Abstract
This study aimed to test the role of hydrogen sulfide (H2S) in the responses regarding the nitric oxide- (NO) and sulfur (S)-mediated improvement in photosynthesis and growth under cadmium (Cd) stress in mustard (Brassica juncea L. cv. Giriraj), and integrate the mechanisms of S, nitrogen (N), and antioxidant metabolism. The plants grown with Cd (200 mg Cd kg−1 soil) exhibited reduced assimilation of S and N and diminished photosynthetic performance, which was associated with higher Cd accumulation-induced excess reactive oxygen species (ROS) production. The application of 100 μM of sodium nitroprusside (SNP, a NO donor) together with a more prominent concentration of S resulted in increased photosynthetic S- and N-use efficiency, production of non-protein thiols and phytochelatins, efficiency of enzymatic (superoxide dismutase, ascorbate peroxidase, and glutathione reductase), non-enzymatic antioxidants (ascorbate and glutathione) limiting Cd accumulation and, thus, reduced oxidative stress (superoxide radical, hydrogen peroxide, and thiobarbituric acid reactive species content). The benefit of NO together with S was manifested through a modulation in H2S production. The use of 100 μM of hypotaurine (HT; H2S scavenger) or 100 μM of cPTIO (2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) in plants treated with NO plus S reversed the action of NO plus S, with a higher reduction in photosynthesis and growth with the use of HT, suggesting that H2S plays a significant role in the NO- and S-mediated alleviation of Cd stress. The interplay of NO and ES with H2S may be used in augmenting the photosynthesis and growth of Cd-grown mustard plants.
1. Introduction
The continuous increase in the demand for agricultural products by an ever-growing human population is perpetually compromising the environment and has exacted a significant price. To this end, heavy metal pollution has emerged as a growing concern across a wide range of terrestrial ecosystems in recent years, posing a threat to both the environment and public health worldwide [1,2]. For plants, heavy metal stress is well-recognized as a potentially hazardous form of abiotic stress. It has generated significant concerns among environmentalists and biologists around the world about the possible impact on cultivated land area, crop quality, and agricultural productivity [1,2,3]. Cadmium (Cd) is placed among the top ten toxic metals in the World Health Organization’s (WHO) report on chemicals of major health concern for humans and other organisms [3,4]. The principal sources of Cd contamination in agricultural soils are mainly anthropogenic, including pigment processing, electroplating, leather tanning, metal polishing, steelmaking, phosphatic fertilizers, and wastewater irrigation [2,3]. Cadmium exerts its detrimental effects on plants by producing excessive reactive oxygen species (ROS), which include free oxidants such as singlet oxygen (1O2), hydroxyl radicals (OH−), superoxide radicals (O2•−), and hydrogen peroxide (H2O2) [5]. Once Cd enters the plant cell, it induces necrosis, stunted growth, altered enzyme activities, chlorosis, and, eventually, cell death [4]. The detrimental impacts of Cd on photosynthesis are directed towards the impaired photosynthetic electron transport chain, chlorophyll biosynthesis, stomatal movements, and enzymes/proteins of light and dark reactions [2,3,5,6]. To confront the cellular ROS levels under stress, plants tend to employ the antioxidant system, which minimizes ROS-accrued consequences including protein and lipid oxidation and maintains cellular redox homeostasis [7]. The major antioxidant enzymes, namely, superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR), as well as non-enzymatic antioxidants, including ascorbate (AsA) and reduced glutathione (GSH), alone or cumulatively scavenge several ROS [8,9,10].
Nitric oxide (NO) is a gaseous signaling molecule and a component of the nitrogen cycle widely known as a “jack of all trades” [11,12,13]. It regulates diverse biological functions in plants, ranging from seed germination to flowering and fruit development [11,13,14]. Under stressed conditions, both exogenously applied or endogenously produced NO modulates the expression of several genes involved in signal transduction, stress sensing, photosynthesis, ROS detoxification, primary metabolism, and apoptosis, thus activating the adaptive stress response of plants. [15,16]. Nitric oxide transmits its bioactivity largely through NO-dependent post-translational modification signaling such as S-nitrosylation (SNO), which regulates important processes through different mechanisms, including cellular localization, enzymatic activity, three-dimensional conformational changes, apoptosis, and protein–protein interaction [14,16,17].
Sulfur (S)—as the fourth essential macronutrient after nitrogen (N), phosphorus (P), and potassium (K)—supports diverse plant responses in both normal and stressful environments [18]. In the S-metabolism pathway, sulfate (SO42−) from soil is assimilated into cysteine (Cys) with the help of the enzymes ATP-sulfurylase (ATP-S) and O-acetylserine (thiol) lyase (OAS-TL). Cysteine synthesis is regarded as the transitional step and branch point for the synthesis of various S metabolites. Moreover, Cys is the primary product of reduced S and serves as a connecting link between S and N metabolism, whereas its precursor O-acetyl serine (OAS) is derived from N and C assimilation pathways [17]. After assimilation, sulfur becomes a constituent of vitamins, cofactors, Fe-S clusters, secondary metabolites (glucosinolates and allyl cysteine sulfoxides), and a large number of S-defensive compounds such as GSH, phytochelatins (PCs), methionine, and hydrogen sulfide (H2S) that have been identified to play an imperative role in combating adverse environmental stresses [9,12,18]. S deficiency in agricultural soils has been reported to be a limiting factor that reduces crop yields and quality as this nutrient is often overlooked by farmers [19]. The persistence of Cd in S-deficient soils further worsens the problem for plants [20]. Since S is an immobile nutrient, a continuous supply of S is needed for the proper growth and development of plants, which can be met by using an effective and inexpensive S source [21].
Several plant species in the Brassica genus are resistant to the toxic effects of heavy metals and thus can be potentially used in phytoremediation [22]. Brassica species can phytoremediate heavy metals via physiological mechanisms such as phytovolatilization, phytostabilization, and phytoextraction [23]. Brassica juncea is an oilseed crop that requires relatively higher S amounts for the synthesis of essential amino acids, oils, and S-rich secondary metabolites (glucosinolates and glycosides) [18]. Indian mustard is also known as leaf mustard or vegetable mustard. Moreover, B. juncea also has many sub-species, such as B. juncea subsp. napiformis (root mustard), B. juncea subsp. tsatsai (big-stem mustard), and B. juncea subsp. integrifolia var. stromata (large-petiole mustard). Due to its greater biomass proportion, B. juncea can hyperaccumulate heavy metals in its vegetative parts. For this reason, it is an excellent hyperaccumulator and chelator of heavy metals such as Cd; however, its phytoextraction capability is genotypically dependent [21]. Moreover, B. juncea plants are capable of synthesizing PCs, GSH, and metallothionines, which aid in metal translocation and chelation [23,24].
In addition to NO, H2S is another gaseous signaling molecule that has received considerable attention in recent years due to its dynamic role in strengthening plants’ defense capabilities under abiotic stress [25,26,27]. H2S is a volatile reduced S compound and its biosynthesis is directly related to the S-related nutritional status of plants [28]. The H2S biosynthetic pathway in plants is parallel to S assimilation, and involves five enzymes of S assimilation, namely, L-cysteine desulfhydrase (L-DES), D-cysteine desulfhydrase (D-DES), sulfite reductase (SiR), cyanoalanine synthase (CAS), and O-acetly-L-serine lyase (OAS-TL) [11]. The H2S in plants plays multiple roles, such as in seed germination, senescence, organogenesis, flowering, root development, and abiotic stress tolerance [25,29]. The putative role of the interplay between NO and H2S under a myriad of environmental conditions is well documented [11,12,13]. H2S may work either upstream or downstream of NO depending on its involvement in processes such as the regulation of stomatal movements under abiotic stresses [6]. NO and H2S have a family of derived molecules known as reactive nitrogen species (RNS) and reactive sulfur species (RSS), respectively, that can interact with a variety of biomolecules such as nucleic acids, proteins, and lipids, and modulate their activity [11]. In recent years, the coordinated action of the two signaling molecules has received considerable attention for its role in metal stress tolerance [5,6,11,12]. However, the direct link between their mode of action and the regulation of plant growth and photosynthetic dynamics in response to Cd stress is largely unknown. On the other hand, major advances in S research in relation to plants, particularly the role of NO in the regulation of S uptake and assimilation under various types of stresses, are well documented [30,31,32], but their interactive impact under Cd stress needs more evidence. Recent research has shown that S and NO interact in plants via H2S, which plays multiple roles in plant responses to drought stress [33]. Despite the extensive research on the roles of NO, S, and H2S in abiotic stress tolerance, little effort has been made to investigate the role of their crosstalk in various physiological and biochemical processes underlying Cd stress tolerance in plants. Therefore, in consideration of these postulations, the current study intends to highlight the impacts and mechanisms underlying the NO- and S-induced regulation of plant growth, photosynthesis, antioxidant potential, S and N assimilation, and Cd accumulation and translocation, as well as the involvement of H2S in the presence of NO and S in the mitigation of Cd stress impacts in plants.
2. Material and Methods
2.1. Plant Material and Growth Conditions
Healthy and uniform mustard (Brassica juncea cv. Giriraj) seeds were procured from the Indian Agricultural Research Institute, New Delhi. Seeds were surface-sterilized with diluted 0.5% sodium hypochlorite solution (v/v) and were later rinsed repetitively with double-distilled water. The experiment was performed in 23 cm diameter earthen pots filled with 5 kg of reconstituted soil with sand: peat: compost (1:4:1, w/w) mixture at the Department of Botany, Aligarh Muslim University, Aligarh, India. Plants were fed with 200 Cd kg−1 soil at the time of seed sowing, while S at 200 mg kg−1 soil was given 15 days prior to sowing in the form of elemental sulfur (ES) and ammonium sulphate (AS). The concentrations of Cd and S are based on the findings of our earlier work [21]. Following the establishment of the seedlings, four plants per pot were maintained. A concentration of 100 µM of NO as sodium nitroprusside (SNP) was applied as foliar spray with a hand sprayer 15 days after sowing (DAS). The treatments included control, Cd (200 mg Cd kg−1 soil), SNP (100 µM), ES (200 mg S kg−1 soil), AS (200 mg S kg−1 soil), Cd + SNP, Cd + ES, Cd + AS, Cd + SNP + ES, and Cd + SNP + AS. Further, to substantiate the role of H2S in NO with S-mediated responses and Cd stress mitigation, another set of experiments was performed wherein 30 mL of 100 µM cPTIO 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; NO scavenger) or 100 µM of hypotaurine (HT; H2S scavenger) were applied to the plants that received Cd plus S at 15 DAS. The experimental design is given in Table 1. The concentrations of NO and H2S scavengers were selected based on the study of Gautam et al. [27]. The pots were kept in a naturally illuminated net-house with day/night temperatures of 23/16 ± 2 °C, 16/8 h light/dark periods, and relative humidity of 60 ± 4%. Recommended basal doses of S, N, phosphorus (P), and potassium (K) (40 mg S + 60 mg N + 17.9 mg P and 17.9 mg K per kg of soil) were supplied at the time of sowing for both the experiments. The treatments in all the experiments were arranged in a factorial, randomized block design with four (n = 4) replicates for each treatment. At 30 DAS, determinations were made regarding various growth and photosynthetic attributes, oxidative stress, antioxidant responses, S and N metabolism, and H2S biosynthesis. For the purpose of determining photosynthetic and growth parameters, one plant per pot was chosen. The same samples in dried form were used to determine concentrations of Cd, N, and S. The remaining plants per pot were used to study various physio-biochemical and histochemical parameters.

Table 1.
Experimental design.
2.2. Plant Growth and Relative Water Content (RWC)
To determine the growth parameters, plants were gently uprooted from pots and washed carefully to remove soil debris from the roots. Plants were kept for drying in a hot air oven at 80 °C until achieving a constant weight to record plants’ dry biomass. Leaf area was measured using a leaf area meter (LA211, Systronics, New Delhi, India).
To determine RWC, fifteen leaves collected from each treatment were weighed to determine their fresh weight (FW). The leaves were then placed in distilled water for 4 h to obtain turgid weight (TW). Later, the same leaves were dried in an oven at 80 °C for 24 h to measure dry weight (DW). The leaf RWC was calculated using the following formula:
RWC (%) = [(FW − DW)/(TW − DW] × 100
2.3. Measurement of Photosynthesis and Related Variables
An Infra gas analyzer (CID-340, Photosynthesis system, Bio-science, Camas, Washington, USA) was used to measure gas exchange parameters, net photosynthesis (PN), stomatal conductance (gs), and intercellular CO2 concentration (Ci) in fully expanded and intact topmost leaves in various treatments and replicates of the plants. The measurements were made on a sunny day between 10:00 and 11:30 h at light-saturating intensity, under photosynthetically active radiation (PAR), above 760 μmol m−2 s−1, and at 360 ± 5 μmol−1 atmospheric CO2 concentration. A SPAD chlorophyll meter was used to calculate the chlorophyll content (SPAD values) in the totally expanded uppermost leaves (502 DL PLUS, Spectrum Technologies, Plainfield, IL, USA).
The activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) was ascertained by adopting the method of Usuda [34]. The details are provided in our earlier work with Mir et al. [21].
Chlorophyll fluorescence was measured with a Mini-PAM chlorophyll fluorometer (Heinz Walz, GmbH, Effeltrich, Germany) and using WinControl-3.29 software with the instructions provided by the manufacturer. All samples were dark-adapted for 30 min prior to fluorescence measurements. Minimal fluorescence (Fo) and maximum fluorescence (Fm) were measured in dark-adapted leaves with a low-measuring beam light intensity, whereas under light-adapted conditions, minimal fluorescence (Fo׳) and maximum fluorescence (Fm׳) were measured in the same leaves with a saturating light intensity together with steady-state fluorescence (Fs). The difference between Fo and Fm was automatically calculated by software as variable fluorescence (Fv). The maximum quantum yield of PS II photochemistry was calculated as a ratio of Fv to Fm. Photochemical quantum yield of photosystem II [Y(II)] and electron transfer rate (ETR) were generated automatically in the dark-acclimated and light-exposed states of the sample using WinControl-3.29 software and a saturation pulse.
