Abstract
Wheat is a cereal grain crop that is commonly cultivated and is a good source of nutrients that are beneficial to human health. In recent years, the productivity of wheat has been steadily declining, with abiotic pressures accounting for almost half of all yield losses. Drought stress is a significant limiting factor for plant development and production around the planet. The influence of polyethylene glycol (PEG) (at concentrations of 5, 10, and 15%)-induced drought stress on the morphological, physiological, and biochemical characteristics of fifteen wheat genotypes was investigated in this work. Overall, it was discovered that morphological and physiological indicators such as germination % and shoot-root lengths during the seedling stage had reduced significantly. The proline content, on the other hand, was shown to be positively correlated with the concentration of PEG treatments. There was a significant difference between the genotypes HD2733, HD2888, and RAJ3765 regarding tolerance to abiotic stress caused by drought. A further finding was that under stressful settings, the first three main components explained 56.65 percent, 65.06 percent, and 72.47 percent of the total variability in PEG treatment levels of five, ten, and fifteen percent, respectively. These collective morphological and physiological parameters, and analyses of their diverse responses, could be used for screening of drought tolerance among the 15 wheat genotypes to select for significant drought tolerance and diverse molecular responses during breeding of stress resistant forms.
1. Introduction
Wheat (Triticum aestivum L.) provides a significant proportion of required dietary calories, minerals, and around 20% of the needed protein for humans [1,2,3]. In 2018, wheat production in India was around 99.7 million tonnes (mt), with an area of 29.58 million hectares (ha) [4]. According to current estimates, the worldwide need for wheat yields is expected to rise by 50% by 2050 to feed the world’s rising population [5]. Productivity in wheat is declining because of the negative impacts of a variety of biotic and abiotic stressors [6,7].
Stresses caused by abiotic variables such as high temperatures, low temperatures, and droughts greatly reduce wheat production, resulting in an average yield loss of around 50% [8,9]. Among the most frequently occurring threats, drought stress is regarded as a severe constraint on agricultural crop production throughout the world [10]. Drought stress has negative effects on the morphological, physiological, and biochemical attributes of the wheat crop [11], and it results in a significant reduction in overall production [6,12]. In the absence of a seasonal or growth stage variation in the crop, genotypes must be evaluated at relevant and often diverse developmental phases [13].
According to predictions, drought periods are expected to become more intense and severe in the foreseeable future [14]. As a result, it is critical to increase our knowledge of how drought stress impacts the functional features of plants and their ecological relationships [15]. Imposing experimental artificial ways to generate drought stress is essential for furthering our knowledge of this phenomenon. Drought stress may be induced artificially in a variety of ways, including restricting water delivery [16], treating with abscisic acid (ABA), and using polyethylene glycol (PEG) [17]. Inducement of drought stress in plants using PEG, a non-ionic water-soluble polymer, is extensively utilized as it is not anticipated to enter the plant cells [18]. Up to this point, several publications have reported the discovery of drought-tolerant wheat genotypes by introducing varying concentrations of PEG-6000 into the plant and observing statistically significant variations in various attributes at the seedling stage [7,19,20,21]. Because it effects the growth and development of wheat via a variety of factors [8], drought stress tolerance is a difficult criterion for wheat performance. When wheat is subjected to moderate to severe water stress, it exhibits significant changes in its morpho-physiological and biochemical characteristics. This is due to changes in plant water relations, cellular oxidative stress, decreased CO2 assimilation, damage to the membranes of affected tissues, and, in some cases, inhibition of enzyme activity [22]. For wheat species, the effects of drought on their morphological, physiological, and biochemical properties change depending on their ploidy level [23].
Lack of soil moisture influences certain morphological characteristics of wheat, including seed germination [11], shoot length [8], root length [8], tillering [24,25,26], spike number, grain number per spike [24,25,26], number of viable tillers per plant [24,25,26], and 1000 grain weight [24,25,26]. These metrics are derived from physiological characteristics such as chlorophyll concentration, relative water content, photosynthetic rate, and membrane stability index. The biochemical proline is vital in managing osmotic pressure and stabilizing cells. As a result, these metrics have the potential to be used as valid indicators for screening and selecting drought-resistant wheat genotypes [27,28]. In these circumstances, the current inquiry was carried out to determine the reaction of 15 wheat genotypes to drought stress conditions generated by PEG. To identify drought-resistant wheat genotypes that may be employed in a breeding programme for the development of drought-tolerant wheat varieties, the effects of different PEG-6000 concentrations on several morphological, physiological, and biochemical characteristics were investigated.
2. Material and Methods
Field experiments were carried out during the wheat growing season of 2016–2017 at the field research laboratory and experimental station, Department of Biotechnology, Sardar Vallabhbhai Patel University of Agriculture & Technology, Meerut, India. Fifteen bread-wheat genotypes were evaluated in this study and their details are given in Table 1. The seeds were procured from Department of Genetics and Plant Breeding, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut.

Table 1.
Details of fifteen genotypes.
2.1. Plant Growth and Drought Treatment
To evaluate the wheat genotypes for various morpho-physiological and biochemical characters under drought stress conditions at the seedling stage, drought stress was induced by polyethylene glycol [19]. Fifty seeds of each genotype were surface sterilized with 0.1% of HgCl2 for 1 min, then washed thrice with distilled water to avoid fungal contamination, and then were placed on Whatman No. 2 filter paper in 90 mm plastic Tarson Aseptic Petri dishes and were moistened with 8 mL of PEG-6000. The petri dishes were covered and incubated at laboratory conditions (27 ± 2 °C) for 15 days with three different concentrations (5%, 10% and 15% in the treatments 1, 2 and 3, respectively) and the untreated seeds were used as a control. After germination, seedlings were transferred to the rainout shelter. Experiments were conducted in a Randomized Block Design in three replicates. The dimensions of each block were 15 × 15 inches (length × breadth) and the space between the two blocks was 6 inches. The crop was maintained in the field using standard agronomic practices. Various morphological, physiological, and biochemical parameters were recorded during the seedling stage, vegetative growth stage and reproductive stage of the plants.
2.2. Observations on the Morphological Parameters
The numbers of germinated seeds were counted and recorded every 24 h for up to 10 days and the germination percentage was calculated (number of germinated seed/total number of seed × 100). The shoot length (ShL), from the shoot apex to the root apex and the root length (RL), from base of the shoot (collar region) to the root apex, were measured after 15 days of germination. Plant height (PH) was measured from the base of the plant to the tip of the spike (including awns). All the measured lengths were recorded in centimetres (cm). The number of tillers plant−1 (NT) and the flag leaf area (FLA) was calculated after 20 days of anthesis stage by the leaf index method [29] as Leaf area = L × W × F; where, L = maximum length (cm), W = maximum width (cm), F = correction factor (0.747). The spike length (SL) in centimetres, spikelet number spike−1 (SPS), number of grain spike−1 (GPS), thousand grain weight (TW) were measured; days to heading (DTH) and days to maturity (DTM) were counted from date of seed treatment to the appearance of ears and browning of ears, respectively.
2.3. Observations on the Physiological and Biochemical Parameters
A Soil Plant Analytical Development (SPAD) chlorophyll meter (Minolta) was used to measure the relative chlorophyll (ChL) content (µg/cm2) of the leaves of each genotype at the seedling stage under stress conditions. An Infra-Red Gas Analyzer (PN), LI-6400 XT (LICOR Inc., Lincoln, NE, USA), was used to measure the photosynthetic rate (Pn) of leaves (µmol/m2sec). The relative water content (RWC) was calculated through the equation
where FW = Fresh weight, TW = Turgid weight and DW = Dry weight of the leaf [30].
The membrane stability index (MSI) was determined by recording the electrical conductivity of leaf leakages in double distilled water at 40 °C and 100 °C [31]. Their electric conductivities were measured with an EC meter (Electrical Conductivity) as C1 and C2 respectively. The membrane stability index = [1 − (C1/C2)] × 100. The free proline content (PC) in leaf tissues were determined by adopting the colorimetric method [32]. The proline content was calculated as per the formula:
where, 36.2311 is the standard curve value of proline, OD = optical density at 520 nm, V = total volume of extract in mL, F = milligram of fresh weight of leaf taken for one proline estimation, 2 = volume of aliquot taken for proline estimation.
