Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Changes in Plant Growth
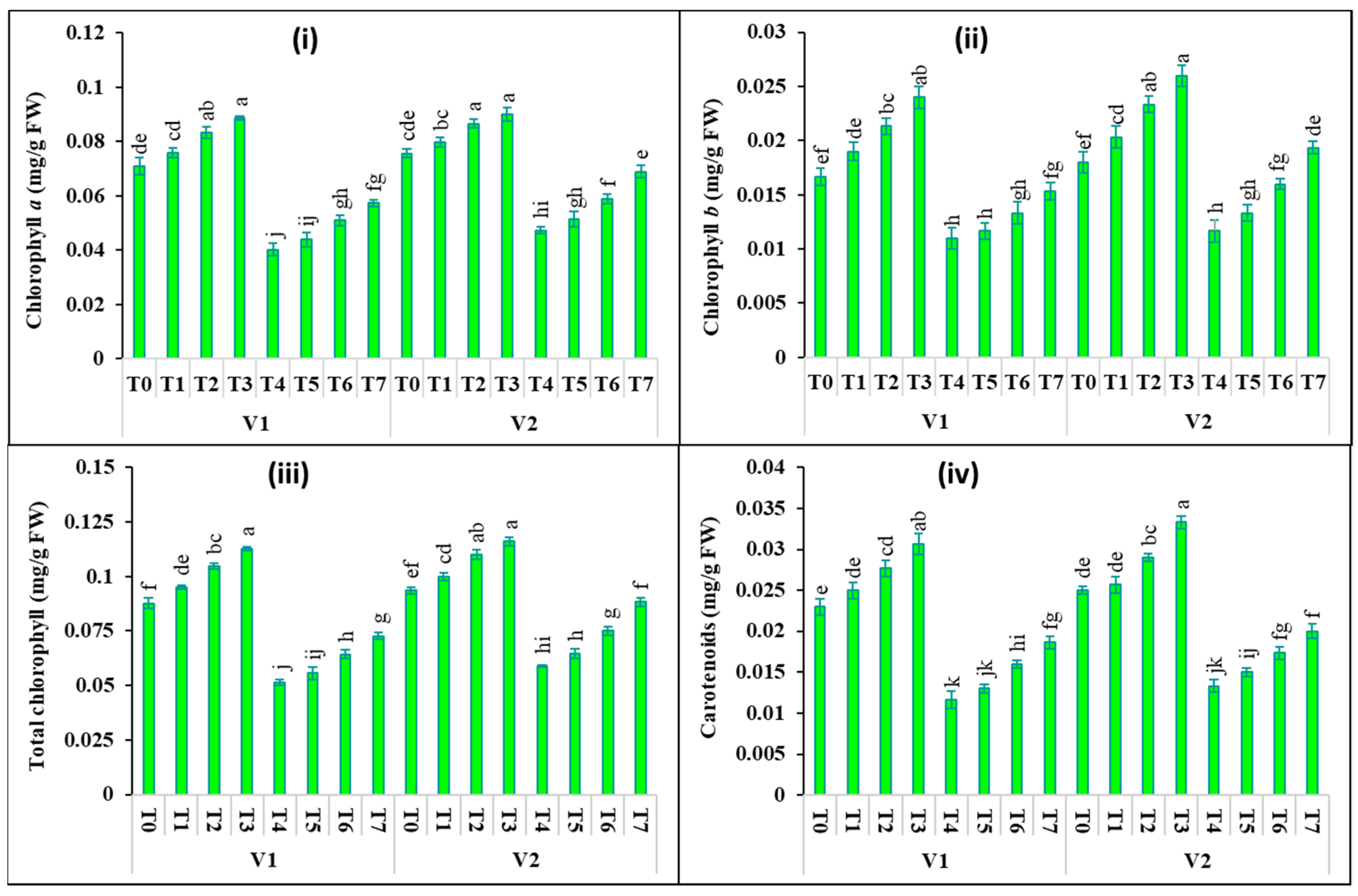
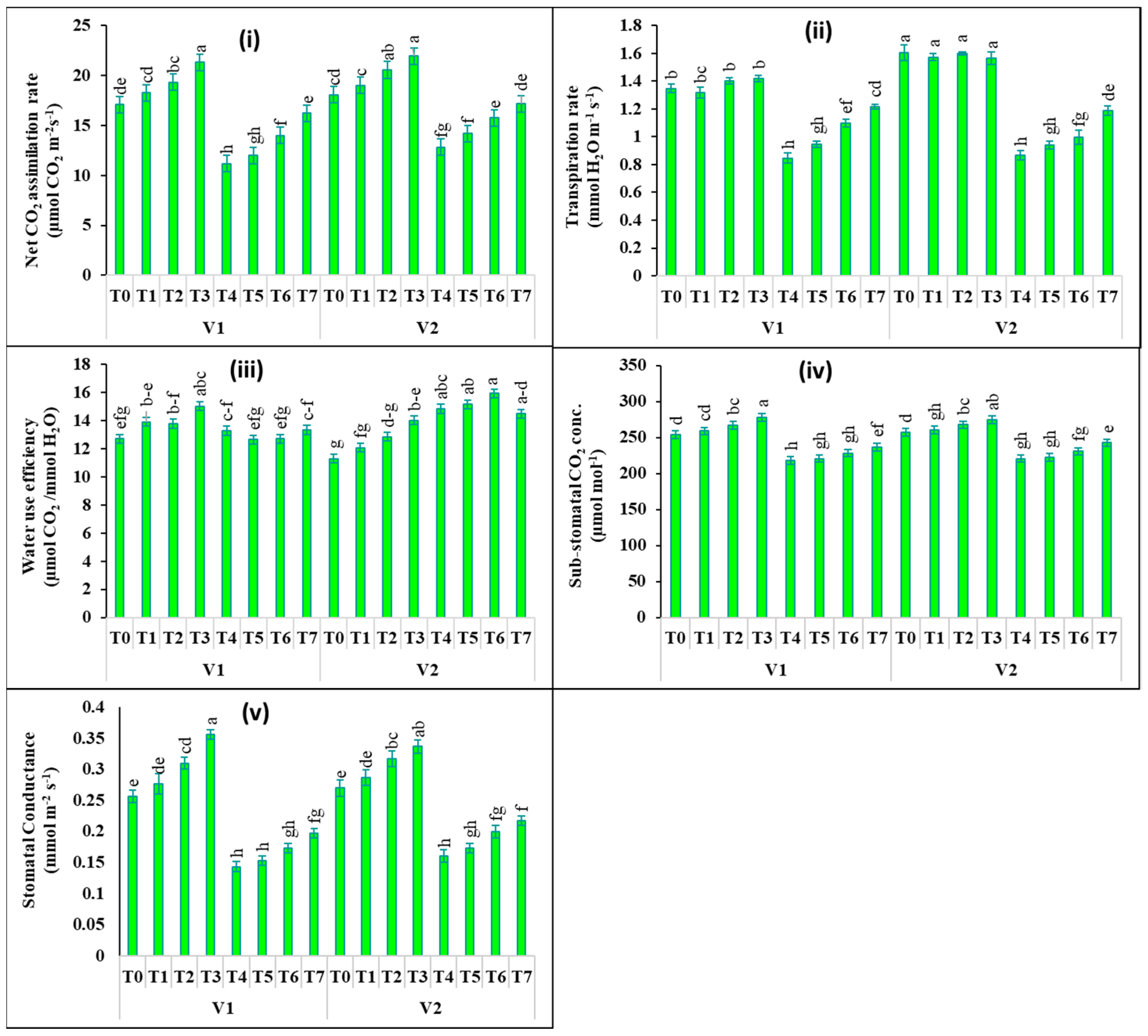
2.2. Changes in Photosynthetic Pigments and Gas Exchange Attributes
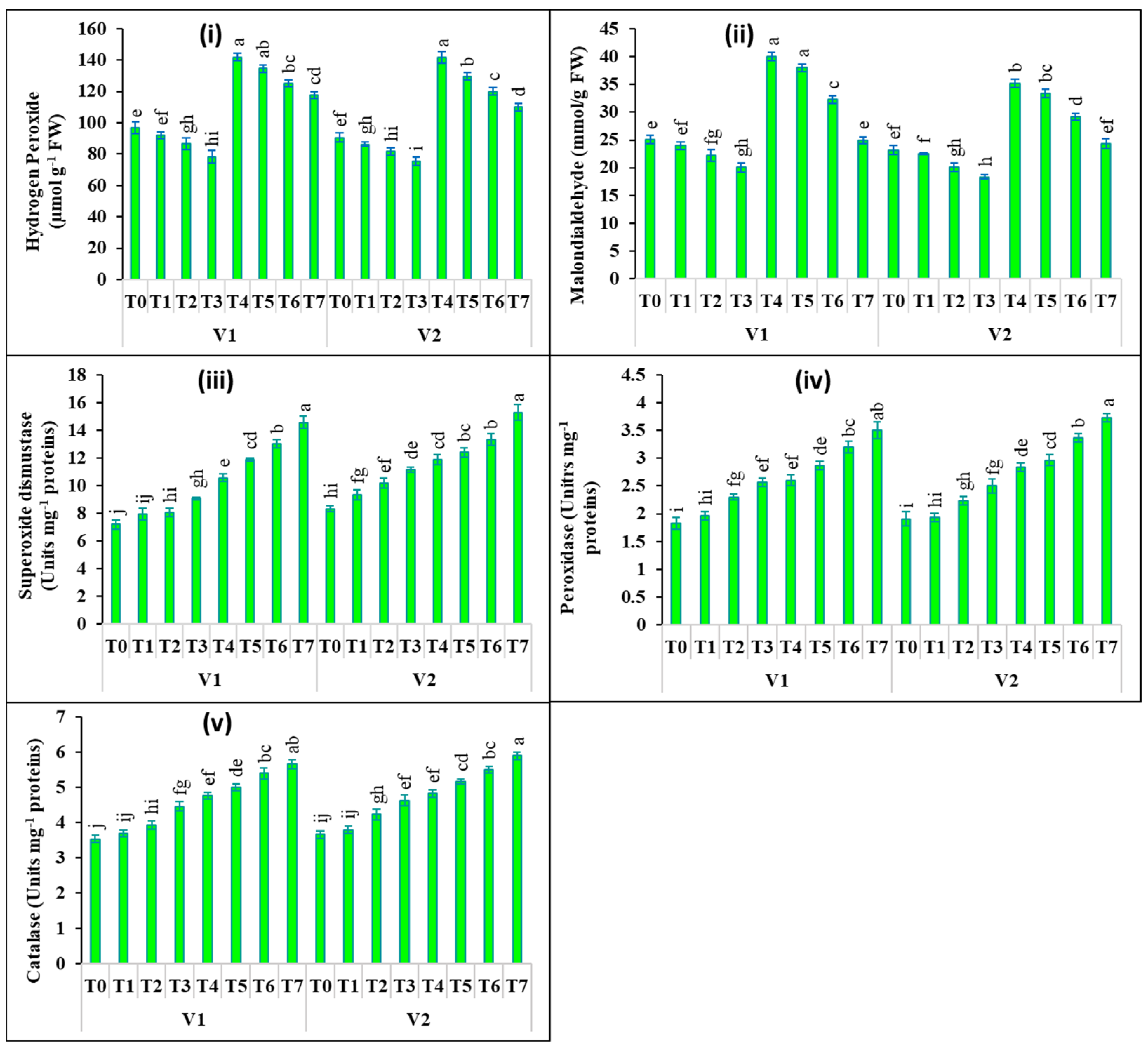
2.3. Changes in Oxidants and Antioxidants Activities
2.4. Changes in Biochemical Attributes
2.5. Changes in the Concentration of Mineral Ions
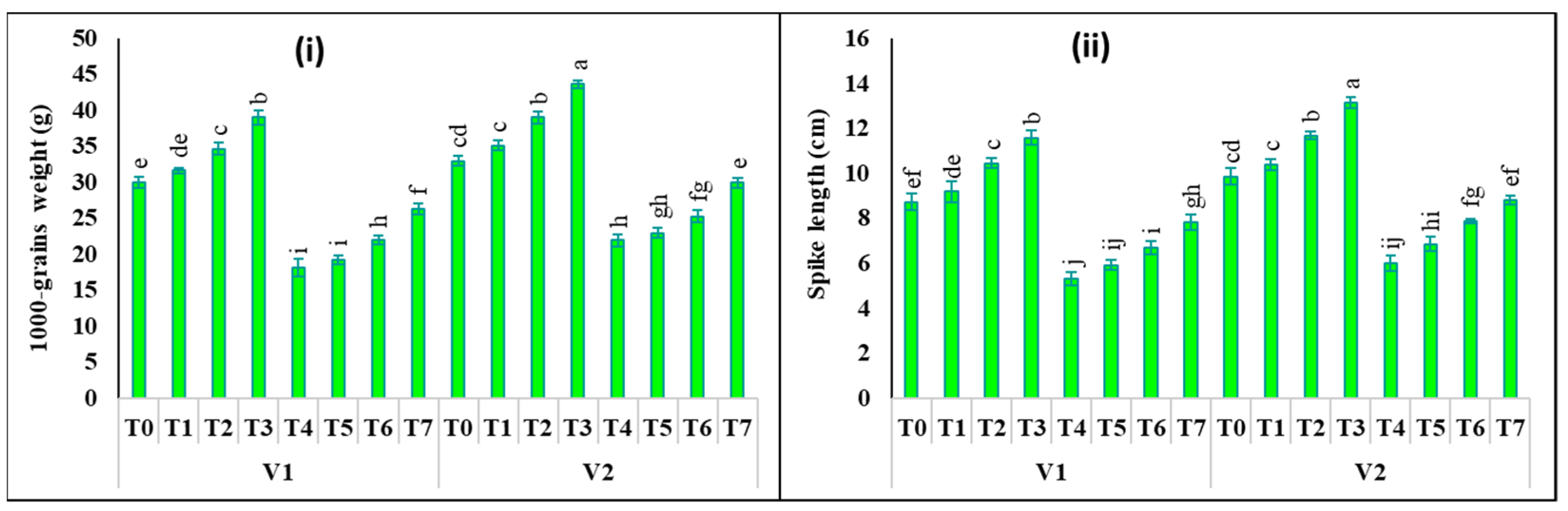
2.6. Changes in Yield Attributes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Design and Treatments
4.3. Estimation of Plant Growth and Biomass
4.4. Estimation of Photosynthetic Pigments and Gas Exchange Properties in Leaves
4.5. Estimation of Oxidants and Antioxidative Enzymes Activities in Leaves
4.6. Estimation of Biochemical Activities
4.7. Estimation of Mineral Ion Contents
4.8. Estimation of Yield-Related Traits
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J. Plant Growth Regul. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Okur, B.; Örçen, N. Soil salinization and climate change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar]