2.4. Assessment of Oxidative Damage
2.4.1. In Vitro Estimation of H2O2 and O2•−
The H2O2 content was determined via assay following the method of Okuda et al. [35]. Using a mortar and pestle, fresh leaves were ground in ice-cold 200 mM perchloric acid, and then centrifuged at 1200× g for ten min. Peroxidase was used to start the reaction, and the resulting increase in absorbance was measured at 590 nm for 3 min.
The method of Wu et al. [36] was used to determine the O2•−content. Fresh leaves (1 g) were ground in 65 mM phosphate buffer containing 1% polyvinylpyrrolidone (PVP) and centrifuged at 5000× g for 15 min. Absorbance was recorded spectrophotometrically at 530 nm, and the O2•− content was accessed from the standard curve.
2.4.2. In Vivo Localization of H2O2 and O2•− and Lipid Peroxidation
The localization of H2O2 and O2•− in leaves was validated via the histochemical method of Kumar et al. [37] with small modifications. To detect the aggregation of H2O2 and O2•−, fresh leaves were stained with nitro blue tetrazolium (NBT) and 3,3-diaminobenzidine (DAB), respectively. Leaves from different treatments were incubated in DAB solution for 8 h. After DAB staining, samples were soaked in absolute ethanol and boiled in a water bath for 15 min to discolor leaf pigments and make the stain visible.
The localization of lipid peroxidation was performed by the method demonstrated by Awasthi et al. [38]. Freshly collected leaf and root samples were washed and immersed in Schiff reagent solution. Afterwards, the samples were rinsed with a sulfite solution to retain the stain’s color. The images were captured using a digital camera.
2.4.3. In Vitro Measurement of Leaf Lipid Peroxidation
Lipid peroxidation in leaves was measured by estimating the content of thiobarbituric acid reactive species (TBARS) following the method of Dhindsa et al. [39] by reading absorbance at 532 nm and correcting for non-specific turbidity by subtracting the absorbance at 600 nm. TBARS content was then calculated using the extinction coefficient (155 Mm−1cm−1).
2.5. Assay of Antioxidant Enzymes
Fresh leaf tissues (0.5 g) were homogenized in chilled mortar and pestle with an extraction buffer containing 0.05% (v/v) Triton × 100, and 1% (w/v) polyvinylpyrrolidone in 100 mM potassium phosphate buffer of pH 7.0. The homogenate was centrifuged at 15,000× g for 20 min at 4 °C. The aliquot obtained from centrifugation was used for enzyme assays of SOD and GR. Ascorbate (2 mM) was added to the supernatant to determine the activity of APX.
2.5.1. SOD (EC 1.15.1.1)
The SOD activity was determined using Giannopolitis and Ries’s [40] procedure by studying the inhibition of photochemical reduction of NBT. At 560 nm, one unit of SOD is the amount of enzyme that inhibits NBT reduction by 50%.
2.5.2. GR (EC 1.6.4.2)
GR was determined based on the detection of GSH-dependent oxidation of NADPH following the protocol of Foyer and Halliwell [41]. The reaction mixture consisted of 0.5 mM of oxidized GSH, 0.2 mM of NADPH, and phosphate buffer (25 mM with pH 7.8). GR activity was calculated by using the extinction coefficient at 6.2 mM−1cm−1. At 25 °C, one unit of enzyme is required to decompose 1.0 mol of NADPH per minute.
2.5.3. APX (EC 1.11.1.11)
The activity of APX was determined using Nakano and Asada’s method [42] by detecting a decrease in ascorbate absorbance at 290 nm. The 1 ml reaction mixture included enzyme extract along with 0.5 mM ascorbate, 0.1 mM H2O2, 0.1 mM EDTA, and 50 mM phosphate buffer of pH 7.0. APX activity was calculated using the extinction coefficient of 2.8 mM−1cm−1. At 25 °C, one unit of enzyme is described as the amount needed to decompose 1 mol of substrate per min.
2.6. Assay of Non-Protein Thiol (NPT) and Phytochelatin (PC) Content
The NPT content was determined using the method provided by De Vos et al. [43]. NPTs were extracted by homogenizing 0.2 g of the samples in 2 mL of 5% sulfosalicylic acid and then centrifuging it for 15 min at 4 °C at 10,000× g. For NPTs’ determination, the reaction mixture consisted of 0.2 mL of the supernatant, 0.15 mL of 10 mM 5,5′-Dithiobis [2-nitrobenzoic acid] (DTNB), and 0.2 M of Tris- HCl (pH 8.2). After incubating the reaction mixture for 20 min, the absorbance at 412 nm was determined spectrophotometrically. The NPT content was determined from the remaining aliquot used for the GSH determination.
The PC content of leaves was calculated by subtracting the GSH content from the total amount of NPTs.
PCs (nmol g−1 FW) = NPT − GSH
2.7. Estimation of Leaf N Content, Nitrate Reductase (NR) Activity, Photosynthetic Sulfur Use Efficiency (p-SUE), and Photosynthetic Nitrogen Use Efficiency (-NUE)
Kjeldahl’s acid–peroxide digestion method was used to determine N content as described by Linder [44]. A 10 mL aliquot of digested leaf material was transferred to a 50 mL volumetric flask and to it 1.0 mL of 10% Na2SiO3 and 2.0 mL of 2.5 N NaOH were added to neutralize acid excess and avoid turbidity. A separate 10 mL graded test tube was employed and to it 5 mL of aliquot from solution and 0.5 mL of Nessler’s agent was added. The content were allowed to settle for 10 min for color development and optical density was recorded spectrophotometrically at 525 nm.
NR (EC 1.6.6.1) activity was determined by adopting the procedure of Kuo et al. [45] by preparing an enzymatic extract. Using liquid nitrogen, fresh leaves (1.0 g) were ground to powder in a chilled mortar and pestle and kept at a storage temperature of −80 °C. After thawing the powder for 10 min at 4 °C, it was homogenized in a blender in 250 mM Tris-HCl buffer of pH 8.5 containing 1 mM of DTT, 10 mM of Cys, 20 μM FAD, 10% (v/v) glycerol, and 1 mM EDTA, and the homogenate was subjected to centrifugation at 10,000× g for 30 min at 4 °C. After 10 min of incubation, spectrophotometric absorbance at 540 nm was measured.
p-SUE was calculated by the ratio of net photosynthesis to S content per unit leaf area. The ratio of net photosynthesis to N content per unit leaf area was used to calculate p-NUE.
2.8. Quantification and Histochemical Localization of Cd
Samples were dried in an oven for two days at 80 °C. The dried tissue was weighed, ground into powder, and the resulting powder was digested with concentrated HNO3/HClO4 (3/1; v/v). Cd content was determined by an atomic absorption spectrophotometer (GBC, 932 Plus; GBC Scientific instruments, Braeside, Australia). The tolerance index (T I as %) was calculated as ratio of dry weights of plants exposed to Cd to those under control conditions.
The histochemical localization of Cd was visualized using dithizone (diphenyl-thiocarbazone) dye (Himedia, India) according to procedure conducted by Seregin and Kozhevnikova, [46] with slight modifications. Tangential sections of roots, stems, and leaves of 30-day-old plants were taken from different treatments. A dithizone staining solution was prepared by dissolving 30 mg of dithizone in 60 mL of acetone and 20 mL of deionized water. To this, one or two drops of glacial acetic acid were added to improve the reaction sensitivity. The sections were reacted with a few drops of dithizone for half an hour and then examined under a light microscope equipped with an Optikam PRO6 digital camera (Optica C-P6, Ponteranica, Italy).
2.9. Assay for S-Assimilating Enzymes, S, Cys, GSH, and AsA Content
The method of Lappartient and Touraine [47] was adopted to determine ATP-S (EC 2.7.7.4) activity in fresh leaves by measuring the molybdate-dependent formation of pyrophosphate. Fresh leaves from each plant’s uppermost whorl were ground at 4 °C in a buffer containing 2 mM of dithiothreitol (DDT), 20 mM of Tris-HCl (pH 8.0), 10 mM of Na2EDTA, and 0.01 g mL−1 of PVP. The homogenate formed was centrifuged at 2000× g for 10 min at 4 °C. The supernatant was used for ATP-S assay. A reaction mixture was created by adding 7 mM of MgCl2, 5 mM of Na2MoO4, 2 mM of Na2ATP, and 0.032 units mL−1 of sulfate-free inorganic pyrophosphate in 80 mM Tris-HCl buffer (pH 8.0). The reaction mixture was incubated for 15 min and phosphate concentration was determined spectrophotometrically at 340 nm.
Content of S was determined in oven-dried samples digested in a mixture of concentrated HNO3 and 60% strength HClO4 (85:15; v/v) using the turbidimetric method of Chesnin and Yien [48]. A 5 mL reaction mixture was prepared by combining 1.0 g BaCl2 and 2.5 mL gum acacia; then, the final volume was increased to 25 mL by adding distilled water, and the contents were thoroughly shaken to completely dissolve BaCl2. The values were recorded spectrophotometrically at 415 nm from the time of turbidity development, and a blank was run after determining each set simultaneously.
Leaf Cys content was determined spectrophotometrically using the method of Gaitonde [49]. Fresh leaves (500 mg) were homogenized in ice-cold perchloric acid (5% w/v). The obtained suspension was centrifuged at 2800× g for 1 h at 5 °C. The supernatant was filtered through Whatman No.1 filter paper. The extinction was recorded at 580 nm and Cys content was determined with reference to a calibration curve obtained under similar conditions for standard Cys.
For the determination of GSH and AsA concentrations, the enzyme-recycling procedure was adopted following the method of Law et al. [50]. The details have been provided earlier by Mir et al. [24]. The extract prepared from grinding of 5 g fresh leaf material using 3.0 mL of 5% (w/v) sulfosalicylic acid with 6.3 mM diethylenetriamine pentacetic acid (DTPA, pH < 1.0) at 4 °C was used for the determination of GSH and NPTs concentrations.
2.10. Determination of Endogenous NO and H2S Content
The endogenous NO content was determined by estimating the nitrite content [51]. The leaf samples (1 g) were homogenized in a chilled mortar using a chilled pestle with extraction buffer (6 mL of 50 mM ice-cold acetic acid buffer (pH 3.6) containing 4% zinc acetate). The enzyme assay sample was centrifuged at 11,500× g for 15 min at 4 °C. After washing twice with extraction buffer, the pellet was again subjected to centrifugation. The supernatant obtained was neutralized with 100 mg of charcoal and then placed on a vortex for filtration. Greiss reagent (1% sulphanilamide and 0.1% N-1napthylethylenediaminedihydrochloride in a 5% H2PO4 solution) was added to 1mL of each filtrate and mixed in equal proportion (1:1). The mixture was then allowed to settle for incubation at room temperature for 30 min. The absorbance of mixture was measured spectrophotometrically at 540 nm and the NO content was calculated using a calibration curve plotted with sodium nitrate as standard.
H2S content was measured in fresh leaves by adopting the method of Nashef et al. [52]. The reaction mixture (2 mL) was prepared with 1.88 mL of extraction phosphate buffer (0.1M; pH 7.0) by adding 20 μL of 5,5′-dithiobis (2-nitrobenzoic acid). The assay mixture was then incubated for two minutes at room temperature. The optical density of the assay mixture was determined at 412 nm. The values were compared with the standard curve of Na2S.
2.11. Determination of L-DES and OAS-TL Activity
The enzymatic activity of L-DES and OAS-TL was determined by taking frozen leaf discs and grinding them in liquid nitrogen following the method of Bloem et al. [53]. The soluble protein for assay of L-DES and OAS-TL activity was extracted by adding 1ml of 20 mM TRIS/HCl (pH 8.0) to 100 mg leaf material and centrifuging it at 7000× g.
The L-DES activity was measured by monitoring the release of sulfide from Cys in a reaction mixture consisting of 1 mL of 0.8 mM L-Cys, 2.5 mM of dithiothreitol, 100 mM of TRIS/HCl (pH 9.0), and enzyme extract. L-cysteine was added to assay mixture to start the reaction. After incubating the enzyme assay for 15 min, the reaction was stopped by adding 10 μL of 20 mM N, N-dimethyl-p-phenylenediamine dihydrochloride dissolved in 7.2 N HCl and 100 μL of 30 mM FeCl3 dissolved in 1.2 N HCl. The absorbance was recorded spectrophotometrically at 670 nm to determine L-DES activity.
For the OAS-TL assay, the reaction mixture consisted of 50 μL of enzyme extract with 1 mL of each of 5 mM of Na2S, 5 mM of OAS, 100 mM of TRIS/HCl (pH 7.5), and 3.33 mM of dithiothreitol. By adding Na2S, the reaction was started, and the samples were left for incubation at 35 °C. After 30 min, the reaction was stopped by adding 1 mL of acidic ninhydrin reagent (0.8% ninhydrin (w/v) in 1:4 concentrated HCl:HOAc) in order to determine the Cys concentration [49]. The samples were then heated at 100 °C for 10 min, and to stabilize color formation, 2 mL of ethanol was added. The absorption was recorded at 560 nm using a spectrophotometer for OAS-TL determination.
2.12. Statistical Analysis
Data were analyzed statistically by analysis of variance (ANOVA) using SPSS v18.0 for Windows (IBM Corporation, New York, NY, USA). Pearson correlation between different variables was performed using OriginPro (v 9.8) for windows. Least significant difference (LSD) was calculated for mean separation for significant differences among treatments at p < 0.05 levels.