2.4. Statistical Analysis
All the data presented are the means of three independent replicates with ±standard error (SE). Data were subjected to analysis of variance (ANOVA) and comparison of means used the Duncan’s Multiple Range test [33] performed by IBM SPSS Statistics v20 (New York, NY, USA). Pearson’s correlation coefficients were calculated separately for the control and the treatments. A principal component analysis (PCA) based on the correlation matrix was performed using SPSS to classify the variation in traits as well as the genotypes. To assess the variation in traits, the PCA biplots and boxplot were generated separately for the control and drought stress setups using R software [34].
3. Results
Analysis of variance (ANOVA) revealed significant differences among the selected wheat genotypes for all the analysed traits, except for MSI and RWC under control and drought stress treatment conditions. The mean squares from the ANOVA and significance of mean comparison (p < 0.05) are given in Table 2.

Table 2.
Mean squares of morphological, physiological, and biochemical traits under control, 5% PEG, 10% PEG and 15% PEG treatment condition.
3.1. Morphological Responses to Drought Stress
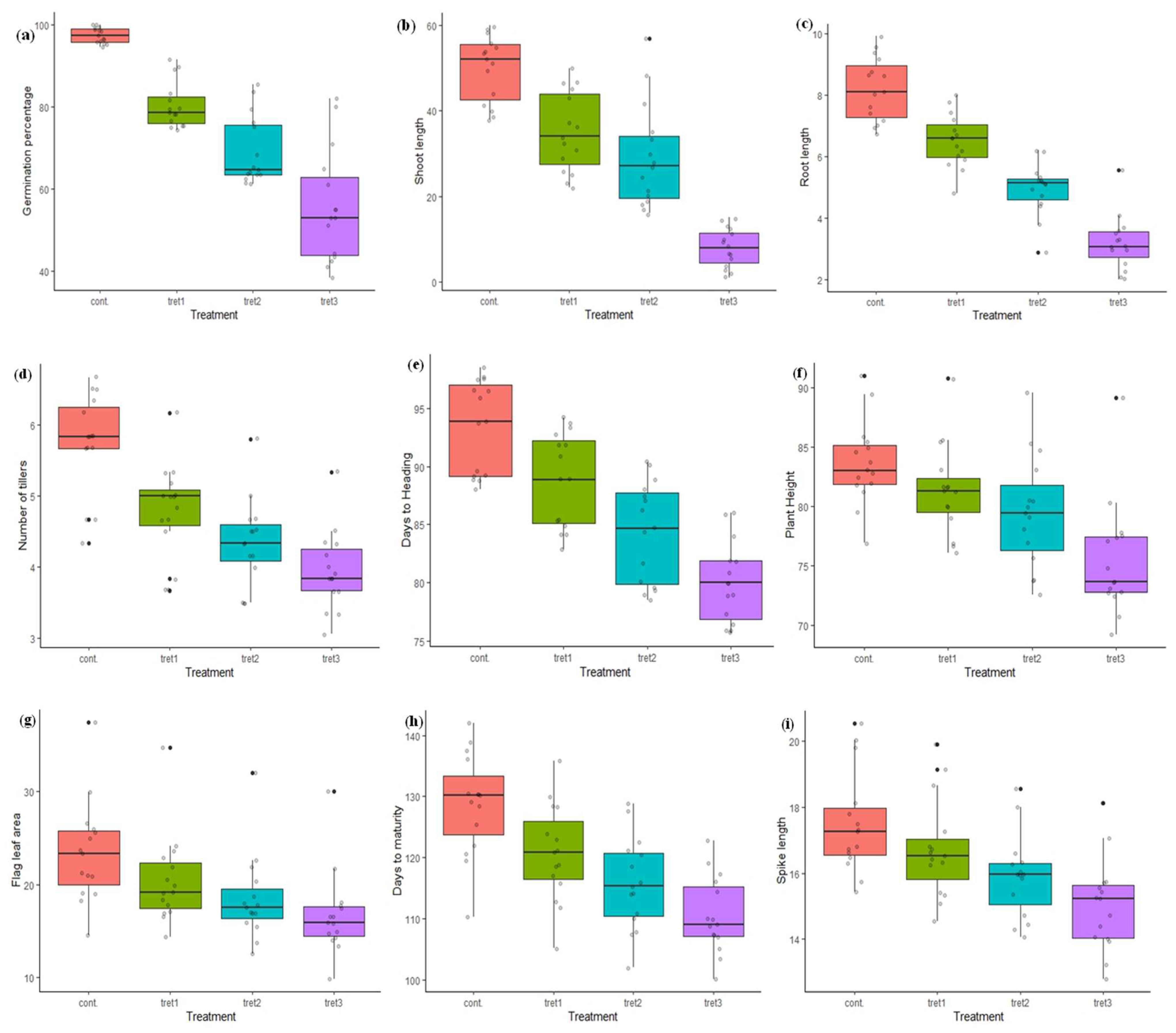
In the present study, the maximum seed GP (100%) was recorded in PBW343 and UP2425, and minimum in K9423 (94%) in control conditions (Table 3, Figure 1). The GP lies between 74.43% (WH1021) and 91.50% (RAJ3765), 61.37% (WH711) and 85.57% (RAJ3765), and 38.53% (DBW71) and 81.97% (RAJ3765), at 5%, 10% and 15% PEG treatment, respectively. The average GP in control conditions was 97.46%, which was reduced to 80.42%, 69.23% and 55.74%, under 5%, 10% and 15% PEG treatments, respectively. The ShL and RL ranged from 9.20 cm (HD3086) to 12.30 cm (DBW17), and 6.73 cm (HD2733) to 9.93 cm (PBW343), respectively in control conditions. The maximum reduction in ShL and RL was observed at the highest induced drought stress (15% of PEG). The average ShL recorded at 0% PEG (control) was 10.32 cm, which was reduced to 27.81%, 45.25% and 58.91% under 5%, 10 % and 15% PEG treatments. The average RL was 8.21 cm in control conditions and found to be reduced on an average to 5.01 cm at the 15% PEG treatment. In control conditions, the minimum NT were 4.33 (DBW17) and maximum 6.67 (HD3086). In treatment cases, NT ranged from 3.67 (WH711) to 6.17 (RAJ3765), 3.50 (WH711, WH1021) to 5.80 (RAJ3765), and 3.17 (WH1021) to 5.33 (RAJ3765), at 5%, 10% and 15% PEG concentration, respectively. The average NT was 5.74 in the control and was reduced to 16.55%, 24.39% and 31.18%, under 5%, 10% and 15% PEG treatments, respectively. The genotypes DBW71, HD3086, PBW343 and RAJ3765, were found to be the best, developing more than 6.50 tillers per plant under control conditions. The genotype RAJ3765 showed a good result with a maximum average NT of 5.33 per plant under all drought stress treatments. The average days to heading was 93.40 days in the control conditions, and reduced by 14.41% at 15% PEG treatment. The maximum number of DTH were 98.50 (DBW17) and minimum were 88.0 (PBW396) in the control conditions. For treatment cases, DTH ranged from 82.83 (PBW396) to 94.17 (DBW17), 78.50 (HUW468) to 90.33 (HD2733) and 75.67 (HUW468) to 86.0 (HD2888) days, at 5%, 10% and 15% PEG concentration, respectively. However, the genotypes HD2733, HD2888 and RAJ3765 were less vulnerable to drought stress with respect to the days to heading. The PH of all the 15 wheat genotypes was significantly decreased compared to the control in all the three stress treatments. The minimum and maximum PH was 76.90 cm (PBW590) and 91.00 cm (RAJ3765), respectively under control conditions. Average PH was 83.64 cm under control conditions, which was decreased by 2.70%, 4.94% and 9.76%, at 5%, 10 % and 15% PEG treatments, respectively. The genotypes HD2733 and UP2425 had a PH more than 85 cm, whereas the genotypes DBW 17, DBW71, HUW468, PBW343 and PBW590 were less than 80 cm at 5% PEG treatment. A PH more than 89.0 cm was recorded in RAJ3765 at 10% and 15% PEG concentration. The genotypes HD2864, HD3086, RAJ3765, UP2425 and WH711 had more than 25 cm2 FLA, whereas, in DBW17, DBW71, PBW343 and WH1021 it was less than 20 cm2 in the control. In treatment conditions, a maximum FLA of 34.73, 32.05 and 30.06 cm2 was recorded for 5%, 10% and 15% PEG in the genotype UP2425. Further, the effect of drought treatments on flag leaf area (FLA) were varied among the genotypes, as an average leaf area was 23.44 cm2 under control and was up to 28.63% less at the higher PEG (15%) treatment.

