; Limor, R.; Stern, N.; Greenman, Y. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 2007, 32, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Investig. 2011, 121, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Wang, L.; Taché, Y. Stress-related alterations of acyl and desacyl ghrelin circulating levels: Mechanisms and functional implications. Peptides 2011, 32, 2208–2217. [Google Scholar] [CrossRef] [Green Version]
- Macht, M. How emotions affect eating: A five-way model. Appetite 2008, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Evers, C.; Stok, F.M.; De Ridder, D.T.D. Feeding Your Feelings: Emotion Regulation Strategies and Emotional Eating. Pers. Soc. Psychol. Bull. 2010, 36, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Taut, D.; Renner, B.; Baban, A. Reappraise the Situation but Express Your Emotions: Impact of Emotion Regulation Strategies on ad libitum Food Intake. Front. Psychol. 2012, 3, 359. [Google Scholar] [CrossRef] [Green Version]
- Evers, C.; Dingemans, A.; Junghans, A.F.; Boevé, A. Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence. Neurosci. Biobehav. Rev. 2018, 92, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bongers, P.; Jansen, A. Emotional Eating Is Not What You Think It Is and Emotional Eating Scales Do Not Measure What You Think They Measure. Front. Psychol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Chao, A.M.; Jastreboff, A.M.; White, M.A.; Grilo, C.M.; Sinha, R. Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity 2017, 25, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.A.; Khan, S.; Briggs, H.; Abelson, J.L. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 2010, 35, 607–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzam, I.; Gilad, S.; Limor, R.; Stern, N.; Greenman, Y. Ghrelin stimulation by hypothalamic–pituitary–adrenal axis activation depends on increasing cortisol levels. Endocr. Connect. 2017, 6, 847–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewagalamulage, S.; Lee, T.; Clarke, I.; Henry, B. Stress, cortisol, and obesity: A role for cortisol responsiveness in identifying individuals prone to obesity. Domest. Anim. Endocrinol. 2016, 56, S112–S120. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Dallman, M.F.; Epel, E.S. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 2011, 36, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Tryon, M.; DeCant, R.; Laugero, K. Having your cake and eating it too: A habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol. Behav. 2013, 114–115, 32–37. [Google Scholar] [CrossRef]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Zellner, D.A.; Loaiza, S.; Gonzalez, Z.; Pita, J.; Morales, J.; Pecora, D.; Wolf, A. Food selection changes under stress. Physiol. Behav. 2006, 87, 789–793. [Google Scholar] [CrossRef]
- Heatherton, T.F.; Herman, C.P.; Polivy, J. Effects of physical threat and ego threat on eating behavior. J. Pers. Soc. Psychol. 1991, 60, 138–143. [Google Scholar] [CrossRef]
- Habhab, S.; Sheldon, J.P.; Loeb, R.C. The relationship between stress, dietary restraint, and food preferences in women. Appetite 2009, 52, 437–444. [Google Scholar] [CrossRef]
- Tannenbaum, B.M.; Brindley, D.N.; Tannenbaum, G.S.; Dallman, M.F.; McArthur, M.D.; Meaney, M.J. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am. J. Physiol. Content 1997, 273, E1168–E1177. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Christiansen, A.M.; Ostrander, M.M.; Jones, A.A.; Jones, K.R.; Choi, D.C.; Krause, E.G.; Evanson, N.K.; Furay, A.R.; Davis, J.F.; et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 20529–20534. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.B.S. Chronic and acute effects of stress on energy balance: Are there appropriate animal models? Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R250–R265. [Google Scholar] [CrossRef] [Green Version]
- Snoek, H.M.; Engels, R.C.; van Strien, T.; Otten, R. Emotional, external and restrained eating behaviour and BMI trajectories in adolescence. Appetite 2013, 67, 81–87. [Google Scholar] [CrossRef]