3. Results
3.1. Effect of SNP and/or S on Plant Growth, Water Status, and Cd Accumulation
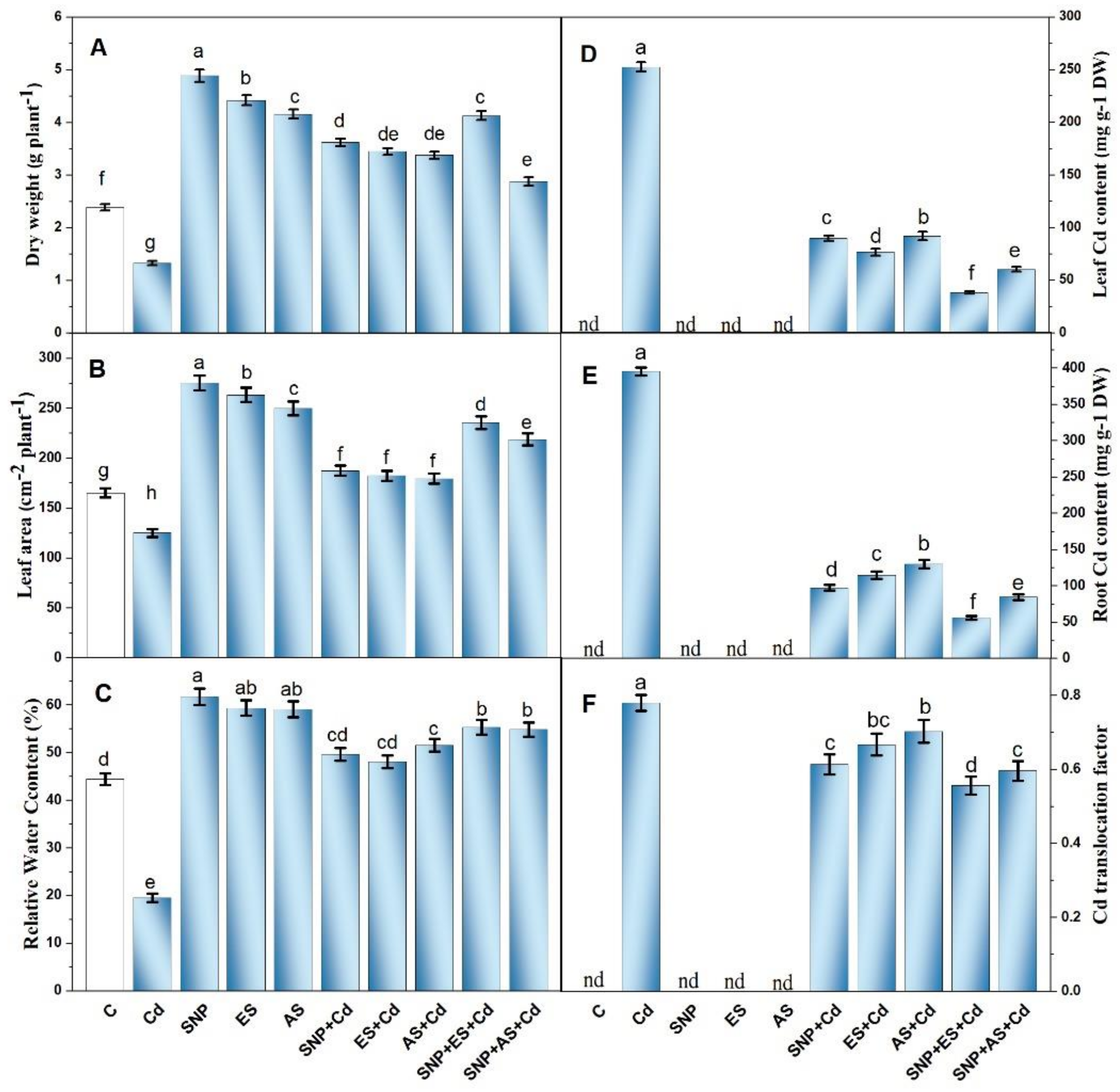
We first examined the plants’ growth metrics including their dry mass and leaf area in order to determine how the exogenous SNP and S forms affected the plants’ development under Cd stress. As shown in Figure 1A,B, Cd200 (200 mg Cd kg−1 soil) substantially reduced the plants’ dry mass (39.2%) and leaf area (11.5%) compared to the control plants. Under non-stressed conditions, the supplementation of 100 μM of SNP, ES, and AS increased the plants’ dry mass by 89.4, 56.1, and 44.2%, and leaf area by 66.7, 59.2, and 51.1%, respectively, compared to the control. However, under Cd stress, the combined SNP, ES, and AN treatment increased the plants’ dry mass over the control plants by 51.4, 44.3, and 41.4%, and leaf area by 13.4, 10.3, and 8.7%, respectively. The collective treatment of SNP plus ES was most effective in moderating the Cd-induced reductions in plant growth and increased the plants’ dry mass by 72.9% and leaf area by 42.5% compared to the control plants (Figure 1A,B).
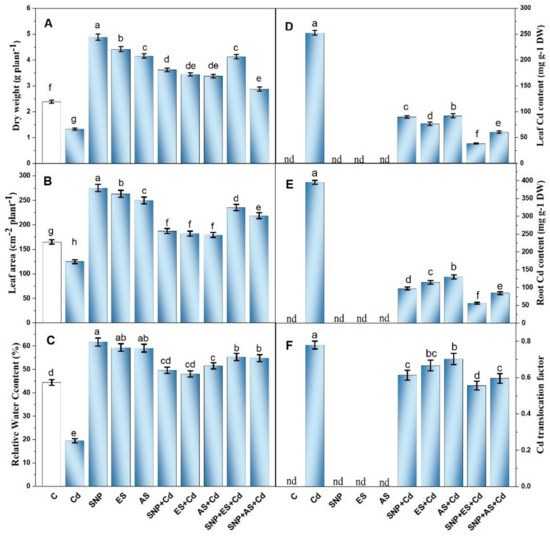
Figure 1.
Plant dry weight (A), leaf area (B), relative water content (C), leaf Cd content (D), root Cd content (E), and Cd translocation factor (F) in mustard (Brassica juncea L. cv. Giriraj) treated with 100 μM of SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in presence or absence of 200 mg Cd kg−1 soil at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data followed by the same letter are not significantly different as determined via LSD test at p < 0.05. C, control; Cd, cadmium; cv., cultivar; SNP, sodium nitroprusside.
The water status of the plants was analyzed by calculating the RWC of leaves. As a consequence of Cd toxicity, the treated plants showed a marked reduction in RWC by 56.5% compared to the control plants. From Figure 1C, it is clear that the SNP-treated plants under unstressed conditions had the highest RWC (66.7%) followed by ES and AS compared to the controls. The individual supplementation of SNP, ES, and AS to the Cd-treated plants partially improved the RWC of their leaves. In particular, the SNP treatment combined with ES maximally increased the RWC by 24.3% compared to the controls (Figure 1C).
The accumulation of Cd was higher in the roots (395.61 μg Cd g−1 DW) than in the leaves (252.4 μg Cd g−1 DW) (Figure 1D,E). The individual treatment of plants with SNP, ES, and AS reduced Cd accumulation in their roots and leaves; however, the greatest reduction was noted with the combined application of SNP with S sources. The collective application of SNP with S sources showed the highest Cd sequestration in the roots and leaves resulting in lower Cd content (Figure 1D,E). The supplementation of SNP plus ES significantly reduced Cd concentration in the roots by 85.4% and in leaves by 84.2%, while SNP + AS treatment accounted for a reduction of 78.3 and 76.6% in the roots and leaves, respectively, compared to the Cd-stressed plants. This was also confirmed by the translocation factor (T F), as only the Cd-treated plants had the highest T F values (0.780) while the plants that received SNP and ES together possessed a 29.6% decline in T F values over the Cd-stressed plants (Figure 1F).
The histochemical localization of Cd in the root, stem, and leaf tissues was revealed through histochemical detection via dithizone dye. The reagent dithizone reacts with Cd and forms an insoluble red salt (Cd-dithizonate) that can be seen as a dark-reddish coloration in various plant tissues. The deep-red coloration was found to vary significantly between the tissues and treatments. As shown in Figure S1A–I, the plants treated with Cd showed an intense Cd accumulation in the roots, stems, and leaves as a result of the reaction of Cd with dithizone and the formation of Cd–dithizone complexes. The metal was mainly accumulated in the cell walls and inter-cellular spaces of the apoplast, as the cytoplasm was slightly colored. On the other hand, the control plants showed no such coloration (Figure S1A,D,G). In line with the quantitative data, the plants co-treated with SNP and ES in the presence of Cd presented a weak reaction with dithizone and a low level of red color development, indicating lower levels of Cd in the root, stem, and leaf tissues when compared to those of the Cd-treated plants (Figure S1C,F,I).
3.2. Effect of SNP and/or S on Photosynthetic Traits under Cd Stress
Under Cd stress, a substantial reduction in chlorophyll content (35.4%), PN (32.3%), Ci (29.9%), gs (21.9%), and Rubisco activity (36.5%) was observed compared to the control plants (Table 2).

Table 2.
Effect of 100 μM of SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil on net photosynthesis, intercellular CO2 concentration, chlorophyll content (SPAD values), Rubisco activity, and electron transfer rate (ETR) in mustard (Brassica juncea cv. Giriraj) at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different as determined by LSD test at p < 0.05. Cd; Cadmium; SNP, sodium nitroprusside.
In the absence of Cd, the plants that received SNP, ES, or AS individually showed higher values with respect to photosynthetic traits compared to the control plants. Furthermore, the plants that received individual doses of SNP, ES, and AS together with Cd ameliorated the Cd inhibition of photosynthesis and partially improved the photosynthetic attributes over the Cd-stressed plants. Notably, the combined treatment of SNP and ES was most effective in countering the photosynthetic repression by Cd and augmented the aforesaid parameters by 66.9, 81.5, 80.8, and 148.1%, respectively, compared to the Cd-treated plants.
The effect of the SNP and S sources (ES and AS) with or without Cd on the chlorophyll fluorescence attributes are given in Table 3. A significant decline (p ≤ 0.05) in Fv/Fm (29.4%), Y (II) (38.5%), and ETR (66.7%) was observed in the plants exposed to Cd over the control plants (Table 3). Under stress-free conditions, the application of SNP, ES, or AS improved these fluorescence parameters, with SNP-treated plants showing the greatest increase (Table 3). Further, in comparison to the control plants, the SNP plus ES application maximally increased Fv/Fm, Y (II), and ETR by 1.1, 2.7, and 6.1-fold in in the presence of Cd (Table 3).

Table 3.
Effect of 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil on maximal PSII efficiency (Fv/Fm), photochemical quantum yield of photosystem II [Y(II)], and electron transfer rate (ETR) of mustard (Brassica juncea cv. Giriraj). Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different as determined by LSD test at p < 0.05. cv., cultivar; Cd; Cadmium; SNP, sodium nitroprusside.
3.3. Effect of SNP and/or S on Cd-Induced Oxidative Stress
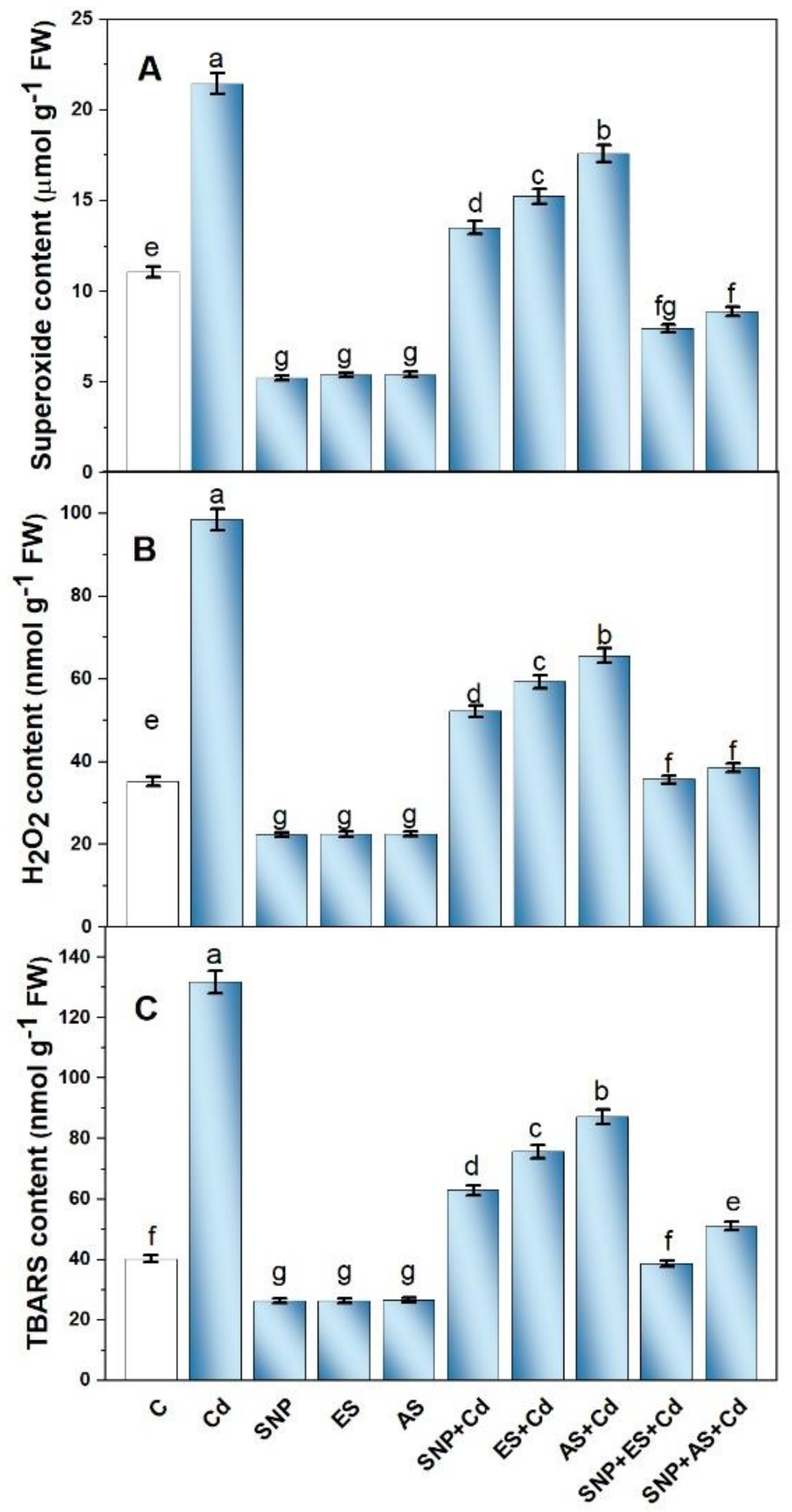
The plants grown with Cd exhibited a significant increase in the content of O2•− (2.2 times), H2O2 (2.1 times), and TBARS (3.2 times) in their leaves when compared to the control plants (Figure 2A–C). The individual treatment of plants with SNP, ES, or AS alleviated Cd-induced oxidative stress; however, the maximum reduction was observed with the combined application of SNP with S sources. The combined application of SNP and ES to the Cd-stressed plants decreased the content of O2•−, H2O2, and TBARS by 63.7, 60.1, and 70.6%, respectively, compared to the Cd-treated plants (Figure 2A–C).