Table 3.
Mean values of morphological traits under control and 5%, 10% and 15% PEG treatment conditions.
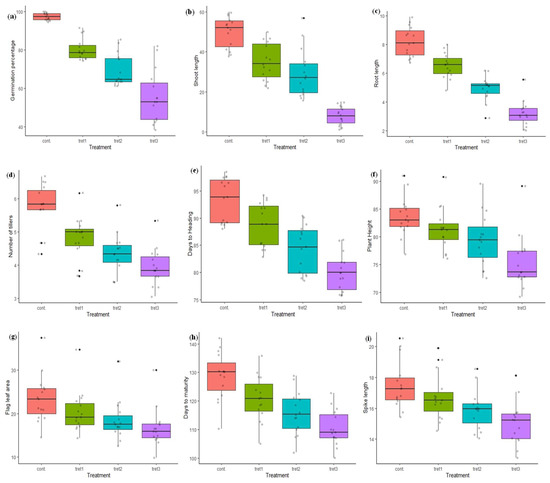
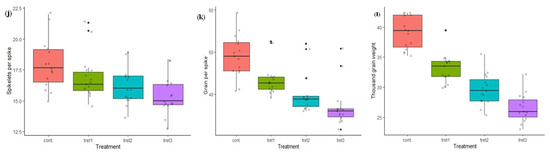
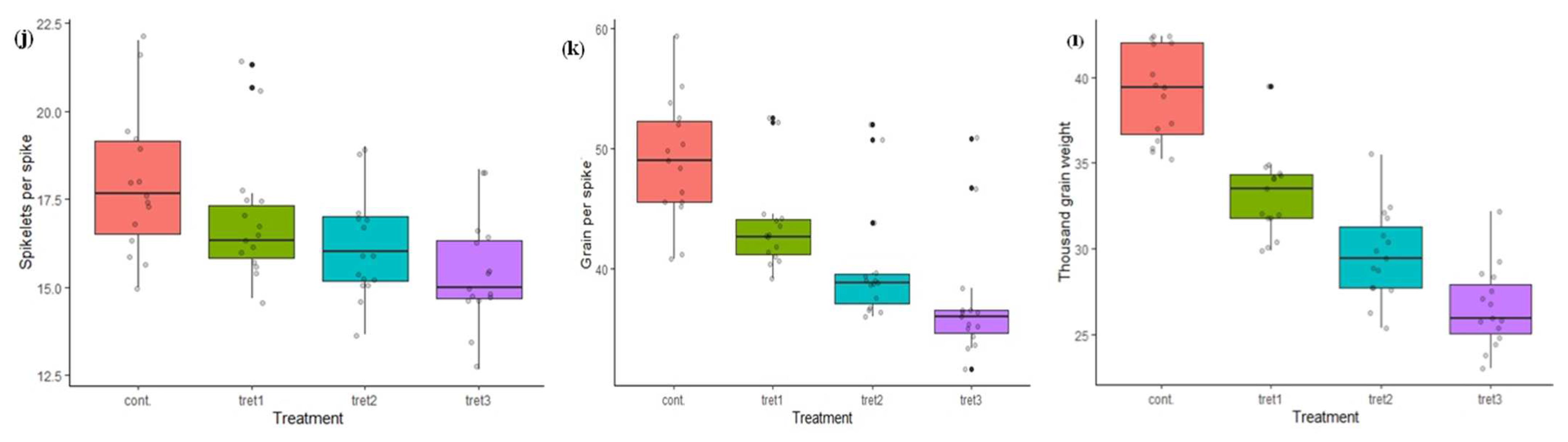
Figure 1.
Variation in morphological traits in control (0%PEG), 5% PEG, 10% PEG and 15% PEG treatment condition: (a) germination percentage, (b) shoot length, (c) root length, (d) no. of tillers, (e) days to heading, (f) plant height, (g) flag leaf area, (h) days to maturity, (i) spike length, (j) spikelets per spike, (k) grain per spike, (l) thousand grain weight.
The days to maturity ranged from 110.33 (HD2888) to 142 days (HD3086) in control conditions, and 105.17 to 135.83 days at the 5% PEG treatment. In further treatments, minimum and maximum DTM were 102.0 to 128.83 days, and 100.17 to 122.83 (RAJ3765) days, in the case of 10% and 15% PEG treatments, respectively. Moreover, the average days to maturity was 128.73 days under control conditions and was found to reduce by up to 14.17% days in the 15% PEG treatment. In control conditions, minimum SL was 15.43 cm (DBW17) and maximum 20.53 cm (HD3086). In treatment cases, SL ranged from 14.53 cm (PBW343) to 19.90 cm (UP2425), 14.07 cm (DBW17) to 18.57 cm (RAJ3765), and 12.80 cm (PBW343) to 18.13 cm (RAJ3765), at 5%, 10% and 15% PEG concentration, respectively. The genotype HD3086 and HD2864 had minimum and maximum SPS in control conditions (15.0 and 22.0) and in 5% PEG (14.67 and 21.33) treatment conditions, but in the case of 10% and 15% PEG treatment, the genotype HD2733 had the maximum 19.0 and 18.33 SPS, respectively. The average SL and SPS recorded in the controls were 17.50 cm and 17.96 cm respectively, SL and SPS were decreased by 4.40%, 5.73% and 9.20%; and 10.30%, 14.17% and 14.27%, under 5%, 10 % and 15% PEG treatments, respectively. However, the genotypes RAJ3765 and HD2888 were less affected under all three drought treatments, and had only a 8.43% and 9.09% decrease in spike lengths and SPS, respectively. The GPS ranged from 40.83 (HD2733) to 59.33 (RAJ3765) in the controls, and 39.17 to 52.50 at 5% PEG treatment. In further treatment, minimum and maximum GPS were 36.0 to 52.0 and 31.67 (PBW343) to 50.83 (HD2888) days, in the case of 10% and 15% PEG treatment, respectively. The average number of grains per spike (GPS) was 48.99 in the controls and was found to reduce by up to 11.08%, 17.98% and 24.33% under 5%, 10 % and 15% PEG treatments, respectively. The TW ranged from 35.22 g (PBW396) to 42.38 g (PBW343) in the control and, the average TW (39.08 g) was recorded in the control and was decreased by 15.15%, 24.16% and 32.01%, at 5%, 10 % and 15% PEG treatments, respectively. The genotypes DBW17, HD2733, HD2888, K9423, PBW343 and WH711 performed better in respect to TW, and recorded more than 40.0 g in the control conditions. The minimum TW was recorded in DBW71 (29.91 g) at 5% PEG, and in PBW396 at 10% and 15% PEG treatment. The maximum TW was in HD2888 under all three drought stress treatments (Table 3, Figure 1).