- Jansen, A.; Nederkoorn, C.; Roefs, A.; Bongers, P.; Teugels, T.; Havermans, R. The proof of the pudding is in the eating: Is the DEBQ—External eating scale a valid measure of external eating? Int. J. Eat. Disord. 2010, 44, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, O.; Kluckner, V.J.; Brandt, S.; Moss, A.; Weck, M.; Florath, I.; Wabitsch, M.; Hebebrand, J.; Schimmelmann, B.G.; Christiansen, H. Restrained and External-Emotional Eating Patterns in Young Overweight Children–Results of the Ulm Birth Cohort Study. PLoS ONE 2014, 9, e105303. [Google Scholar] [CrossRef] [Green Version]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Groesz, L.M.; McCoy, S.; Carl, J.; Saslow, L.; Stewart, J.; Adler, N.; Laraia, B.; Epel, E. What is eating you? Stress and the drive to eat. Appetite 2012, 58, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Kistenmacher, A.; Goetsch, J.; Ullmann, D.; Wardzinski, E.K.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Psychosocial stress promotes food intake and enhances the neuroenergetic level in men. Stress 2018, 21, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Henze, G.-I.; Zänkert, S.; Urschler, D.F.; Hiltl, T.J.; Kudielka, B.M.; Pruessner, J.C.; Wüst, S. Testing the ecological validity of the Trier Social Stress Test: Association with real-life exam stress. Psychoneuroendocrinology 2017, 75, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Pabst, S.; Brand, M.; Wolf, O.T. Stress effects on framed decisions: There are differences for gains and losses. Front. Behav. Neurosci. 2013, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Wemm, S.E.; Wulfert, E. Effects of Acute Stress on Decision Making. Appl. Psychophysiol. Biofeedback 2017, 42, 1–12. [Google Scholar] [CrossRef] [Green Version]
- DelParigi, A.; Tschöp, M.; Heiman, M.L.; Salbe, A.D.; Vozarova, B.; Sell, S.M.; Bunt, J.C.; Tataranni, P.A. High Circulating Ghrelin: A Potential Cause for Hyperphagia and Obesity in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 5461–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatansever-Ozen, S.; Tiryaki-Sonmez, G.; Bugdayci, G.; Ozen, G. The Effects of Exercise on Food Intake and Hunger: Relationship with Acylated Ghrelin and Leptin. J. Sports Sci. Med. 2011, 10, 283–291. [Google Scholar] [PubMed]
- Abu-Farha, M.; Dehbi, M.; Noronha, F.; Tiss, A.; Alarouj, M.; Behbehani, K.; Bennakhi, A.; Elkum, N. Gender Differences in Ghrelin Association with Cardiometabolic Risk Factors in Arab Population. Int. J. Endocrinol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.L.; Dimaraki, E.V.; Jessup, S.K.; Symons, K.V.; Ermolenko, M.; Jaffe, C.A. Ghrelin Secretion in Humans Is Sexually Dimorphic, Suppressed by Somatostatin, and Not Affected by the Ambient Growth Hormone Levels. J. Clin. Endocrinol. Metab. 2003, 88, 2180–2184. [Google Scholar] [CrossRef] [Green Version]
- Sakao, Y.; Ohashi, N.; Sugimoto, M.; Ichikawa, H.; Sahara, S.; Tsuji, T.; Kato, A.; Fujigaki, Y.; Sugimoto, K.; Furuta, T.; et al. Gender Differences in Plasma Ghrelin Levels in Hemodialysis Patients. Ther. Apher. Dial. 2019, 23, 65–72. [Google Scholar] [CrossRef]
- Von Dawans, B.; Kirschbaum, C.; Heinrichs, M. The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology 2011, 36, 514–522. [Google Scholar] [CrossRef]
- Wüst, S.; Federenko, I.S.; Van Rossum, E.F.; Koper, J.W.; Hellhammer, D.H. Habituation of cortisol responses to repeated psychosocial stress—further characterization and impact of genetic factors. Psychoneuroendocrinology 2005, 30, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Prussner, J.C.; Stone, A.A.; Federenko, I.; Gaab, J.; Lintz, D.; Schommer, N.; Hellhammer, D.H. Persistent High Cortisol Responses to Repeated Psychological Stress in a Subpopulation of Healthy Men. Psychosom. Med. 1995, 57, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; von Känel, R.; Preckel, D.; Zgraggen, L.; Mischler, K.; Fischer, J.E. Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biol. Psychol. 2006, 72, 147–153. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, L.; McCrickerd, K.; Yeomans, M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015, 41, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Rust, P.; Hasenegger, V.; König, J. Österreichischer Ernährungsberich 2017; Wien, Universität Wien und Bundesministerium für Gesundheit und Frauen: Vienna, Austria, 2017. [Google Scholar]
- Slimani, N.; Ferrari, P.; Ocké, M.; Welch, A.; Boeing, H.; Van Liere, M.; Pala, V.; Amiano, P.; Lagiou, A.; Mattisson, I.; et al. Standardization of the 24-h diet recall calibration method used in the European Prospective Investigation into Cancer and Nutrition (EPIC): General concepts and preliminary results. Eur. J. Clin. Nutr. 2000, 54, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, I.; Hartmann, B.; Grotz, A.; Stang, K. Max Rubner-Institut-Bundesforschungsinstitut Für Ernährung und Lebensmittel (MRI) Bundeslebensmittelschlüssel (BLS)-Version 3.02 2010. Available online: https://www.nutritional-software.at/assets/downloads/550fe483/bls_3.0_handbuch.pdf (accessed on 8 October 2018).