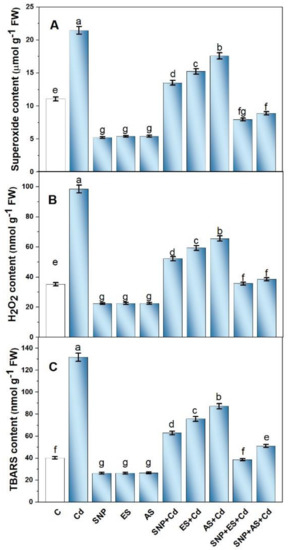
Figure 2.
Superoxide content (A), H2O2 content (B), and TBARS content (C) in mustard (Brassica juncea L. cv. Giriraj) treated with 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data followed by the same letter are not significantly different as determined by LSD test at p < 0.05. C, control; Cd, cadmium; cv., cultivar; FW, fresh weight; H2O2, hydrogen peroxide; SNP, sodium nitroprusside; TBARS, thiobarbituric acid reactive substances.
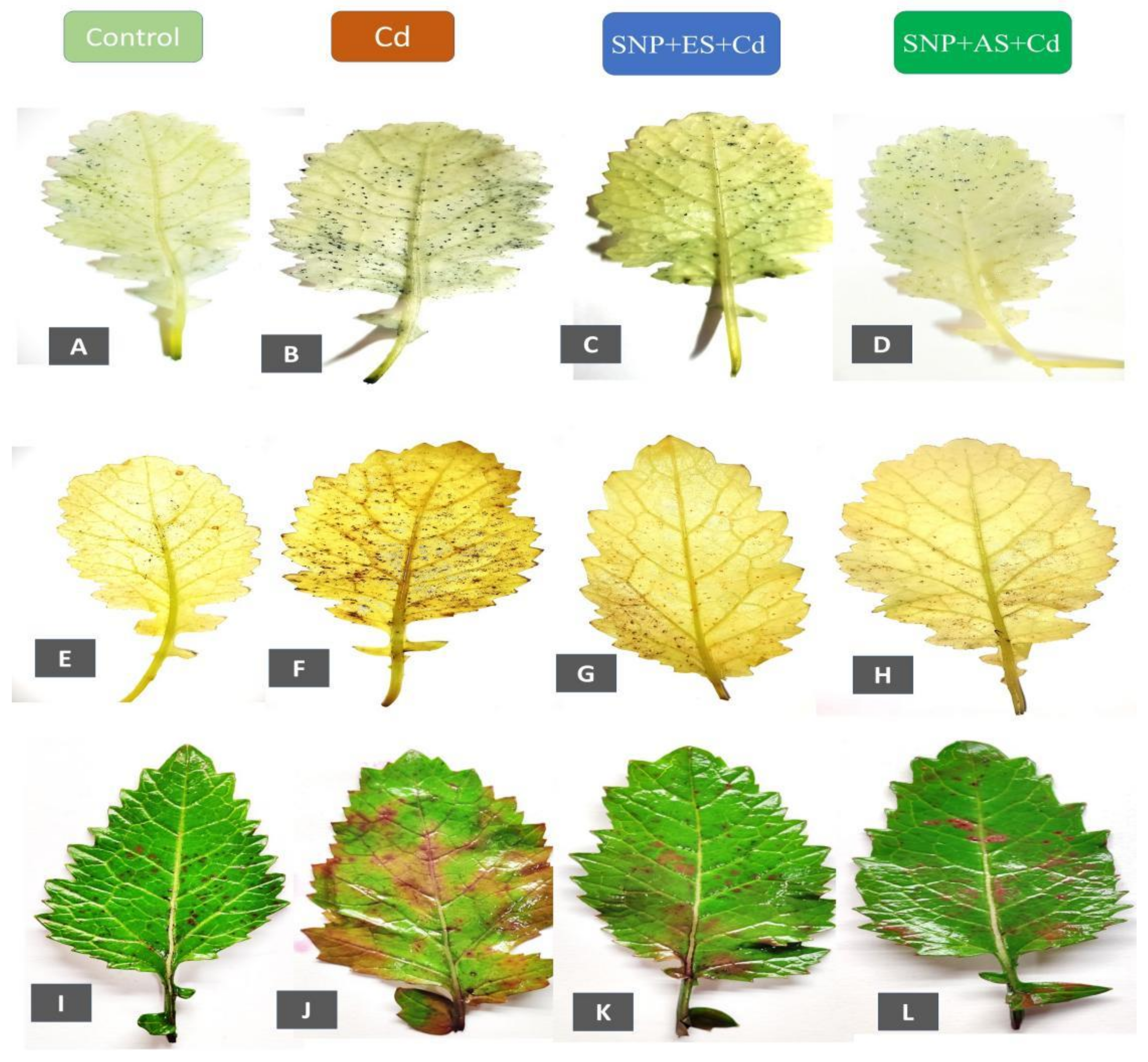
The representative images of the control, Cd-stressed, SNP + ES + Cd-treated, and SNP + AS + Cd-treated plants are presented in Figure 3A–L. The level of O2•− was visualized with NBT staining, which revealed the presence of a dark-blue-colored formazan formation in the Cd-stressed leaves (Figure 3B). The effect of SNP with AS showed fewer dark-blue stains on the leaves in contrast to the Cd-stressed plants (Figure 3D). However, the plants that received SNP plus ES or AS with Cd showed the presence of few spots and were similar to the control (Figure 3C). The degree of H2O2 accumulation was visualized by DAB staining, which clearly indicated the formation of deep-brown spots on the leaves of the Cd-treated plants. The spots were less apparent in the Cd-stressed plants treated with SNP + ES and SNP plus AS (Figure 3G,H). Further, the leaves from the Cd-treated plants exhibited an enhanced level of pink-colored formazan staining, indicating an enhanced level of lipid peroxidation (Figure 3J). However, the leaves of the plants treated with SNP + ES and SNP + AS showed less coloration than the Cd-treated plants, and thus lower lipid peroxidation (Figure 3K,L). The absence of dark-blue, deep-brown, and pink coloration in the leaves strongly suggested the ameliorative role of the cumulative effect of SNP with S sources and, particularly, SNP + ES in reducing the Cd-induced oxidative burden in the plants.

Figure 3.
Histochemical detection of: superoxide anion (O2•−) with NBT (A–D); hydrogen peroxide (H2O2) with DAB (E–H), and lipid peroxidation using Schiff’s reagent (I–L) in leaves of mustard (Brassica juncea L. cv. Giriraj) treated with control (A,E,I) 200 mg Cd kg−1 soil; (B,F,J) 100 μM of SNP (NO donor) + 200 mg S kg−1 soil of elemental sulfur (ES) in presence of Cd (C,G,K); and 100 μM of SNP + 200 mg S kg−1 soil of ammonium sulfate (AS) in presence of Cd (D,H,L) at 30 days after sowing. Cd; Cadmium; cv., cultivar; DAB, 3,3′-diamiobenzidine; ES, elemental sulfur; H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate; NBT, nitro blue tetrazolium; SNP, sodium nitroprusside.
3.4. Effect of SNP and/or S on Antioxidants under Cd Stress
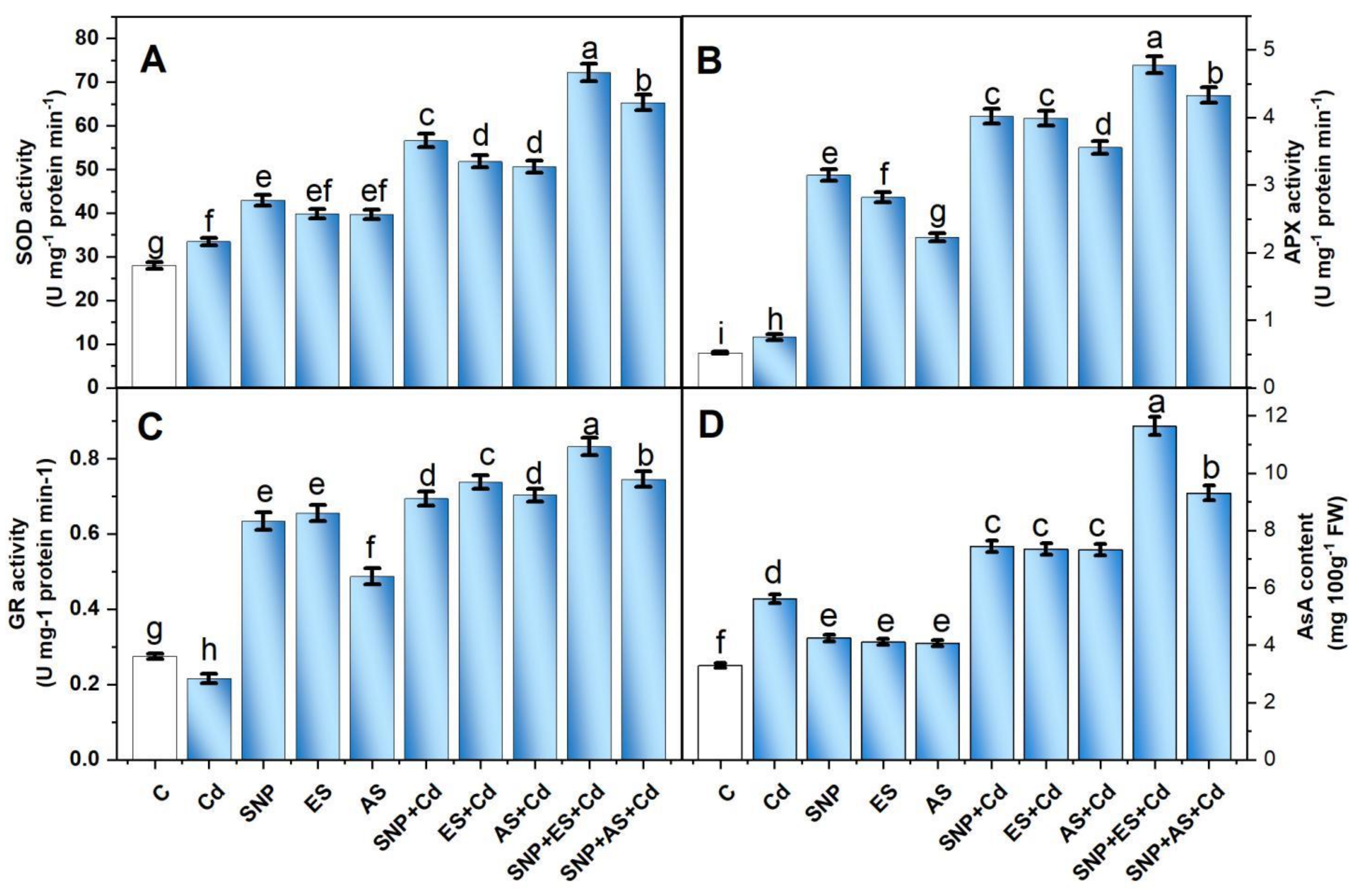
Compared to the controls, the plants grown with Cd showed a subtle but significant increase in SOD and APX activity, as well as an increase in AsA content (Figure 4A–C). The increase in the activity of SOD and APX and in AsA content was 19.7, 44.2, and 70.5%, respectively, over the untreated controls. The activity of the antioxidant enzymes and AsA content increased further with the individual application of SNP, ES, and AS in the Cd-stressed condition. Meanwhile, the maximum increment was observed with the co-treatment of SNP with S sources in the presence of Cd.
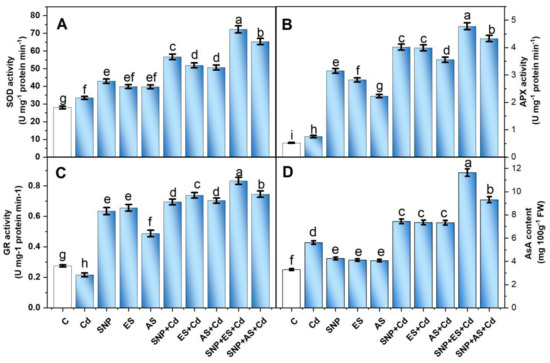
Figure 4.
Activity of SOD (A), APX (B), GR (C), and content of AsA (D) in mustard (Brassica juncea L. cv. Giriraj) treated with 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data followed by the same letter are not significantly different as determined by LSD test at p < 0.05. AsA, ascorbate; APX, ascorbate peroxidase; C, control; Cd, cadmium; cv., cultivar; GR, glutathione reductase; SNP, sodium nitroprusside; SOD, superoxide dismutase.
The plants that received SNP and ES together in the presence of Cd increased the SOD, APX, GR, and AsA content by 2.1, 6.3, 2.9, and 2.0 times, respectively, compared to the Cd-treated plants. Likewise, the plants treated with SNP + AS under Cd stress increased the activity of SOD, APX, and GR by 1.9, 5.7, and 3.2 times, respectively, and the AsA content by 1.6 times compared to the stressed plants (Figure 4A–C).
3.5. Effect of SNP and/or S on S-Assimilation under Cd Stress
The effects of Cd on the enzymes of S assimilation and the content of foliar S, Cys, and GSH are given in Table 3. The activity of ATP-S and OASTL increased in response to Cd stress by 18.1 and 32.1%, respectively, while GR activity exhibited a decrease of 6.9% compared to the controls (Table 4). The individual treatment of the plants with SNP, ES, or AS showed increased activity of all the S-metabolism enzymes both in stressed and non-stressed conditions. However, the co-supplementation of SNP + ES or SNP + AS with Cd generated the maximum increase. The plants supplied with SNP plus AS with Cd increased the activity of ATP-S and OASTL by 1.5 and 2.1 times with respect to the Cd-treated plants. On the other hand, the plants that received SNP plus ES with Cd maximally enhanced the activity of ATP-S and OASTL enzymes by 1.7 and 2.6 times compared to the Cd-stressed plants (Table 4).