3.2. Physiological and Biochemical Response to Drought Stress
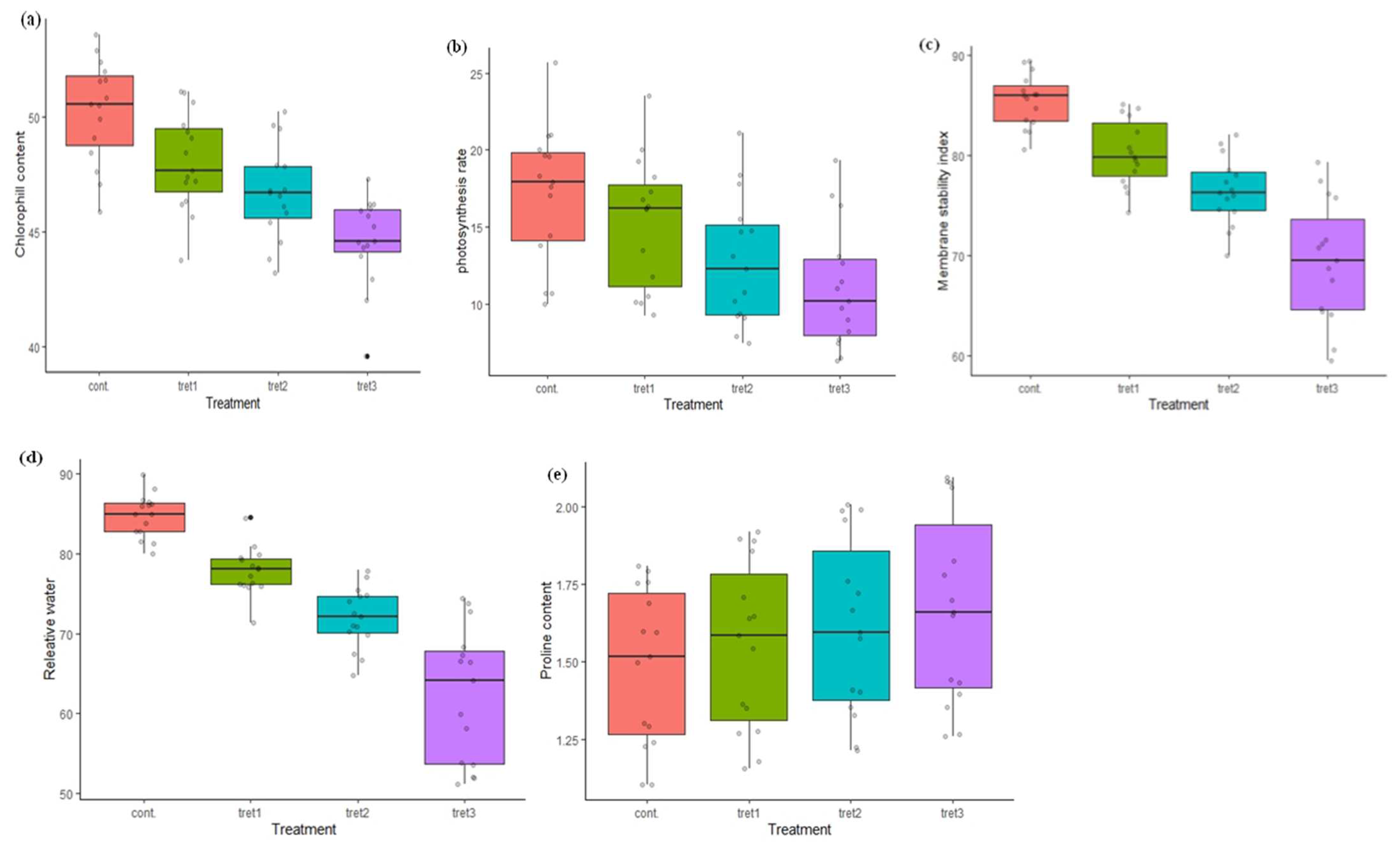
The chlorophyll (ChL) content ranged from 45.88 (UP2425) to 53.57 (WH1021) µg/cm2 in control conditions (Table 4, Figure 2). The genotype WH1021 showed maximum ChL content 51.08 µg/cm2 at 5% PEG treatment and reduced to 44.40 µg/cm2 under 15% PEG treatment. The maximum ChL content was 47.30 µg/cm2 in HD2888 and the minimum 39.62 µg/cm2 in PBW343 at 15% PEG treatment. The average ChL content was 50.26 µg/cm2 in control conditions, and reduced by 4.42%, 7.02% and 11.26%, under 5%, 10 % and 15% PEG treatments, respectively. The genotypes K9423 and HD2733 were less affected, even at the highest level of stress treatment. The photosynthetic rate (Pn) was found to reduce under drought stress conditions. In the control condition, the minimum Pn was 10.0 (WH711) and the maximum 25.67 µmol/m2sec (RAJ3765) (Table 4, Figure 2). In treatment cases, Pn ranged from 9.27 (WH711) to 23.48 (RAJ3765) µmol/m2sec, 7.48 (PBW343) to 21.12 (RAJ3765) µmol/m2sec and 6.50 (WH711) to 19.35 (RAJ3765), at 5%, 10% and 15% PEG concentration, respectively. An average Pn of 17.16 µmol/m2sec was observed under the control conditions, which was decreased by 11.01%, 25.52% and 35.49%, under 5%, 10% and 15% PEG treatments, respectively. The maximum reduction in Pn of 40.31% was observed in the genotype DBW17 at 15% PEG treatment, whereas the genotypes HD2888 and HD2733 were least affected and showed only a 18.90% and 21.70% reduction.

Table 4.
Mean values of physiological and biochemical traits under control and 5%, 10% and 15% PEG treatment condition.
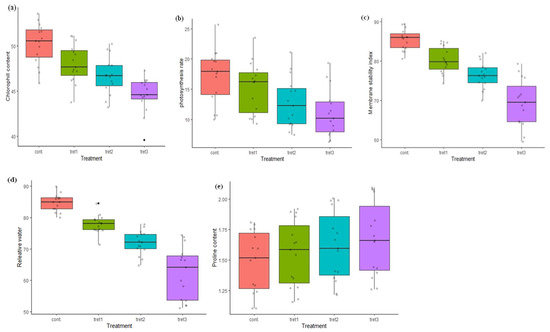
Figure 2.
Variation in physiological and biochemical traits in Control (0% PEG), 5% PEG, 10% PEG and 15% PEG treatment conditions: (a) Chlorophyll content, (b) Photosynthesis rate, (c) Membrane stability index, (d) Relative water content, (e) Proline content.
The maximum MSI 89.42% was recorded in HD288 and the minimum in UP2425 (80.55%) in control conditions (Table 4, Figure 2). The MSI lies between 74.30% (WH711) and 85.14% (HD2888), 69.99% (WH711) and 82.09% (HD2888), and 60.60% (PBW343) and 79.29% (HD2888), at 5%, 10% and 15% PEG treatment, respectively. The average MSI under the control treatments was 85.48%, and it gradually decreased, being 6.14%, 10.59% and 18.78% less under the stress treatments. Among all the genotypes tested, HD2888 and RAJ3765 were least affected and were reduced by 11.32% and 12.40% respectively. The RWC ranged from 80.0% (PBW590) to 89.92 (WH711) in control conditions and 71.39% (WH1021) to 84.52% at 5% PEG treatment. In further treatments, the minimum and maximum RWC was 64.74% (DBW71) to 77.89% (RAJ3765) and 51.14% (PBW343) to 74.34% (HD2888), in the case of 10% and 15% PEG treatment, respectively (Table 4, Figure 2). A significant reduction in the RWC was observed as the stress was increased. The average RWC was 84.77% in the control conditions, which was 26.58% less under the stress treatments at 15% PEG. The maximum proline content under control conditions was observed in the genotype HD2733 (1.81µM/gfw), and this increased to 1.92 µM/gfw, 2.01 µM/gfw and 2.08 µM/gfw, at 5%, 10 % and 15% PEG, respectively. The minimum proline content was in the genotype UP2425 (1.10 µM/gfw) under the control conditions and increased to 1.15 µM/gfw, 1.21 µM/gfw and 1.26 µM/gfw, at 5%, 10 % and 15% PEG treatment, respectively (Table 4, Figure 2).