- Schulz, P.; Schlotz, W.; Becker, P. Trierer Inventar Zum Chronischen Stress (TICS): Trier Inventory for Chronic Stress (TICS); Hogrefe: Göttingen, Germany, 2004. [Google Scholar]
- Schwabe, L.; Dalm, S.; Schächinger, H.; Oitzl, M.S. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiol. Learn. Mem. 2008, 90, 495–503. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.; Bergers, G.; Defares, P. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Grunert, S. Ein Inventar zur Erfassung von Selbstaussagen zum Ernährungsverhalten. Diagnostica 1989, 31, 167–179. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Breyer, B.; Bluemke, M. Deutsche Version der Positive and Negative Affect Schedule PANAS (GESIS Panel); ZIS—GESIS Leibniz Institute for the Social Sciences: Mannheim, German, 2016. [Google Scholar] [CrossRef]


| Mean (SD) | Test Day Mean (SD) | Control Day Mean (SD) | p Value | |
|---|---|---|---|---|
| Age (years) | 23 (3) | |||
| BMI (kg/m2) | 22.5 (1.3) | |||
| Work (h/week) | 21 (11.31) | |||
| Sport (h/week) | 5.33 (4.19) | |||
| RMR (kJ/24 h) | 7326.90 (512.87) | |||
| EI on previous day (kJ/d) | 10.831 (3.683) | 11.077 (4.774) | NS | |
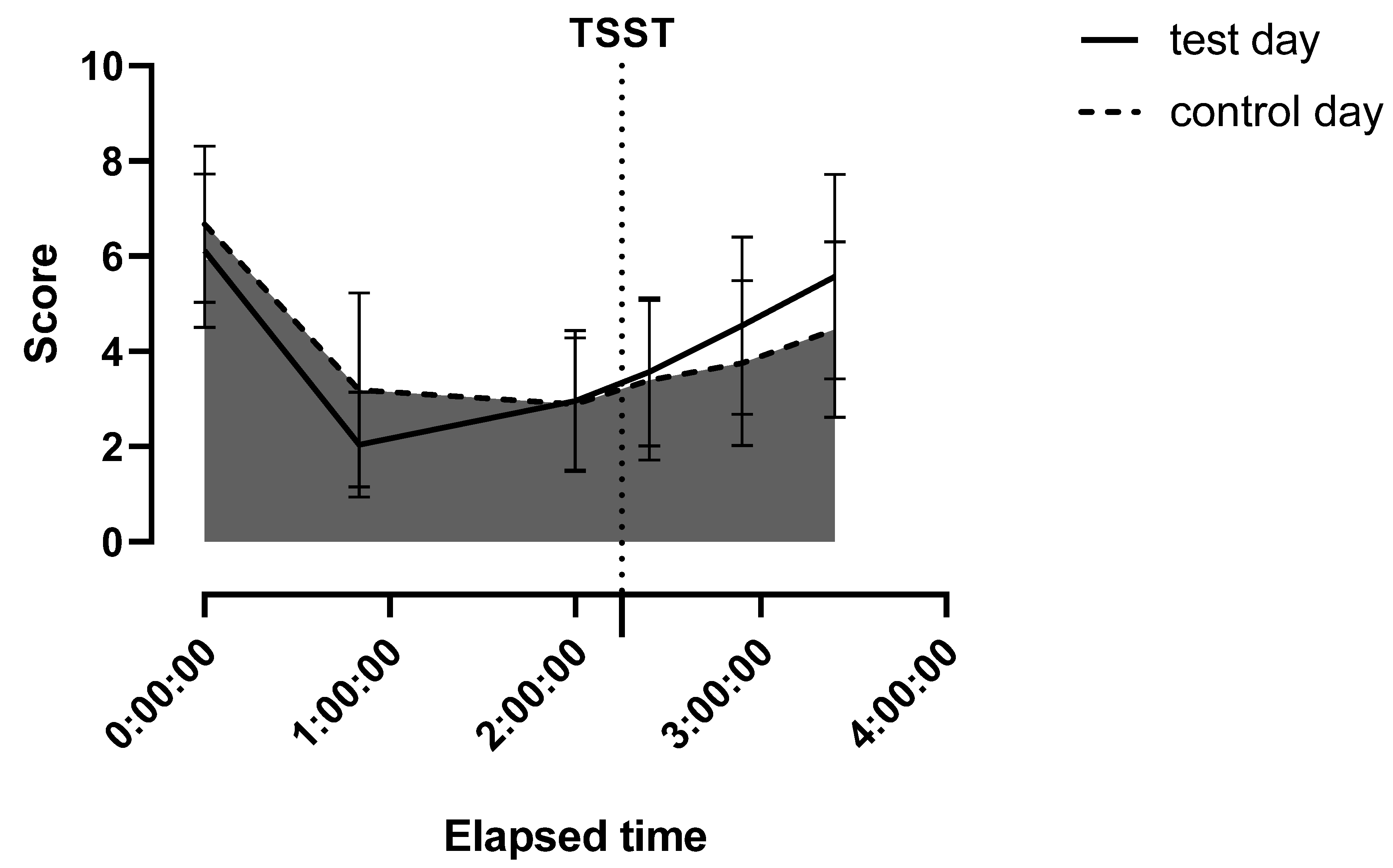