Table 4.
Effect of 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil on activity of ATP-S, OAS-TL, and content of S, cysteine, and GSH in mustard (Brassica juncea L. cv. Giriraj) at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data with the same letter are not significantly different as determined by LSD test at p ≤ 0.05. ATP-S, ATP-sulfurylase; Cys, cysteine; Cd, cadmium; DW, dry weight; FW, fresh weight; GSH, reduced glutathione; OAS-TL, O-acetylserine (thiol) lyase; S, sulfur; SNP, sodium nitroprusside.
The foliar content of S, Cys, and GSH showed differential responses towards Cd. The content of S and GSH declined by 28.2% and 24.1%, respectively, whereas the Cys content increased by 20.5% with respect to the control (Table 4). Applying SNP, ES, or AS increased the content of S, Cys, and GSH to a greater extent under normal conditions. Under Cd stress, the individual treatment of SNP, ES, or AS amended the content of S, Cys, and GSH and recovered the losses relative to the Cd-treated plants. The cumulative effect of SNP with ES or AS modulated the negative impact of Cd on the content of S, Cys, and GSH and improved it maximally by 2.5, 2.9, and 3.0 times in comparison to the Cd-exposed plants. The combined effect of SNP and AS also showed a similar trend, but the increase in S-containing metabolites was lower than the combined SNP plus ES treatment (Table 4).
3.6. Effect of SNP and/or S on N Assimilation, p-SUE, p-NUE, and NPT and PC Content
The content of N and NR activity diminished significantly by 31.6 and 33.9%, respectively, with Cd compared to the respective control (Table 5). The application of SNP or S individually to stress-free plants enhanced N content and NR activity conspicuously with respect to the control. Similarly, in the Cd-stressed plants, SNP or supplemental S sources reduced the impacts of Cd. The SNP treatment provoked a maximum increase, whereas both S sources did not differ significantly. The simultaneous application of SNP and ES provoked the greatest responses and increased N content and NR activity by 2.5 times and 4.4 times, respectively, with respect to the Cd-stressed plants (Table 5).

Table 5.
Effect of 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil on nitrogen content, NR activity, photosynthetic-NUE, photosynthetic-SUE, NPT content, and PC content in mustard (Brassica juncea L. cv. Giriraj). Data are presented as treatment mean ± SE (n = 4). Data followed by the same letter are not significantly different as determined by LSD test at p ≤ 0.05. Cd, cadmium; DW, dry weight; FW, fresh weight; NR, nitrate reductase; NPT, non-protein thiol; NUE, nitrogen use efficiency; PCs, phytochelatins; SNP, sodium nitroprusside; SUE, sulfur use efficiency.
The p-NUE and p-SUE displayed a reduction of 27.7 and 22.9%, respectively, under the influence of Cd (Table 5). The p-NUE and p-SUE were highest in the ES-treated plants under non-stressed conditions, showing increases of 2.1 and 2.4 times, respectively, compared to the SNP and AS treatments with respect to the control. Generally, in the Cd-stressed plants, the supplementation of SNP or S sources, individually or in combination, improved the P-NUE and P-SUE prominently when compared to the Cd-stressed plants. With the SNP + ES treatment, P-NUE and P-SUE displayed notable increments of 2.3-fold and 2.2-fold, respectively, compared to the stressed plants (Table 5).
Only the Cd-treated plants showed a small increase in NPT and PC content when compared to the controls (Table 5). NPT content increased further with SNP, ES, or AS treatment both in optimal as well as stressed plants compared to the control. However, the SNP, ES, or AS treatments individually could not increase the PC content in the unstressed plants. The maximum increase in the NPT and PC content was observed under the collective treatment of SNP with S sources. In particular, the SNP plus ES treatment led to a maximum increase in NPT (2.3 times) and PC (1.9 times) content when compared to the Cd-treated plants (Table 5).
3.7. Confocal Laser Microscopy
Cd-induced H2O2 accretion was visualized in the roots of the plants stained with H2DCFDA, an indicator for ROS, predominantly H2O2, in cells (Figure 5A–D). H2DCFDA enters passively inside the cells and, on oxidation with H2O2, the non-fluorescent H2DCFDA turns into the highly fluorescent 2′,7-dichlorofluorescein (DCF).
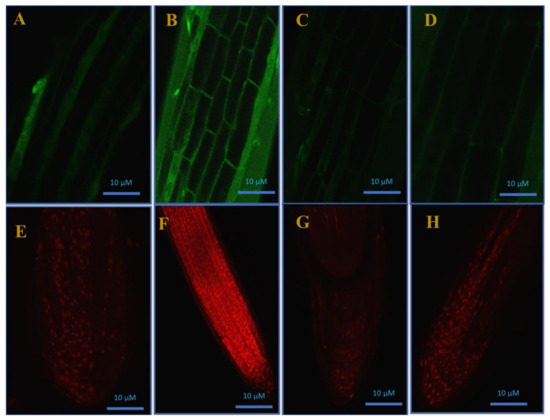
Figure 5.
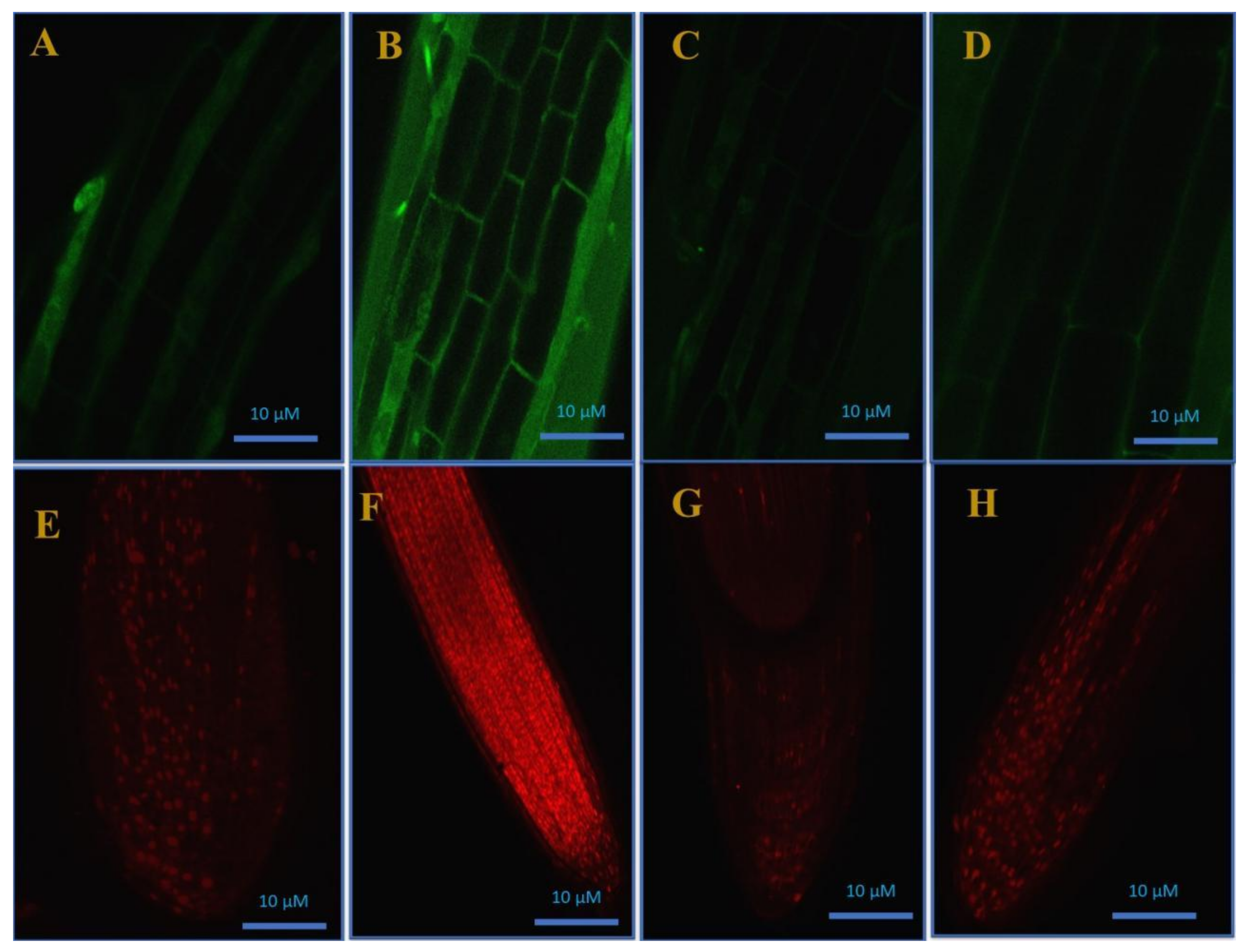
Confocal microscopic images of H2O2 formation in roots using H2DCFDA staining (A–D) and cell viability test (E–H) by propidium iodide staining in roots of mustard (Brassica juncea cv. Giriraj) in control (A,E), in 200 mg Cd kg−1 soil (B,F), in 100 μM of SNP (NO donor) + 200 mg S kg−1 soil of elemental sulfur (ES) in presence of Cd (C,G), and in 100 μM SNP + 200 mg S kg−1 soil of ammonium sulfate (AS) in presence of Cd (D,H) at 30 days after sowing. C, control; Cd, cadmium; cv., cultivar; H2DCFDA, 2’,7’-dichlorodihydrofluorescein diacetate; SNP, sodium nitroprusside.
From our results, the root cells of the Cd-stressed plants exhibited a higher intensity of green fluorescence (Figure 5B), while, compared to the Cd-stressed plants, the plants supplemented with SNP and S sources exhibited a lower intensity of green fluorescence and were more or less identical to the control plants (Figure 5C,D). PI is a staining dye that penetrates damaged cell membranes and stains nucleic acids, which become visible inside the dead cells as red fluorescent spots. In the current study, the root cells of the Cd-stressed plants were less viable. However, Cd-induced cell death was reversed by SNP + ES and SNP + AS application, and the plants exhibited a similar response as that of the control plants (Figure 5E–H).
3.8. How SNP and/or S Influence Endogenous NO and H2S Production
Table 4 shows that the plants grown with Cd displayed a significant reduction in NO content by 47.4% compared to the control plants. The application of SNP, ES, and AS increased the endogenous NO content both in the stressed and non-stressed plants, with the maximum content observed in non-stressed plants in comparison to the control. Further, the combined application of SNP and ES in the Cd-stressed plants remarkably increased NO generation; specifically, NO generation was increased by 3.5 times when compared to only the Cd-treated plants (Table 6).

Table 6.
Effect of 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil on NO content, H2S content, and L-DES activity in mustard (Brassica juncea L. cv. Giriraj) at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data followed by the same letter are not significantly different as determined by LSD test at p ≤ 0.05. Cd, cadmium; FW, fresh weight; H2S, hydrogen sulfide; L-DES, L-cysteine dedulfhydrase; NO, nitric oxide; SNP, sodium nitroprusside.
The plants’ H2S metabolism increased with the application of SNP and S sources by increasing the endogenous H2S content and activity of L-DES (Table 6). Compared to the respective controls, Cd stress induced a 21% increase in H2S content and a 18.4% increase in L-DES activity. H2S metabolism was found to be highest in the non-stressed plants, and SNP treatment generated greater responses than ES or AS in comparison to the controls. The inclusion of SNP, ES, or AS individually in stressed plants showed a slight increase in H2S and L-DES activity. H2S formation and L-DES activity increased even further under combined treatment, particularly for the SNP plus ES treatment with Cd, which showed an increment of 3.5 and 2.0 times, respectively, relative to the Cd-treated plants (Table 6).
3.9. Validation of Role of H2S in SNP plus S-Mediated Response by Inhibiting NO and H2S under Cd Stress
As a part of the analysis to confirm the potential involvement of H2S in NO and S uptake (as ES, the most responsive S source) in stress acclimation, HT (H2S synthesis inhibitor) and cPTIO (NO scavenger) were used (Table 7). The Cd-stressed plants exhibited a significant reduction in dry weight (40.1%), PN (29.3%), P-SUE (50.2%), and NO content (49.9%); however, there was a slight increase in endogenous H2S content (36.7%) compared to the control plants (Table 7). The SNP + ES treatment—even in the presence of Cd—exhibited maximal increases in dry weight, PN, P-SUE, NO content, and H2S content compared to the respective control plants. The inclusion of HT and cPTIO significantly reduced dry weight by 100 and 72.8%, PN by 88.9 and 50.8%, and p-SUE by 85.6 and 33.2%, respectively, when compared to the control plants (Table 7). From the results, it is clear that the inhibition of H2S inhibited photosynthesis and growth more prominently even in the presence of NO. The inhibition of NO using cPTIO had lesser impacts.

Table 7.
Effect of 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) in the presence of 200 mg Cd kg−1 soil on plant dry weight, net photosynthesis, photosynthetic-SUE, H2S content, and NO content in mustard (Brassica juncea L. cv. Giriraj) treated with 100 µM hypotaurine (HT) or 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) at 30 days after sowing. Data are presented as treatment mean ± SE (n = 4). Data followed by the same letter are not significantly different as determined by LSD test at p < 0.05. FW, fresh weight; H2S, hydrogen sulfide; NO, nitric oxide; SUE, sulfur use efficiency.