3.3. Correlation of Traits
Significant correlations were observed in all traits compared between the control and drought stress treatments (Table 5 and Table 6). The GP had a significant positive correlation with ShL, PH, DTH in the control condition, whereas it showed a negatively correlation with RL in the case of 5% PEG. Otherwise, in all the treatments, the GP had a significant positive correlation with all the traits except DTH, FLA and DTM. In the control conditions, ShL was significantly negatively correlated with FLA and ChL. In contrast, significant positive correlations of ShL were observed with DTH, GPS, TW, Pn in 5% PEG; with PH, SPS, Proline in 10% PEG; and with RL, DTH, SPS, GPS, TW, Pn, MSI, RWC and Proline in 15% PEG. The RL had a significant negative correlation with PH, SL, and SPS in the control and with Proline in the 5% PEG. Moreover, NT was negatively correlated with the proline content in the control, whereas, under the 5% PEG treatment, it had a negative correlation with DOH and a positive correlation with SL. DTH showed a significantly positive correlation with TW in control as well as drought stress conditions, whereas, negatively correlated with Pn and DTM.

Table 5.
Pearson’s correlation coefficients (r) describing the association of morphological, physiological and biochemical traits of 15 wheat genotypes evaluated under drought control (lower diagonal) and 5% PEG (upper diagonal) conditions.

Table 6.
Pearson’s correlation coefficients(r) describing association of morphological, physiological and biochemical traits of 15 wheat genotypes evaluated under drought 10% PEG (lower diagonal) and 15% PEG (upper diagonal) treatment conditions.
Similarly, PH was positively correlated with SL under both stress and control conditions, whereas, with FLA, DTM and Pn, a positive correlation was observed only under drought stress conditions. On other hand, SL had significant positive correlation with FLA, SPS and DOM in control and PEG treatment conditions. However, DOM was negatively correlated with TW in 5% and 10% PEG, whereas, TW was positively correlated with Pn, MSI and proline content in treatment conditions. In the control and all the three treatments, Pn had a significant positive correlation with MSI and proline content, whereas, with ChL and RWC the correlation was observed only in 5% and 10% PEG. The proline content had a significant negative correlation with all physiological traits and yield related traits like SPS, GPS and TW in drought condition.
3.4. Principal Component Analysis (PCA)
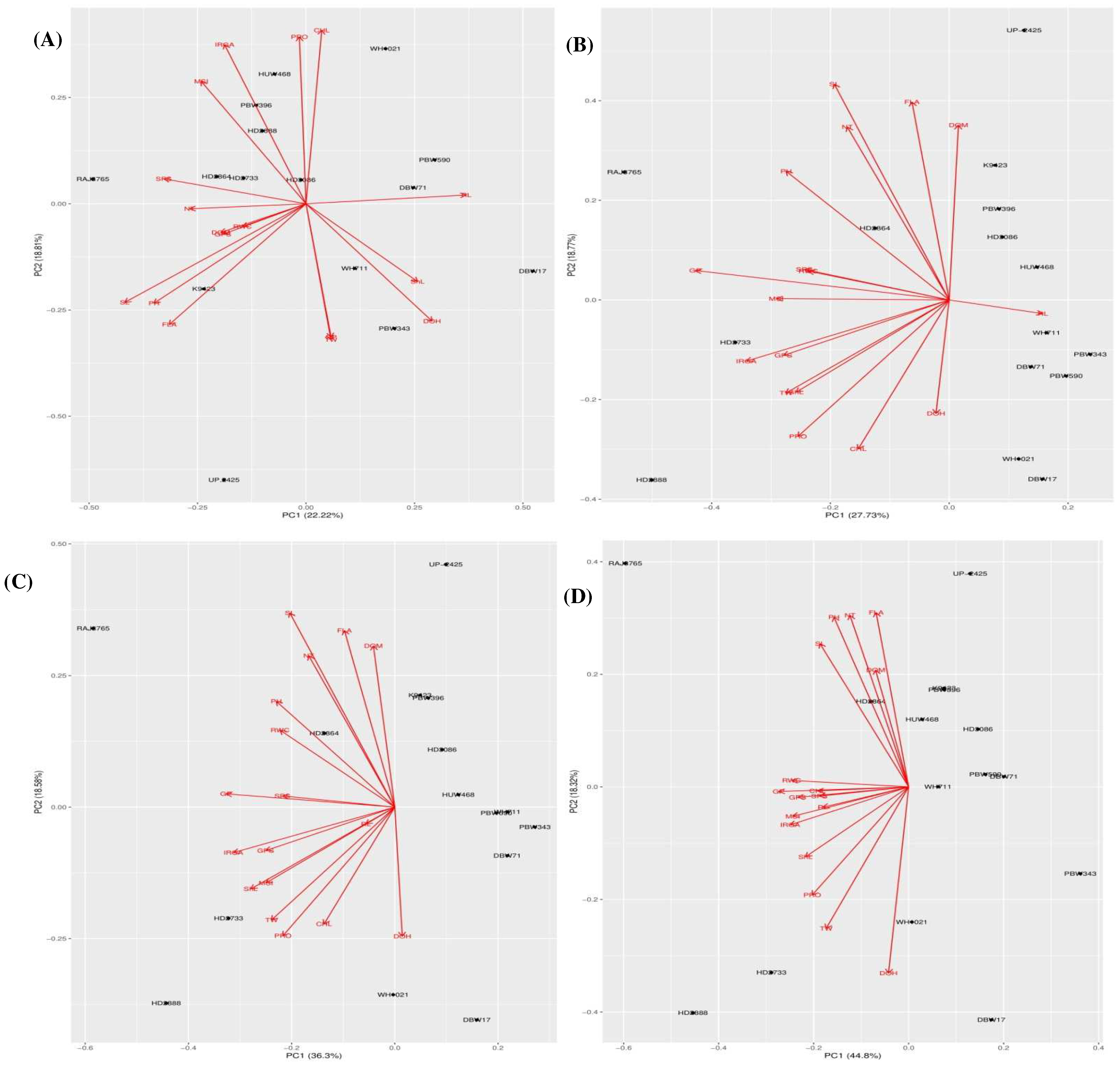
The first three components explained 53.29% of the total variation under the control conditions (Table 7). The first component (PC1) accounted for 22.22% of the variation, mostly affected by SL, PH, SPS and FLA. The most effective traits in the second component (PC2) were SL, PH, FLA and DOH. The third component (PC3) was mostly influenced with the variation of ShL and Pn. In drought stress conditions, the first three principal components explained 57.65%, 65.06% and 72.47% of the total variability in Treatment 1 (5%), Treatment 2 (10%) and Treatment 3 (15%), respectively (Table 7 and Table 8). In Treatment 1, the first two principal components accounted for 46.51% of total cumulative variation. The variables GP, Pn, MSI, GPS, TW and PH had high positive loading into the PC1, while PC2 was mostly affected by PH, SL and FLA followed by DOM and NT. The third component had high correlations with TW, ShL and RL variables. In treatments 2 and 3, the first two principal components had 54.88% and 63.12% total cumulative variations respectively. In treatment 2, the GP, Pn, ShL, and GPS in PC1; SL, FLA and DOM in PC2; while the DOH in PC3 were found as the most effective traits. Similarly, in treatment 3, the GP, Pn, RWC, MSI and GPS had high positive loading into the PC1; while FLA, NT and PH in PC2; followed by ShL and RL in PC3. The relationships between the different traits and genotypes with the respective principal components are further illustrated by the principal component biplots for the control and drought treatment conditions (Figure 3A–D).

Table 7.
Rotated component matrix of morphological, physiochemical and biochemical traits of 15 wheat genotypes under control and 5% PEG treatment conditions. Abbreviations: see Table 5.

Table 8.
Rotated component matrix of morphological, physiochemical, and biochemical traits of 15 wheat genotypes under 10% PEG and 10%PEG treatment conditions. Abbreviations: see Table 5.