| Basal VAS score | 6.12 (1.61) | 6.67 (1.65) | NS | |
| Before testing, VAS score | 2.96 (1.49) | 2.89 (1.39) | NS | |
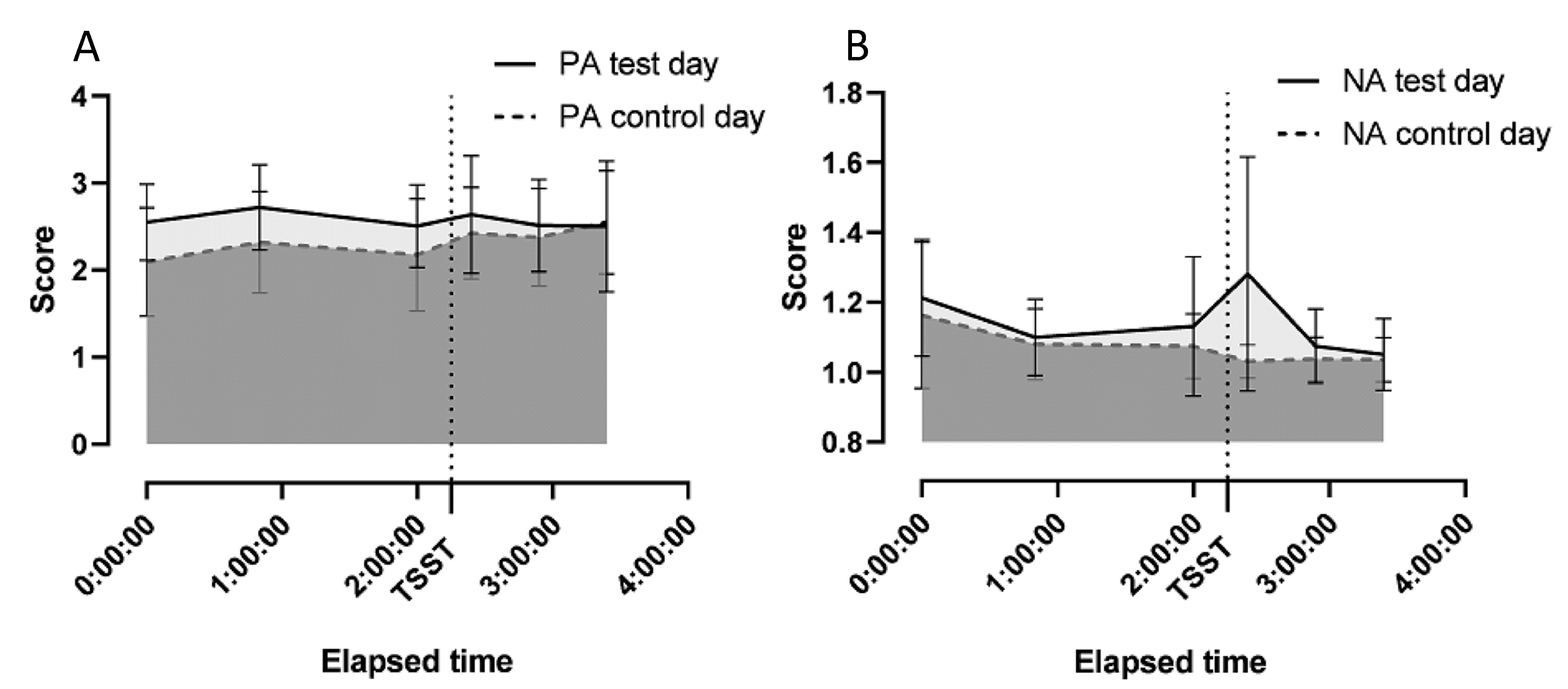
| Basal PA score | 2.55 (0.11) | 2.09 (0.16) | 0.008 | |
| Before testing, PA score | 2.51 (0.47) | 2.18 (0.64) | 0.030 | |
| Basal NA score | 1.21 (0.04) | 1.16 (0.05) | NS | |
| Before testing, NA score | 1.13 (0.20) | 1.08 (0.09) | NS | |
| Heart rate during TSST-G (bpm; n = 5) | 108 (16) | 92 (13) | <0.0001 |
| Mean (SD) | p Value (Mean Comparison) | Correlation | p Value (Correlation) | |
|---|---|---|---|---|
| Restrained Eating | 1.86 (0.48) | <0.001 a | 0.20 | NS c |
| Emotional Eating | 1.73 (0.42) | <0.001 b | −0.39 | NS d |
| External Eating | 3.05 (0.52) | 0.21 | NS e |
| SSCS | Median | p Value | |
|---|---|---|---|
| Mean (n = 16) | 68.5 | ||
| SD | 25.20 | ||
| Median | 60.50 | ||
| Working participants (n = 6) | 62.00 | NS | |
| Non-working participants (n = 10) | 60.50 | ||
| Concerned eaters (n = 6) | 60.00 | NS | |
| Less-concerned eaters (n = 10) | 63.00 | ||
| Test or Control Day | Chronic Stress Exposure | Estimated Mean (SD) | 95% CI | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| test | low | 129.67 (19.95) | 86.67 | 172.66 |