3.10. Relationship
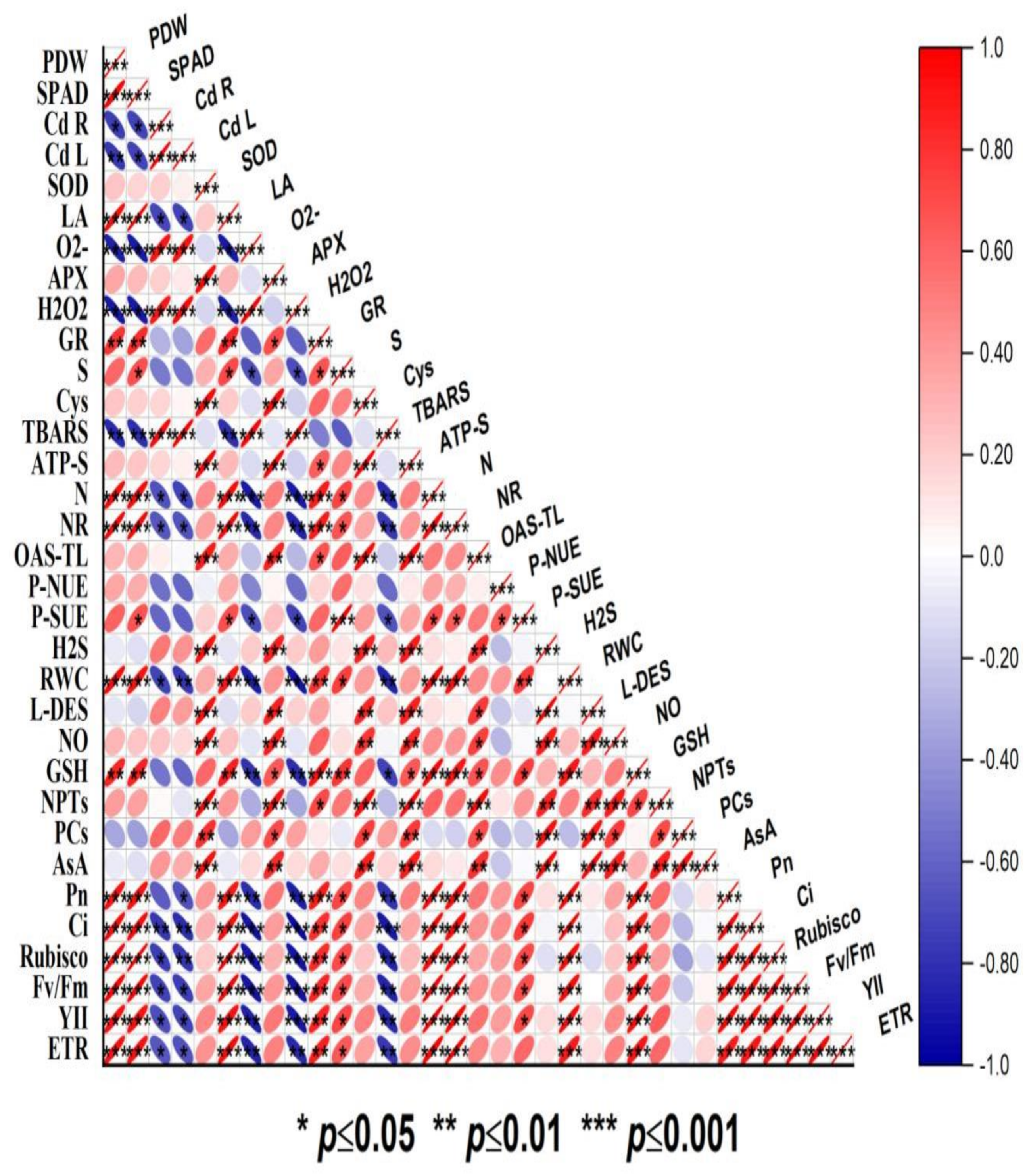
A Pearson correlation analysis was conducted to investigate the relationship between the different variables evaluated in the present study (Figure 6). The Cd concentration in the roots and leaves showed an emphatically strong correlation with oxidative stress parameters such as O2•−, H2O2, and TBARS content. The oxidative stress indicators (O2•−, H2O2, and TBARS content) were negatively correlated (p ≥ 0.05, p ≥ 0.01, and p ≥ 0.001) with plant dry biomass, leaf area, chlorophyll content, PN, Ci, Rubisco activity, and ETR. On the other hand, the antioxidants (SOD, APX, GR, GSH, and AsA), NPTs, and PCs showed a significant (p ≥ 0.05, p ≥ 0.01, and p ≥ 0.001) positive correlation with plant growth and photosynthetic attributes. Above all, endogenous NO and H2S showed a strong positive correlation with leaf S content, photosynthetic SUE and NUE, and the enzymes and metabolites of S and N assimilation. Thus, the correlation depicted a close association between NO, S, and H2S and plant growth, photosynthesis, antioxidant potential, and N and S-assimilation under Cd stress (Figure 6).
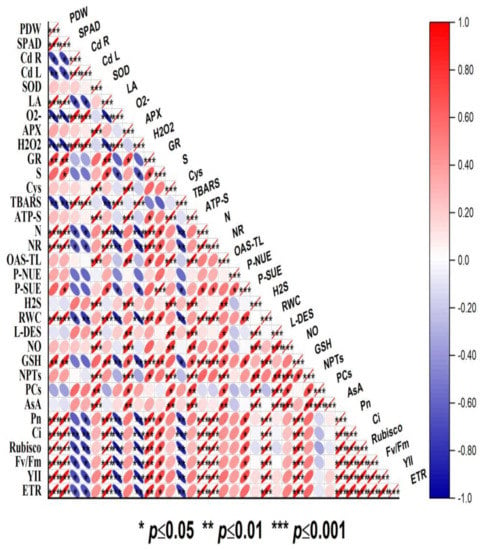
Figure 6.
Pearson correlation matrix represents correlations at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001 between different growth, photosynthesis, oxidative stress, antioxidant metabolism, and S and N metabolism parameters in mustard (Brassica juncea L. cv. Giriraj) treated with 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1. Scale represents Pearson correlation (r) values that range from −1 to +1. Net photosynthesis (PN), intercellular CO2 concentration (Cint), Rubisco activity, reduced glutathione (GSH), ATP-sulfurylase (ATP-S), O-acetylserine (thiol) lyase (OAS-TL) nitrate reductase (NR), fresh weight (FW), dry weight (DW), glutathione reductase (GR), non-protein thiols (NPT), nitric oxide (NO), H2S, hydrogen sulfide; L-DES, L-cysteine dedulfhydrase; ascorbate (AsA), dehydroasorbate reductase (DHAR), superoxide dismutase (SOD), ascorbate peroxidase (APX), phytochelatins (PCs), cadmium root (Cd R), cadmium leaf (Cd L), H2O2 content, electrolyte leakage (EL), superoxide content (O2), and thiobarbituric acid reactive substances (TBARS) content.
3.11. Principal Component Analysis (PCA)
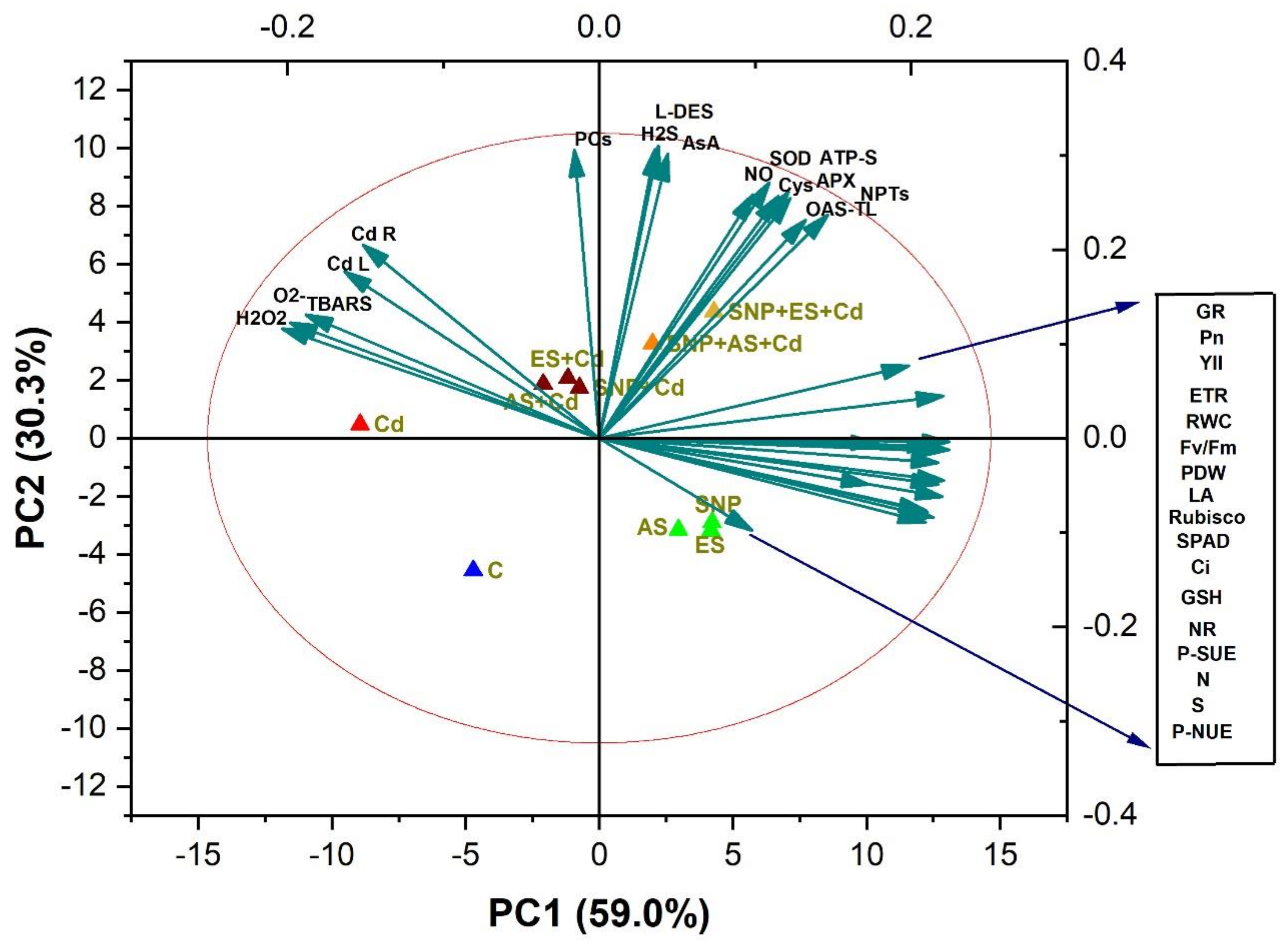
A PCA was carried out to determine the degree of data variability and the relationship between the different treatments and parameters in the B. juncea cultivar Giriraj (Figure 7). The two components (PC1 and PC2) described 89.3% of the data variability under the influence of different treatments (Figure 7). PC1, the first component, contributed 59.0% of the total variation, and the second component, PC2, accounted for 30.3% of the total variation. The oxidative stress biomarkers such as O2•−, H2O2, and TBARS and were clustered together with Cd content in the root and leaves. The loading plot showed a positive relationship between the parameters of plant growth; photosynthesis; chlorophyll fluorescence; the activity of GR and NR; the content of N, S, and GSH; and P-SUE and P-NUE (Figure 7). The biplot was divided into three clusters. Plant growth and photosynthetic attributes were negatively correlated with each other, and antioxidants were in between them, suggesting their role in mitigating Cd stress. Moreover, endogenous NO and H2S showed a positive correlation with antioxidants. Among the treatments, Cd was close to oxidative stress and Cd accumulation, whereas SNP + ES + Cd showed a strong correlation with antioxidants, indicating the role of the SNP + ES + Cd treatment in mitigating Cd stress (Figure 7).
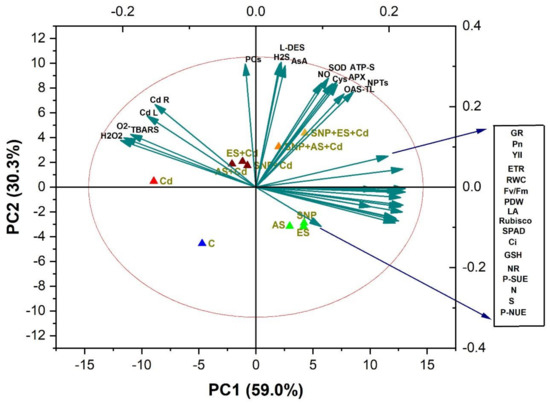
Figure 7.
Biplots of principal component analysis (PCA) represent the relationship among different variables and treatments in mustard (Brassica juncea L. cv. Giriraj) treated with 100 μM SNP (NO donor) and 200 mg S kg−1 soil of elemental sulfur (ES) or ammonium sulfate (AS) in the presence or absence of 200 mg Cd kg−1 soil. The variables included net photosynthesis (PN), intercellular CO2 concentration (Cint), Rubisco activity, reduced glutathione (GSH), ATP-sulfurylase (ATP-S), O-acetylserine (thiol) lyase (OAS-TL) nitrate reductase (NR), fresh weight (FW), dry weight (DW), glutathione reductase (GR), non-protein thiols (NPT), nitric oxide (NO), hydrogen sulfide (H2S), L-cysteine dedulfhydrase (L-DES), ascorbate (AsA), dehydroasorbate reductase (DHAR), superoxide dismutase (SOD), ascorbate peroxidase (APX), phytochelatins (PCs), cadmium root (Cd R), cadmium leaf (Cd L), H2O2 content, electrolyte leakage (EL), superoxide content (O2), and thiobarbituric acid reactive substances (TBARS) content.
4. Discussion
Cadmium is a well-known soil pollutant that harms plants by inhibiting their growth and photosynthesis owing to the excessive degree of ROS aggregation caused by its presence [2]. In recent years, studies on the roles of plant growth regulators and mineral nutrients in managing Cd toxicity have grown exponentially [4,6,9,17]. Both NO and S play promising roles in synthesizing various metabolites that activate downstream signal transduction pathways and eradicate Cd toxicity [54,55]. As a sustainable approach, this study revealed synergism between NO with S that mitigated Cd phytotoxicity in mustard plants via H2S participation and, thus, modulated various physiological and biochemical mechanisms associated with Cd tolerance. The combined application of SNP with ES (as an S source) maximally protected the plants against Cd-induced damage. Many sulfate-based fertilizer sources provide SO42− in a readily available form to crops during their initial growth, but they are prone to leaching and may not be available at later stages of growth [56]. Therefore, there is a need for an inexpensive and more responsive source of S, which can be met by using ES. ES, as an S fertilizer, has higher S content, can stay in soil for longer periods, and improves plant tolerance to environmental stresses by transforming into volatile sulfur species and reactive sulfur species. On the other hand, when compared to other NO donors, SNP provides superior NO generation and supplies NO for a significantly longer period of time [56,57].