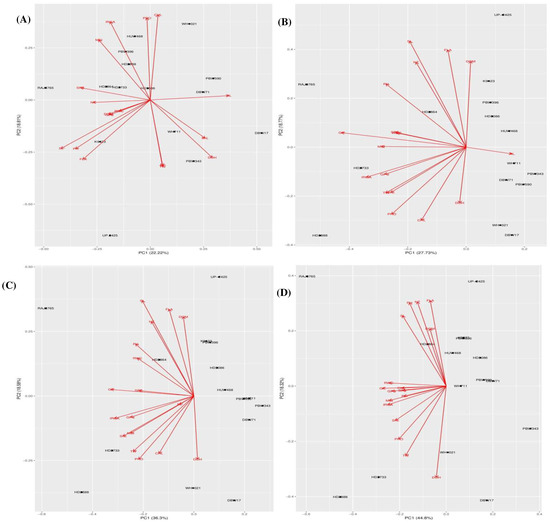
Figure 3.
(A) Principal component biplot showing genotypic grouping under control conditions. (B). Principal component biplot showing genotypic grouping under 5%PEG treatment condition. (C). Principal component biplot showing genotypic grouping under 10% PEG treatment condition. (D). Principal component biplot showing genotypic grouping under 15% PEG treatment condition. Where Germination count (GC), shoot length (ShL), root length (RL), number of tillers plant−1 (NT), days to heading (DOH), plant height (PH), flag leaf area (FLA), days to maturity (DOM), spike length (SL), spikelet number spike−1 (SPS), number of grain per spike (GPS), Thousand Grain Weight (TW), Photosynthesis rate (IRGA), chlorophyll (CHL), Membrane stability index (MSI) and Relative water content (RWC) and proline (PRO).
4. Discussion
Drought stress is known to cause a reduction in values for morphological traits (shoot length, root length, no. of tillers, days to heading, spike length, plant height and thousand grain weights) and affect the biological yield [19]. The wheat genotypes that were significantly tolerant to drought stress had major changes in their root system, photosynthetic rate and efficient utilization of available water. In the present study, germination percentage and seedling growth was significantly reduced with increase in the concentration of the PEG treatment. Similar findings have also been reported, where there was 98–100% germination under control conditions [11,35] but significant decreases from a maximum of 64% [36] to a lowest of 36% [37] observed with increased stress levels. The genotypes RAJ3765, HD288 and HD2733 performed better and showed maximum GP at higher PEG treatments. The induced drought stress significantly reduced the shoot and root lengths of wheat genotypes. A reduction in ShL and RL, ranging from 11.66 cm to 1.0 cm and 11.83 to 1.34 cm, with an increase in drought stress has been observed [8,11,38]. The reduction in the shoot/root lengths might be due to some disturbance posed by the osmotic stress conditions in cell division and elongation [19,39]. The number of tillers per plant has a direct contribution towards grain yield in wheat [40], and thus, it is an important trait to measure. In this study, the average number of tillers per plant was 5.74 and was found to reduce with increasing levels of drought stress. A reduction in the average tiller numbers from 4.45 to 3.36 due to severe drought stress has also been reported [26,41]. The drought stress caused reduction in PH and FLA of between 9.76% and 28.63% under stress conditions. A drastic reduction in FLA, up to 30% under stress conditions, was observed in previous studies [42]. Under drought stress, the reduction in plant height could be attributed to a decline in the cell enlargement and more leaf senescence [23,43] and the reduction in cell expansion and production of cells both are known to contribute to a loss in leaf area [44]. In the present study, a reduction of 8–16 days in DTH and 10–23 days in the number of days to maturity was observed. Likewise, 7–18 days early heading in drought conditions was also reported [42]. In accordance with the previous reports, the number of days to maturity was found to reduce as stress levels were increased. A reduced number of days to heading and days to maturity also play an important role in drought stress tolerance as they allow for drought escape [19,45,46,47]. However, the plant cycle should not be too short, because such traits will compromise yields. The average DTM under drought stress treatment condition was 98.97 days, which was slightly lower than in the control (103.13 days) [48]. It was earlier found that, the susceptible and tolerant genotypes that show early maturity under stress conditions, manifest the escape mechanism of the genotype for drought tolerance [28]. Besides, drought stress is also known to cause reduction in the spike length (SL), number of grains per spike and spikelets per spike [49,50]. The drought stress also significantly affects the grain filling, thus leading to reduced grain size and a smaller number of grains [51,52]. So ultimately this causes reduction in grain and biological yields [19,53,54]. Previously, about 19.8% reduction in the number of grains per spike under drought stress condition have been reported [50].
The varied responses by morphological and physiological features in the wheat genotypes are assumed to be attributable to differences in genotype of each variety. The genotypes HD2888 and RAJ3765 were less affected in terms of the quantitative traits like SL, SPS, GPS and TW. Fewer effects on these traits under different drought stress conditions can be considered as the phenotype of tolerant genotypes [40]. The studies on physiological responses of wheat varieties to drought stress are essential to understand the mechanisms of drought resistance. Drought induces significant alterations in wheat physiology [55]. Previous studies have showed that water stress significantly decreased the ChL content and values of other physiological traits during the different developmental stages of wheat [16,56,57]. Among all the genotypes tested, PBW343 was found to be the most sensitive to drought stress, with an observed 21.62% reduction in ChL content, otherwise HD2888 was the least affected, reduced by only 8.24%. The genotypes with highest chlorophyll content under drought stress were classified as resistant, and those with lowest ChL content as the susceptible genotypes [27]. The reduction in Pn from 20 µmol/m2sec to 6µmol/m2sec with the increase in the level of PEG-6000 concentration recorded previously [16]. In the current findings, the maximum reduction in Pn of 40.31% was observed in the genotype DBW17 at 15% PEG treatment, whereas the genotypes HD2888 and HD2733 were least affected and showed only 18.90% and 21.70% reductions. Senescence is accelerated by drought stress, which accelerates chlorophyll breakdown, resulting in a reduction in photosynthesis and the reduction in Pn ultimately leads to yield loss [54,58,59].
Under drought stress conditions, the RWC is an important indicator of the water status in wheat [60]. The drought stress could reduce the RWC up to 43% (from 88 to 45%) in bread wheat [61].
As water stress has adverse effects both on membrane structure and function [62], measurement of the membrane stability index has been considered as an important scale for selecting the drought tolerant wheat genotypes [28]. Previously, a significant decrease in MSI from 85% in the control to 50% in drought stress treatments has been reported [63]. Most importantly, the accumulation of proline in plant cells plays a crucial role in fighting drought stress due to its ability to oppose oxidative stress and is considered to be an important strategy to overcome the effects of water stress [64]. It was observed that the amount of proline content increased with the increase in the level of drought stress [65] and the genotype with the highest proline content performed better under stress conditions [28]. A significant increase of proline up to 1.37 µM/gfw was recorded under drought stress conditions [66].
A significant correlation between the yield related traits in normal and drought stress conditions may be considered as target traits during the selection process [67,68]. Significant positive correlation between cell MSI and yield related traits and, spike length with PH and SPS under both stressed and control conditions have been reported [26,69]. In the current study also, significant positive correlations were found between the morphological traits related to yield (TW, SL, SPS, GPS) and physiological traits (Pn, RWC, MSI) in treatment conditions.
Hence, the measurement of these traits may also be used as an important scale for selecting drought tolerant wheat genotypes [28]. The high correlation between a trait and component indicates that the trait is associated with the direction of the minimum or maximum amount of variability in the data set [70]. PCA biplots have been used by many researchers for the comparison of different genotypes [71,72], and some were able to reveal that the bread wheat genotypes with the larger PCA1 and lower PCA2 scores will give high yields (stable genotypes) and genotypes with the lower PCA1 and larger PCA2 scores had low yield (unstable genotype) [73,74,75].
5. Conclusions
A large range of genotypic diversity exists and confers a wide response to PEG-stimulated drought stress in wheat genotypes, according to the findings of the current research (Figure 1). PEG concentrations were observed to decrease with increasing PEG concentrations in all treatment conditions except the proline content, which was shown to rise with increasing PEG concentrations. The relationship between physiological and yield related (SPS, GPS, and TW) features was shown to be statistically significant and favourable. Evaluation of these characteristics, as well as the build-up of proline content, may be regarded as a method for the successful selection of drought resistant wheat cultivars in future research. GP, Pn, MSI, GPS, and TW were all shown to be impacted by PEG treatment under the drought treatment scenarios, suggesting that these characteristics might be used as marker traits to assess the genotypes for drought stress under the conditions studied. The genotype RAJ3765 showed favourable results in all the drought stress treatments tested, and it would be an excellent source for future research into the mechanisms of drought resistance in wheat, if it were available.