| high | 71.74 (23.15) | 22.70 | 120.78 | |
| control | low | 99.15 (19.78) | 56.36 | 141.94 |
| high | 125.17 (21.79) | 78.18 | 172.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahrngruber-Velasquez, C.; Duszka, K.; König, J. The Impact of Chronic Stress and Eating Concern on Acylated Ghrelin Following Acute Psychological Stress in Healthy Men. Stresses 2021, 1, 16-29. https://doi.org/10.3390/stresses1010003
Fahrngruber-Velasquez C, Duszka K, König J. The Impact of Chronic Stress and Eating Concern on Acylated Ghrelin Following Acute Psychological Stress in Healthy Men. Stresses. 2021; 1(1):16-29. https://doi.org/10.3390/stresses1010003
Chicago/Turabian StyleFahrngruber-Velasquez, Christine, Kalina Duszka, and Jürgen König. 2021. "The Impact of Chronic Stress and Eating Concern on Acylated Ghrelin Following Acute Psychological Stress in Healthy Men" Stresses 1, no. 1: 16-29. https://doi.org/10.3390/stresses1010003
APA StyleFahrngruber-Velasquez, C., Duszka, K., & König, J. (2021). The Impact of Chronic Stress and Eating Concern on Acylated Ghrelin Following Acute Psychological Stress in Healthy Men. Stresses, 1(1), 16-29. https://doi.org/10.3390/stresses1010003