The sacrifice of plant growth in response to metal stress is a common phenomenon during a plant’s life cycle [1]. The accumulation of Cd in plant tissues reduces growth as Cd interferes with the activity of many enzymes associated with carbon and N assimilation [10]. Hence, minimizing Cd uptake and accumulation is critical for maintaining proper growth and reducing Cd phytotoxicity in plants. In our study, the reduction in growth after Cd exposure can be linked to Cd stress, as diminished plant growth in Cd-polluted soils is a direct measure of Cd toxicity [4]. The maximum plant dry mass and leaf area were recorded in the plants grown with the combined application of SNP and ES in the presence of Cd (Figure 1A,B). ES is known to decrease the pH of the soil by microbial oxidation, which leads to the production of H2SO4 [56]. This process precipitates divalent cations such as Cd [57], and makes sulfate available for plant uptake, which could be one of the reasons for the higher plant growth observed with ES supplementation through increased S-assimilation in our study. The growth of plants is largely regulated by the target of rapamycin (TOR) pathway, which depends on the availability of amino acids, such as Cys, and glucose. The availability of S regulates plant growth largely by the glucose–TOR signaling pathway [58]. Nitric oxide, on the other hand, is also an efficient growth promoter under various abiotic stresses, as previously reported in salt stress [30], heat stress [8], Cr stress [12], and Cu stress [13]. NO shows functional crosstalk with cytokinin and increases plant growth by enhancing the rate of cell division, cell wall extensibility, and the production of new tissues [59]. NO-mediated plant growth can also be correlated to an increased antioxidant response that reverses the inhibitory effect of Cd on plant growth [54]. Additionally, both NO and ES are involved in the uptake of essential mineral nutrients required for plant growth [31,54,55,60]. One of the first symptoms of Cd toxicity in plants is the perturbance of plant–water relations, which causes osmotic stress [1]. Both NO and ES induce the synthesis of various osmolytes, such as proline and glycine betaine, which improve the RWC of plants [55]. The enhanced RWC contributes to plant growth through increasing photosynthesis by enhancing stomatal conductance and the transpiration rate. Thus, NO and ES are involved in improving plant growth by increasing mineral nutrient acquisition, reducing cellular ROS content, and regulating the water status of plants under higher Cd regimes.
Since chloroplasts constitute one of the prime sites of ROS formation, they are commonly more sensitive organelles to Cd-induced damage [2]. The modulation of photosynthetic efficacy in response to Cd stress is critical for plant survival, and a plant accepts this challenge by activating the antioxidant response, which limits the severity of ROS towards photosynthetic organelles and improves gas exchange attributes, chlorophyll content, and chlorophyll fluorescence to counter Cd stress. The results pertaining to photosynthesis showed that photosynthetic (chlorophyll content, PN, Ci, gs, and Rubisco activity) and chlorophyll fluorescence (Fv/Fm, Y (II), and ETR) attributes were severely affected by the Cd treatment (Table 2 and Table 3). Exogenously applied SNP and/or S sources modulated the Cd-induced inhibition of photosynthetic parameters. A maximum increase in photosynthesis was achieved with the combined treatment of NO plus ES under Cd-stressed conditions (Table 2 and Table 3). The reason for the increased degree of photosynthesis under Cd stress with ES involves an S-mediated increase in N assimilation that allocates more N to Rubisco and other enzymes of the Calvin cycle, which is parallel to the results of the study conducted by Per et al. [55]. NO, on the other hand, regulates stomatal movements, increases Rubisco activity, maintains PSII integrity, and improves chlorophyll biosynthesis in normal as well as stressed conditions [10,12,13,27]. Plants that cannot sufficiently biosynthesize NO exhibit an increased upregulation of genes associated with chlorophyll degradation, which can be reversed by exogenous NO application [61]. Both NO and ES lead to an increase in PN and Ci under Cd stress by maintaining gs and thus increasing the activity of the Rubisco enzyme under Cd stress [54]. The decline in PN may be linked to decreased sub-cellular gs, which can be reversed after NO and ES supplementation [31]. Thus, the increased carboxylation in our study is the sum of stomatal and non-stomatal limitations by maintaining gs and increasing the activity of the Rubisco enzyme. Furthermore, the combined application of ES and SNP maximized GSH production, which provides a shielding effect to the photosynthetic apparatus and an important regulatory loop for NO bioactivity via GSNO formation [62]. Notably, NO and S applications have also shown positive roles in improving chlorophyll fluorescence parameters such as Fv/Fm, Y (II), and ETR, which indicates the higher activity of the reaction center under Cd stress [63].
The process of S assimilation ensures an adequate flux of important growth and defensive metabolites that are required for metal detoxification, growth, and photosynthesis [18]. Reduced S absorption or S insufficiency in plants can cause alterations in physiological and biochemical processes, resulting in a slew of changes in plant growth and photosynthetic responses [20]. In the present study, the plants grown under Cd-stressed conditions exhibited a significant decrease in the enzymes of S assimilation (ATP-S, OASTL, and GR), S metabolites (GSH, Cys, NPTs, and PCs), and leaf S content (Table 3 and Table 4). However, the combined treatment of NO + ES generated the maximum increase in the above parameters in the presence of Cd (Table 3). Both NO and/or S were observed to be effective in enhancing S assimilation in some previous studies focused on salt stress [31], Cd stress [54,55], As stress [28], and Cr stress [12]. Elemental S is a slow-releasing S fertilizer with high S content [57]. Notably, when compared with other S forms, it makes more SO42− available in the soil due to microbial oxidation, which could be a reason for the rapid S uptake resulting in higher S assimilation as reported in our study [60]. NO is known to increase S assimilation by modulating the activities of enzymes related to S assimilation via the post-translational modification of Cys residues [64]. This biosynthetic pathway is essential to plants and cannot be separated from general metabolism because it produces essential amino acids such as Cys and methionine, which are then used to synthesize a variety of S metabolites such as GSH, ethylene, NPTs, and PCs that contribute to the biochemical adaptation mechanisms to metal stress [18]. Cysteine, a product of S assimilation, acts as precursor for the synthesis of GSH. The enhanced synthesis of GSH and PCs reduces the bioaccumulation and translocation of Cd in roots and leaves [9]. Despite the fact that B. juncea is a hyperaccumulator of heavy metals, the toxicity of the Cd 200 mg kg−1 soil was obvious in the different studied variables. From our results, the plant receiving NO and ES had a lower degree of TF and Cd accumulation in its roots and shoots, respectively, with higher content of NPTs and PCs, suggesting the Cd chelation capacity of these metabolites. The S-assisted reduction in Cd toxicity could be attributed to regulatory mechanisms of S assimilation towards uptake, translocation, and detoxification, thus establishing metal homeostasis in the B. juncea plants [20,55]. Moreover, ES is an ideal amendment for reducing Cd accumulation by reducing its bioavailability in soil and increasing the microbial activity in Cd-polluted soils [57]. As observed in ES, sulfate must be activated before the S assimilation process can begin, which is accomplished by ATP-S. A study by Wangeline et al. [65] showed that overexpression of ATP-S improved the phytoextraction capacity of B. juncea plants for twelve heavy metals, including Cd. Cys acts as a substrate and branch point for the biosynthesis of methionine, glucosinolates, camalexin, polyamines (spermine, spermidine, and putrescine), H2S, and ethylene, which play a broad role in regulating various plant abiotic stress responses [18,23]. OAS-TL is the precursor enzyme for Cys biosynthesis that shows enhanced activity in the presence of NO and S [55].
Cysteine forms a connecting link between S and N metabolism, wherein S assimilation shows a close relationship with N requirement and N metabolism; thus, the availability of one element affects the other [5,17,24]. Both S and NO have been reported to increase N content, NR activity, and N metabolism, which corroborates our results [21,23]. Soil-applied S increases nutrient uptake, including N [17]. Additionally, a deficiency of S inhibits the N-assimilatory pathway, as reported in barley plants [66]. As N forms the backbone for various metabolites of S assimilation, the S assimilation pathway relies on N metabolism for incorporating an N skeleton into S-containing amino acids and proteins [24]. NR is the rate-limiting enzyme of N assimilation and plays a key role in NO biosynthesis by reducing nitrate to NO [67]. A perusal of the relevant data reveals that the application of NO and ES to Cd-stressed plants enhances NR activity and endogenous NO content, which is parallel to the results of our study [55]. GSH, via GSNO formation, regulates the activity of various target enzymes such as NR [68]. The higher levels of S and N assimilation after NO and ES application in our study can also be substantiated by the corresponding increased levels of photosynthetic S and NUE (Table 4). The results revealed that the B. juncea cultivar Giriraj used in this study is also a highly photosynthetic SUE and NUE cultivar by exhibiting greater inherent S and N assimilation capacity and showing more responsiveness to NO and ES. Hence, our results imply that S and NO upregulated S- and N-assimilatory pathways and increased the levels of their metabolites, which played a prominent role in eradicating Cd-induced stress.
In the present study, oxidative stress parameters (O2•− and H2O2) and TBARS levels were found to be increased in the leaves of the Cd-treated plants (Figure 2 and Figure 3). The altered oxidative stress biomarkers and TBARS levels influenced by Cd may perturb the membrane capacity for ion exchange, cellular redox homeostasis, and overall cellular functioning [17]. Reports suggest that NO and ES mediate several antioxidant enzyme expressions, such as SOD, APX, and GR, that detoxify excess O2•− and H2O2 and decrease lipid peroxidation [31,54]. The increase in antioxidants with ES could be related to improved S assimilation, which increases the pool of S-containing amino acids required for their biosynthesis [55]. Either exogenous or endogenous NO induces antioxidant expression, presenting NO as a specific regulator, an anti-stress manager, and a master regulator of plants under various environmental cues [4]. Since the level of antioxidant formation was maximal in the combined application of SNP + ES, it has become clear that NO and ES respond to oxidative stress in a coordinated manner under Cd stress. Additionally, our results also demonstrated that the SNP + ES supplementation led to an increase in the AsA and GSH content that kept cellular ROS under check and the cells free from Cd toxicity via the AsA-GSH pathway (Figure 5C, Table 3). This pathway detoxifies the excessive concentration of H2O2 via APX and forms H2O, and its regenerating systems maintain the cellular AsA and GSH pools [24]. Importantly, GSH is a product of S assimilation and a powerful antioxidant that maintains cellular redox homeostasis and quenches excessive ROS levels under stressed conditions [55,63]. Therefore, SNP + ES-mediated oxidative stress resilience might be due to the combined function of antioxidants in B. juncea plants.
H2S mitigates the syndromes associated with heavy metal toxicity, such as those caused by Cd, B, Cu, As, Cr, and Al [5,6,69,70]. Under Cd stress, H2S regulates an array of plant responses, including stomatal movements, seed germination, root growth, photosynthetic functions, ROS homeostasis, and underlying signal transduction pathways for Cd detoxification [6,11,71]. The molecular mechanism of H2S involves the PTMs of Cys residues of proteins through persulfidation, which regulates the antioxidant system (GR, SOD, and APX) and other stress-responsive proteins [72]. In the present study, the endogenous content of H2S was maximal in the plants treated simultaneously with SNP + ES under Cd stress (Table 5). The increase may be due to demand-driven enhanced activities of enzymes of H2S biosynthesis (OAS-TL and L-DES) (Table 5). Cys accumulation in plant cells is directly linked to H2S biosynthesis and serves as a catabolite during H2S release [28]. Since ES can supply sulphate for longer periods of time in soil, it is not surprising that under stress, Cys and H2S content were highest with this treatment alone and in combination with NO. On the other hand, Khan et al. [73] deduced that GSNO (a NO donor) application improved the activities of H2S-synthesizing enzymes and Cys content, which maintained Cys homeostasis for the enhanced synthesis of H2S for the proper functioning of cellular machinery under stressful conditions. However, this effect of NO on H2S formation was reversed by the application of an NO scavenger (cPTIO), thereby confirming the involvement of NO as an upstream signal to H2S. Other authors [12,26,63] have also validated the NO-induced biosynthesis of H2S using the NO scavenger (cPTIO) or H2S scavenger/inhibitor (HT and PAG) that reduced the activities of H2S biosynthesis enzymes and H2S content. Both NO and H2S are equally efficient ROS scavengers and act either directly or indirectly by strengthening the antioxidant system through increasing the levels of antioxidant enzymes (SOD, APX, and GR), GSH content, and the metabolism of ROS (H2O2 and O2•−), and reducing membrane lipid peroxidation by regulating intercellular responses [11].
H2S biosynthesis and signaling is completely dependent on the availability of S [12]. The availability of sufficient sulfate directly affects the amount of H2S generated [28]. Sulfur deprivation under stressed conditions in plants can halt the biosynthesis of H2S and plants’ capability to counter stress [26]. Noteworthily, S played an interim regulatory role in NO and H2S crosstalk to fortify the plants’ defense capabilities in order to fight Cd stress in the current study. Shreds of evidence describe that H2S requires endogenous NO to activate the Cd detoxification system by mediating signal transduction [12,27]; however, it has not yet been described how exogenous S amendment functions as an intermediary. Moreover, the modus operandi of NO and H2S-mediated Cd phytotoxicity regulation is unclear and requires further investigation. Our findings suggest that an optimal supply of S and NO under stressed conditions in plants can result in the enhanced production of H2S and that its crosstalk with NO can help plants counter environmental challenges such as Cd stress. It can be inferred from our results that NO, S, and H2S work in harmony and play a decisive role in withstanding and countering Cd toxicity by upregulating antioxidant potential and PCs formation, which lowers oxidative burden and improves growth and photosynthesis.