Author Contributions
Data curation, V.S. (Vandana Sharma) and P.K.; Formal analysis, B.Y.B.; Funding acquisition, B.Y.B. and V.S. (Vaishali Shami); Investigation, V.S. (Vandana Sharma) and S.R.; Methodology, A.K. and P.K.; Resources, V.S. (Vandana Sharma), A.C., A.M. and V.S. (Vaishali Shami); Software, A.K., A.M. and P.K.; Supervision, V.S. (Vaishali Shami); Visualization, A.C. and P.K.; Writing—original draft, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Acknowledgments
All the authors are thankful to Sardar Vallabhbhai Patel University of agriculture and technology for providing laboratory facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aparna, S.; Patel, K.; Patel, S.; Pinto, S. Wheat and Its Application in Dairy Products: A Review. Research & Reviews. J. Dairy Sci. Technol. 2015, 4, 19–34. [Google Scholar]
- Shewry, P.R. The HEALTHGRAIN programme opens new opportunities for improving wheat for nutrition and health. Nutr. Bullet. 2009, 34, 225–231. [Google Scholar] [CrossRef]
- Kugler, K.G.; Siegwart, G.; Nussbaumer, T.; Ametz, C.; Spannagl, M.; Steiner, B.; Lemmens, M.; Mayer, K.F.; Buerstmayr, H.; Schweiger, W. Quantitative trait loci-dependent analysis of a gene co-expression network associated with Fusarium head blight resistance in bread wheat (Triticum aestivum L.). BMC Genom. 2013, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 September 2020).
- Allen, A.M.; Winfield, M.O.; Burridge, A.J.; Downie, R.C.; Benbow, H.R.; Barker, G.L.; Wilkinson, P.A.; Coghill1, J.; Waterfall, C.; Davassi, A.; et al. Characterization of a Wheat Breeders’ Array suitable for high-throughput SNP genotyping of global accessions of hexaploid bread wheat (Triticum aestivum). Plant Biotechnol. J. 2017, 15, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, I.; Rana, R.M.; Shoaib, U.R.; Haidery, Q.; Ahmad, F.; Ijaz, A.; Umar, H.M.I. A comprehensive review of effects of water stress and tolerance in wheat (Triticum aestivum L.). Trop. Plant Res. 2015, 2, 271–275. [Google Scholar]
- Ahmad, I.; Khaliq, I.; Mahmood, N.; Khan, N.; Secretariat, E.F. Morphological and physiological criteria for drought tolerance at seedling stage in wheat. J. Anim. Plant Sci. 2015, 25, 1041–1048. [Google Scholar]
- Prakash, V.; Tiwari, S.; Shukla, R.S.; Tripathi, N.; Sapre, S. Evaluation of Drought Stress Tolerance Efficiency of Wheat (Triticum aestivum L.) Genotypes at Germination and Seedling Stages. Int. J. Bio-Resour. Stress Manag. 2015, 6, 41–46. [Google Scholar] [CrossRef]
- Kajla, M.; Yadav, V.K.; Khokhar, J.; Singh, S.; Chhokar, R.S.; Meena, R.P.; Sharma, R.K. Increase in wheat production through management of abiotic stresses: A review. J. Appl. Nat. Sci. 2015, 7, 1070–1080. [Google Scholar] [CrossRef]
- Peymaninia, Y.; Valizadeh, M.; Shahryari, R.A.M.; Ahmadizadeh, M. Evaluation of morphophysiological responses of wheat genotypes against drought stress in presence of a Leonardite derived humic fertilizer under greenhouse condition. J. Anim. Plant Sci. 2012, 22, 1142–1149. [Google Scholar]
- Chachar, M.H.; Chachar, N.A.; Chachar, Q.; Mujtaba, S.M.; Chachar, S.; Chachar, Z. Physiological characterization of six wheat genotypes for drought tolerance. Int. J. Res. Granthaalayah 2016, 4, 184–196. [Google Scholar]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Borner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.M.; Ossola, A.; Leishman, M.R.; Ellsworth, D.S. A simple method for simulating drought effects on plants. Front. Plant Sci. 2020, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zheng, X.; Liu, H.; Able, J.A.; Yang, H.; Zhao, H.; Liu, M. Effects of Drought Stress on Pollen Sterility, Grain Yield, Abscisic Acid and Protective Enzymes in Two Winter Wheat Cultivars. Front. Plant Sci. 2017, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Ni, Z.; Peng, H.; Dong, G.; Sun, Q. Ectopic overexpression of wheat TaSrg6 gene confers water stress tolerance in Arabidopsis. Plant Sci. 2007, 172, 1079–1086. [Google Scholar] [CrossRef]
- Djibril, S.; Mohamed, O.K.; Diaga, D.; Diegane, D.; Abaya, B.F.; Maurice, S.; Alain, B. Growth and development of date palm (Phoenix dactylifera L.) Seedlings under drought and salinity stresses. Afr. J. Biotechnol. 2005, 4, 968–972. [Google Scholar]
- Bayoumi, T.Y.; Eid, M.H.; Metwali, E.M. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. Afr. J. Biotechnol. 2008, 7, 2341–2352. [Google Scholar]
- Rana, M.S.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R. Effect of polyethylene glycol induced water Stress on germination and seedling growth of wheat (Triticum aestivum). Agriculturists 2017, 15, 81–91. [Google Scholar] [CrossRef][Green Version]
- Datir, S.S.; Inamdar, A. Biochemical Responses of Wheat Cultivars to PEG-Induced Drought Stress. Russ. Agric. Sci. 2019, 45, 5–12. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Turner, N.C.; Liu, Y.X.; Siddique, K.H.; Xiong, Y.C. Effects of drought stress on morphological, physiological and biochemical characteristics of wheat species differing in ploidy level. Funct. Plant Biol. 2017, 44, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Plaut, Z.; Butow, B.J.; Blumenthal, C.S.; Wrigley, C.W. Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crop. Res. 2004, 86, 185–198. [Google Scholar] [CrossRef]
- Blum, A. Mitigation of Drought Stress by Crop Management. 2005. Available online: https://plantstress.com/drought/ (accessed on 8 September 2020).