- Tian, F.; Hou, M.; Qiu, Y.; Zhang, T.; Yuan, Y. Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma 2020, 357, 113961. [Google Scholar] [CrossRef]
- Singh, A. Alternative management options for irrigation-induced salinization and waterlogging under different climatic conditions. Ecol. Indic. 2018, 90, 184–192. [Google Scholar] [CrossRef]
- Wang, F.; Yang, S.; Wei, Y.; Shi, Q.; Ding, J. Characterizing soil salinity at multiple depth using electromagnetic induction and remote sensing data with random forests: A case study in Tarim River Basin of southern Xinjiang, China. Sci. Total Environ. 2021, 754, 142030. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Shi, Z.; Biswas, A.; Yang, S.; Ding, J. Multi-algorithm comparison for predicting soil salinity. Geoderma 2020, 365, 114211. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Haas, M.; Schreiber, M.; Mascher, M. Domestication and crop evolution of wheat and barley: Genes, genomics, and future directions. J. Integr. Plant Biol. 2019, 61, 204–225. [Google Scholar] [CrossRef] [Green Version]
- Nouri, H.; Borujeni, S.C.; Nirola, R.; Hassanli, A.; Beecham, S.; Alaghmand, S.; Chris; Mulcahy, D. Application of green remediation on soil salinity treatment: A review on halophytoremediation. Process Saf. Environ. Prot. 2017, 107, 94–107. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Klessig, D.F. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Le Thanh, T.; Thumanu, K.; Wongkaew, S.; Boonkerd, N.; Teaumroong, N.; Phansak, P.; Buensanteai, N. Salicylic acid-induced accumulation of biochemical components associated with resistance against Xanthomonas oryzae pv. oryzae in rice. J. Plant Interact. 2017, 12, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef] [Green Version]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Naeem, M.; Basit, A.; Ahmad, I.; Mohamed, H.I.; Wasila, H. Effect of Salicylic Acid and Salinity Stress on the Performance of Tomato Plants. Healthy Plants 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, M.; Sarvestani, Z.T.; Emam, Y.; Bidgoli, A.M.; Sorooshzadeh, A. Foliar-applied GR24 and salicylic acid enhanced wheat drought tolerance. Russ. J. Plant Physiol. 