The involvement of H2S in NO- and S-mediated stress responses was validated through the use of the scavengers of NO (cPTIO) and the H2S biosynthesis inhibitor (HT). The supplementation of cPTIO and HT to plants reduced their dry weights, PN, and P-SUE, and the reduction was greater in the plants treated with HT, showing the importance of H2S. The plants grown with SNP plus S and treated with HT exhibited a greater decrease in photosynthesis and plant dry weight. This suggests the essentiality of H2S for the maintenance of photosynthesis and growth. In contrast, inhibiting NO with cPTIO did not maximally inhibit photosynthesis and growth. From the results, it is clear that the NO- and S-mediated alleviation of Cd toxicity requires the optimization of H2S generation, wherein H2S acts as a downstream signal in inducing N and S assimilation and improving the antioxidant potential of mustard plants.
5. Conclusions
In the present study, the NO- and ES-induced endogenous roles of H2S were evaluated with respect to overcoming the toxic effects of Cd in mustard plants. The cadmium in soil potentiated its toxicity by suppressing plant growth, photosynthesis, and the antioxidant capacity of the plants. Together, NO and ES regulate Cd accumulation, growth, photosynthetic efficiency, and the antioxidant mechanism of plants by modulating the S and N metabolism pathways that provide resilience from Cd phytotoxicity. Further, the activities of L-DES and OAS-TL were increased, Cys content was enriched, and the biosynthesis of H2S was greatly improved when SNP and ES were given simultaneously. By functioning as a downstream signal to NO and S, it was discovered that H2S is involved in protecting plants from Cd-induced stress. It was observed that H2S acted as a downstream signal in the NO- and S-mediated alleviation of Cd stress in mustard plants. Thus, the triad of NO, ES, and H2S effectively alleviated the harmful effects of Cd in mustard plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/stresses2040037/s1, Figure S1: Histochemical localization of Cd in roots (A–C), stem (D–F) and Leaves (G–I) in mustard (Brassica juncea L. cv. Giriraj) treated with control (A,D,G), 200 mg Cd kg−1 soil, (B,E,H) and 100 μM SNP (NO donor) + 200 mg S kg−1 soil of elemental sulfur (ES) (C,F,I). cv., cultivar; SNP, sodium nitroprusside.
Author Contributions
Conceptualization; A.M.; investigation, original draft preparation and writing, I.R.M.; data curation and biochemical analysis, I.R.M. and B.A.R.; editing and content improvement, A.M. and N.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
A.M. gratefully acknowledges the necessary research and instrumentation grants received by the Department of Science and Technology SERB (Project code: SB/YS/LS-108/2014) and Start-Up Grant by the University Grants Commission (UGC), New Delhi, India.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the graphs provided in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 52, 675–726. [Google Scholar] [CrossRef]
- Shiyu, Q.I.N.; Hongen, L.I.U.; Zhaojun, N.I.E.; Rengel, Z.; Wei, G.A.O.; Chang, L.I.; Peng, Z.H.A.O. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar]
- Mir, I.R.; Gautam, H.; Anjum, N.A.; Masood, A.; Khan, N.A. Calcium and nitric oxide signaling in plant cadmium stress tolerance: A cross talk. S. Afr. J. Bot. 2022, 150, 387–403. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Sehar, Z.; Anjum, N.A.; Masood, A.; Khan, N.A. The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol. Biochem. 2020, 155, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Shivaraj, S.M.; Vats, S.; Bhat, J.A.; Dhakte, P.; Goyal, V.; Khatri, P.; Deshmukh, R. Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol. Plant. 2020, 168, 437–455. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Ethylene-nitrogen synergism induces tolerance to copper stress by modulating antioxidant system and nitrogen metabolism and improves photosynthetic capacity in mustard. Environ. Sci. Pollut. Res. 2022, 25, 49029–49049. [Google Scholar] [CrossRef]
- Rasheed, F.; Mir, I.R.; Sehar, Z.; Fatma, M.; Gautam, H.; Khan, S.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. Nitric Oxide and Salicylic Acid Regulate Glutathione and Ethylene Production to Enhance Heat Stress Acclimation in Wheat Involving Sulfur Assimilation. Plants 2022, 11, 3131. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.F.; Li, J.; Xiong, J.; Zhou, L.N.; He, S.L.; Liu, H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019, 9, 7397. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J.Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric oxide pre-treatment advances seed germination and alleviates copper-induced photosynthetic inhibition in Indian mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Barroso, J.B. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front. Plant Sci. 2016, 7, 152. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Mobin, M.; Anjum, N.A.; Khan, N.A. Modulation and significance of nitrogen and sulfur metabolism in cadmium challenged plants. Plant Growth Regul. 2016, 78, 1–11. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Gautam, H.; Majid, A.; Anjum, N.A.; Masood, A.; Khan, N.A. Appraisal of functional significance of sulfur assimilatory products in plants under elevated metal accumulation. Crop Pasture Sci. 2022, 73, 573–584. [Google Scholar] [CrossRef]
- Rego, T.J.; Sahrawat, K.L.; Wani, S.P.; Pardhasaradhi, G. Widespread deficiencies of sulfur, boron, and zinc in Indian semi-arid tropical soils: On-farm crop responses. J. Plant Nutr. 2007, 30, 1569–1583. [Google Scholar] [CrossRef]
- Bashir, H.; Ahmad, J.; Bagheri, R.; Nauman, M.; Qureshi, M.I. Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot. 2013, 94, 19–32. [Google Scholar] [CrossRef]
- Mir, I.R.; Rather, B.A.; Masood, A.; Majid, A.; Sehar, Z.; Anjum, N.A.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Soil sulfur sources differentially enhance cadmium tolerance in Indian mustard (Brassica juncea L.). Soil Syst. 2021, 5, 29. [Google Scholar] [CrossRef]
- Bortoloti, G.A.; Baron, D. Phytoremediation of toxic heavy metals by brassica plants: A biochemical and physiological approach. Environ. Adv. 2022, 8, 100204. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Umar, S.; Ahmad, I.; Duarte, A.C.; Pereira, E. Improving growth and productivity of oleiferous Brassicas under changing environment: Significance of nitrogen and sulphur nutrition, and underlying mechanisms. Sci. World J. 2012, 2012, 657808. [Google Scholar] [CrossRef]
- Mir, I.R.; Rather, B.A.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitrogen sources mitigate cadmium phytotoxicity differentially by modulating cellular buffers, N-assimilation, non-protein thiols, and phytochelatins in mustard (Brassica juncea L.). J. Soil Sci. Plant Nutr. 2022, 18, 1–21. [Google Scholar] [CrossRef]
- Corpas, F.J. Hydrogen sulfide: A new warrior against abiotic stress. Trends Plant Sci. 2019, 24, 983–988. [Google Scholar] [CrossRef]
- Kushwaha, B.K.; Singh, V.P. Mitigation of chromium (VI) toxicity by additional sulfur in some vegetable crops involves glutathione and hydrogen sulfide. Plant Physiol. Biochem. 2020, 155, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Gautam, H.; Fatma, M.; Sehar, Z.; Mir, I.R.; Khan, N.A. Hydrogen sulfide, ethylene, and nitric oxide regulate redox homeostasis and protect photosynthetic metabolism under high temperature stress in rice plants. Antioxidants 2022, 11, 1478. [Google Scholar] [CrossRef]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Protective mechanisms of sulfur against arsenic phytotoxicity in Brassica napus by regulating thiol biosynthesis, sulfur-assimilation, photosynthesis, and antioxidant response. Plant Physiol. Biochem. 2022, 188, 1–11. [Google Scholar] [CrossRef]
- Alsahli, A.A.; Bhat, J.A.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Hydrogen sulfide (H2S) mitigates arsenic (As)-induced toxicity in pea (Pisum sativum L.) plants by regulating osmoregulation, antioxidant defense system, ascorbate glutathione cycle and glyoxalase system. J. Plant Growth Regul. 2020, 40, 2515–2531. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Rasheed, F.; Khan, N.A. Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. Crop J. 2016, 4, 153–161. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prasad, S.M.; Singh, V.P. Additional calcium and sulfur manages hexavalent chromium toxicity in Solanum lycopersicum L. and Solanum melongena L. seedlings by involving nitric oxide. J. Hazard. Mat. 2020, 39, 122607. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; AlZuaibr, F.M.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Al-Muwayhi, M.A.; Al-Haque, H.N. Hydrogen sulfide-mediated activation of O-Acetylserine (thiol) Lyase and L/D-Cysteine desulfhydrase enhance dehydration tolerance in Eruca sativa mill. Int. J. Mol. Sci. 2018, 19, 3981. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Okuda, T.; Masuda, Y.; Yamanka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide 780 and H2O2 accumulation in Brassica juncea seedlings. Biol. Protoc. 2014, 4, e1108. [Google Scholar]
- Awasthi, J.P.; Saha, B.; Chowardhara, B.; Devi, S.S.; Borgohain, P.; Panda, S.K. Qualitative analysis of lipid peroxidation in plants under multiple stress through schiff’s reagent: A histochemical approach. Bio-protocol 2018, 8, e2807. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- De Vos, C.R.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992, 98, 853–858. [Google Scholar] [CrossRef]
- Lindner, R.C. Rapid analytical method for some of the more common organic substances of plant and soil. Plant Physiol. 1944, 19, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.M.; Warner, R.L.; Kleinhofs, A. In vitro stability of nitrate reductase from barley leaves. Phytochemistry 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Histochemical methods for detection of heavy metals and strontium in the tissues of higher plants. Russian J. Plant Physiol. 2011, 58, 721–727. [Google Scholar] [CrossRef]
- Lappartient, A.G.; Touraine, B. Demand-driven control of root ATP sulfurylase activity and SO42-uptake in intact canola (the role of phloem-translocated glutathione). Plant Physiol. 1996, 111, 147–157. [Google Scholar] [CrossRef]
- Chesnin, L.; Yien, C.H. 1950. Turbidimetric determination of available sulphates. Soil Sci. Soc. Am. Proc. 1950, 15, 149–151. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. Environ. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Nashef, A.S.; Osuga, D.T.; Feeney, R.E. Determination of hydrogen sulfide with 5,5′-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal. Biochem. 1977, 79, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Bloem, E.; Riemenschneider, A.; Volker, J.; Papenbrock, J.; Schmidt, A.; Salac, I.; Schnug, E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 2004, 55, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Sehar, Z.; Khan, N.A. Gibberellic acid and sulfur-mediated reversal of cadmium-inhibited photosynthetic performance in mungbean (Vigna radiata L.) involves nitric oxide. J. Plant Growth Regul. 2020, 39, 1605–1615. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef]
- Sun, L.; Song, K.; Shi, L.; Duan, D.; Zhang, H.; Sun, Y.; Qin, Q.; Xue, Y. Influence of elemental sulfur on cadmium bioavailability, microbial community in paddy soil and Cd accumulation in rice plants. Sci. Rep. 2021, 11, 11468. [Google Scholar] [CrossRef]
- Dong, Y.; Silbermann, M.; Speiser, A.; Forieri, I.; Linster, E.; Poschet, G.; Wirtz, M. Sulfur availability regulates plant growth via glucose-TOR signaling. Nat. Commun. 2017, 8, 1174. [Google Scholar] [CrossRef]
- Lehotai, N.; Feigl, G.; Koós, Á.; Molnár, Á.; Ördög, A.; Pető, A.; Erdei, L.; Kolbert, Z. Nitric oxide–cytokinin interplay influences selenite sensitivity in Arabidopsis. Plant Cell Rep. 2016, 35, 2181–2195. [Google Scholar] [CrossRef]
- Kaya, M.; Zeliha, K.; Erdal, I. Effects of elemental sulfur and sulfur containing waste on nutrient concentrations and growth of bean and corn plants grown on a calcareous soil. Afr. J. Biotechnol. 2009, 8, 4481–4489. [Google Scholar]
- Liu, F.; Guo, F.Q. Nitric oxide deficiency accelerates chlorophyll breakdown and stability loss of thylakoid membranes during dark-induced leaf senescence in Arabidopsis. PLoS ONE 2013, 8, e56345. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, I.; Lindermayr, C. Nitric oxide-based protein modification: Formation and site-specificity of protein S-nitrosylation. Front. Plant Sci. 2013, 4, 137. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; AlSolami, M.A.; Alamri, S.; Hu, Y.; Ali, H.M.; Al-Ghamdi, A. Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol. Biochem. 2020, 156, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.C.; Jiménez, A.; Sevilla, F. Thioredoxin network in plant mitochondria: Cysteine S-posttranslational modifications and stress conditions. Front. Plant Sci. 2020, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Wangeline, A.L.; Burkhead, J.L.; Hale, K.L.; Lindblom, S.D.; Terry, N.; Pilon, M.; Pilon-Smits, E.A. Overexpression of ATP sulfurylase in Indian mustard—Effects on tolerance and accumulation of twelve metals. J. Environ. Qual. 2004, 33, 54–60. [Google Scholar]
- Veliz, C.G.; Roberts, I.N.; Criado, M.V.; Caputo, C. Sulphur deficiency inhibits nitrogen assimilation and recycling in barley plants. Biol. Plant. 2017, 61, 675–684. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, P.; Li, S.; Li, K.Z.; Xu, H.N. Nitrate reductase-dependent NO production is involved in H2S-induced nitrate stress tolerance in tomato via activation of antioxidant enzymes. Sci. Hort. 2018, 229, 207–214. [Google Scholar] [CrossRef]
- Lea, U.S.; Leydecker, M.T.; Quillere’, I.; Meyer, C.; Lillo, C. Posttranslational regulation of nitrate reductase strongly affects the levels of free amino acids and nitrate, whereas transcriptional regulation has only minor influence. Plant Physiol. 2006, 140, 1085–1094. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Wang, B.L.; Shi, L.; Li, Y.X.; Zhang, W.H. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 2010, 231, 1301–1309. [Google Scholar] [CrossRef]
- Wang, H.R.; Che, Y.H.; Wang, Z.H.; Zhang, B.N.; Huang, D.; Feng, F.; Ao, H. The multiple effects of hydrogen sulfide on cadmium toxicity in tobacco may be interacted with CaM signal transduction. J. Hazard. Mat. 2021, 403, 123651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, C.; Wang, X.; Liu, C.; Ding, X.; Ma, P.; Jia, H. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to Copper Oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol. Biochem. 2020, 156, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).