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [PubMed]
- Arjenaki, F.G.; Jabbari, R.; Morshedi, A. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int. J. Agric. Crop Sci. 2012, 4, 726–729. [Google Scholar]
- Kadam, S.; Shukla, Y.; Subhash, N.; Singh, C.; Suthar, K. Screening of Wheat Genotypes (Triticum durum L.) in Response to Drought Stress by Some Physiological and Biochemical Indices. Int. J. Pure App. Biosci. 2017, 5, 969–977. [Google Scholar]
- Stickler, F.C.; Wearden, S.; Pauli, A.W. Leaf area determination in grain sorghum. Agron. J. 1961, 53, 178–188. [Google Scholar] [CrossRef]
- Schonfeld, M.A.; Johnson, R.C.; Carver, B.F.; Mornhinweg, D.W. Water relations in winter wheat as drought resistance indicators. Crop. Sci. 1988, 28, 526–531. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer: New York, NY, USA, 2002. [Google Scholar]
- Duan, H.; Zhu, Y.; Li, J.; Ding, W.; Wang, H.; Jiang, L.; Zhou, Y. Effects of Drought Stress on Growth and Development of Wheat Seedlings. Int. J. Agric. Biol. 2017, 19, 1119–1124. [Google Scholar] [CrossRef]
- Jajarmi, V. Effect of water stress on germination indices in seven wheat cultivar. World Acad. Sci. Eng. Technol. 2009, 49, 105–106. [Google Scholar]
- Rauf, M.; Munir, M.; ul Hassan, M.; Ahmad, M.; Afzal, M. Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr. J. Biotechnol. 2007, 6, 8. [Google Scholar]
- Almaghrabi, O.A. Impact of drought stress on germination and seedling growth parameters of some wheat cultivars. Life Sci. J. 2012, 9, 590–598. [Google Scholar]
- Fraser, T.; Silk, W.; Rosr, T. Effect of low water potential on cortical cell length in growing region on maize roots. Plant Physiol. 1990, 93, 648–651. [Google Scholar] [CrossRef]
- Khan, N.; Naqvi, F.N. Effect of water stress in bread wheat hexaploids. Curr. Res. J. Biol. Sci. 2011, 3, 487–498. [Google Scholar]
- Khakwani, A.A.; Dennett, M.D.; Munir, M. Drought tolerance screening of wheat varieties by inducing water stress conditions. Songklanakarin J. Sci. Technol. 2011, 33, 135–142. [Google Scholar]
- Bazzaz, M.M.; Mahmud, A.A.; Khan, M.S.A. Effects of Water Stress on Morpho-Phenological Changes in Wheat Genotypes. Glob. J. Sci. Front. Res. D Agric. Vet. 2014, 14, 91–99. [Google Scholar]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef]
- Alves, A.A.; Setter, T.L. Response of cassava leaf area expansion to water deficit: Cell proliferation, cell expansion and delayed development. Ann. Bot. 2004, 94, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sial, M.A.; Dahot, M.U.; Arain, M.A.; Markhand, G.S.; Mangrio, S.M.; Naqvi, M.H.; Laghari, K.A.; Mirbahar, A.A. Effect of water stress on yield and yield components of semi-dwarf bread wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 1715–1728. [Google Scholar]
- Khakwani, A.A.; Dennett, M.D.; Munir, M.; Abid, M. Growth and yield response of wheat varieties to water stress at booting and anthesis stages of development. Pak. J. Bot 2012, 44, 879–886. [Google Scholar]
- Lopes, M.S.; Reynolds, M.P.; Jalal-Kamali, M.R.; Moussa, M.; Feltaous, Y.; Tahir, I.S.A.; Baum, M. The yield correlations of selectable physiological traits in a population of advanced spring wheat lines grown in warm and drought environments. Field Crop. Res. 2012, 128, 129–136. [Google Scholar] [CrossRef]
- Mansouri, A.; Oudjehih, B.; Benbelkacem, A.; Fellahi, Z.E.A.; Bouzerzour, H. Variation and Relationships among Agronomic Traits in Durum Wheat [Triticum turgidum (L.) Thell. ssp. turgidum conv. durum (Desf.) MacKey] under South Mediterranean Growth Conditions: Stepwise and Path Analyses. Int. J. Agron. 2018, 2018, 8191749. [Google Scholar] [CrossRef]
- Eid, M.H. Estimation of heritability and genetic advance of yield traits in wheat (Triticum aestivum L.) under drought condition. Int. J. Genet. Mol. Biol. 2009, 1, 115–120. [Google Scholar]
- Kilic, H.; Yagbasanlar, T. The effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum ssp. durum) cultivars. Not. Bot. Horti Agrobot. 2010, 38, 164–170. [Google Scholar]
- Liu, Y.; Bowman, B.C.; Hu, Y.G.; Liang, X.; Zhao, W.; Wheeler, J.; Chen, J. Evaluation of Agronomic Traits and Drought Tolerance of Winter Wheat Accessions from the USDA-ARS National Small Grains Collection. Agronomy 2017, 7, 51. [Google Scholar] [CrossRef]
- Pireivatlou, S.A.; Yazdansepas, A. Evaluation of wheat (Triticum aestivum L.) genotypes under pre-and post-anthesis drought stress conditions. J. Agric. Sci. Technol. 2010, 10, 109–121. [Google Scholar]
- Noreen, S.; Fatima, K.; Athar, H.U.R.; Ahmad, S.; Hussain, K. Enhancement of physio-biochemical parameters of wheat through exogenous application of salicylic acid under drought stress. J. Anim. Plant Sci. 2017, 27, 153–163. [Google Scholar]
- Ding, J.; Huang, Z.; Zhu, M.; Li, C.; Zhu, X.; Guo, W. Does cyclic water stress damage wheat yield more than a single stress. PLoS ONE 2018, 13, e0195535. [Google Scholar] [CrossRef]
- Muhammad, K.N.; Ahmad, M.; Muhammad, K.; Muhammad, K.; Nawaz, S.; Muhammad, S.I. Physiological Responses of Wheat (Triticum aestivum L.) to Drought Stress. Int. J. Plant Soil Sci. 2015, 6, 1–9. [Google Scholar]
- Saeidi, M.; Abdoli, M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J. Agric. Sci. Technol. 2015, 17, 885–898. [Google Scholar]
- Shamsi, K. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010, 8, 1051–1060. [Google Scholar]
- Ashraf, M.A.; Ashraf, M. Salt-induced variation in some potential physiochemical attributes of two genetically diverse spring wheat (Triticum aestivum L.) cultivars: Photosynthesis and photosystem II efficiency. Pak. J. Bot. 2012, 44, 53–64. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gupta, S.; Gupta, N.K.; Arora, A.; Agarwal, V.P.; Purohit, A.K. Effect of water stress on photosynthetic attributes, membrane stability and yield in contrasting wheat genotypes. Indian J. Plant Physiol. 2012, 17, 22–27. [Google Scholar]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Zhan, H.X.; Chang, Z.J.; Wei, A.L.; Zhang, X.J.; Li, X. Impact of drought to wheat physiological index. J. Shanxi Agric. Sci. 2011, 39, 1049–1051. [Google Scholar]
- Singh, N.P.; Pal, P.K.; Vaishali, S.K. Morpho-physiological characterization of Indian wheat genotypes and their evaluation under drought condition. Afr. J. Biotechnol. 2014, 13, 20. [Google Scholar]
- Dodig, D.; Zorić, M.; Kandić, V.; Perović, D.; Šurlan-Momirović, G. Comparison of responses to drought stress of 100 wheat accessions and landraces to identify opportunities for improving wheat drought resistance. Plant Breed. 2012, 131, 369–379. [Google Scholar] [CrossRef]
- Sareen, S.; Tyagi, B.S.; Sarial, A.K.; Tiwari, V.; Sharma, I. Trait analysis, diversity, and genotype x environment interaction in some wheat landraces evaluated under drought and heat stress conditions. Chil. J. Agric. Res. 2014, 74, 135–142. [Google Scholar] [CrossRef]
- Farshadfar, E.; Mohammadi, M.; Haghparast, R. Diallel analysis of agronomic, physiological and metabolite indicators of drought tolerance in bread wheat (Triticum aestivum L.). Int. J. Plant Breed. 2011, 1, 42–47. [Google Scholar]
- Khamssi, N.N.; Najaphy, A. Physiological and biochemical responses of durum wheat under mild terminal drought stress. Cell Mol. Biol. 2018, 64, 59–63. [Google Scholar] [CrossRef]
- Parchin, R.A.; Najaphy, A.; Farshadfar, E.; Hokmalipour, S. Assessment of drought tolerance in genotypes of wheat by multivariate analysis. World Appl. Sci. J. 2013, 22, 594–600. [Google Scholar]
- Shivramakrishnan, R.; Vinoth, R.; Arora, A.; Singh, G.P.; Kumar, B.; Singh, V.P. Characterization of wheat genotypes for stay green and physiological traits by principal component analysis under drought condition. Int. J. Agric. Sci. 2016, 12, 245–251. [Google Scholar] [CrossRef]
- Kaya, Y.; Palta, C.; Taner, S. Additive main effects and multiplicative interactions analysis of yield performances in bread wheat genotypes across environments. Turk. J. Agric. For. 2002, 26, 275–279. [Google Scholar]
- Dadbakhsh, A.; Yazdansepas, A.; Ahmadizadeh, M. Study drought stress on yield of wheat (Triticum aestivum L.) genotypes by drought tolerance indices. Adv. Environ. Biol. 2011, 5, 1804–1810. [Google Scholar]
- Alshahi, R.A.; Omidi, M.; Talei, A.R.; YazdiSamadi, B. Evaluation of bread wheat genotypes for drought tolerance. Electron. J. Crop Prod. 2010, 3, 159–171. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).