2020, 67, 733–739. [Google Scholar] [CrossRef]
- Min, K.; Lee, S.-R. Exogenous salicylic acid alleviates freeze-thaw injury of cabbage (Brassica oleracea L.) leaves. Sustainability 2021, 13, 11437. [Google Scholar] [CrossRef]
- El Dakak, R.A.; Hassan, I.A. The alleviative effects of salicylic acid on physiological indices and defense mechanisms of maize (Zea mays L. Giza 2) stressed with cadmium. Environ. Process. 2020, 7, 873–884. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Muscolo, A.; Rehman, A. Crop diversification and saline water irrigation as potential strategies to save freshwater resources and reclamation of marginal soils—A review. Environ. Sci. Pollut. Res. 2020, 27, 28695–28729. [Google Scholar] [CrossRef]
- Ami, K.; Planchais, S.; Cabassa, C.; Guivarc’h, A.; Véry, A.-A.; Khelifi, M.; Djebbar, R.; Abrous-Belbachir, O.; Carol, P. Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol. Plant. 2020, 42, 21. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; Abdelgawad, Z.A.; El-Bassiouny, H.M.S. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S. Afr. J. Bot. 2016, 103, 275–282. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The integration of activity in saline environments: Problems and perspectives. Funct. Plant Biol. 2013, 40, 759–774. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Aragon, R.; Haro, R.; Benito, B.; Rodríguez-Navarro, A. Salt intolerance in Arabidopsis: Shoot and root sodium toxicity, and inhibition by sodium-plus-potassium overaccumulation. Planta 2016, 243, 97–114. [Google Scholar] [CrossRef]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef]
- Mehboob, W.; Rehman, H.U.; Basra, S.M.A.; Afzal, I.; Abbas, M.A.; Naeem, M.; Abbas, M. Seed priming improves the per-formance of late sown spring maize (Zea mays) through better crop stand and physiological attributes. Int. J. Agric. Biol. 2015, 17, 491–498. [Google Scholar] [CrossRef]
- Azeem, M.; Qasim, M.; Abbasi, M.W.; Tayyab; Sultana, R.; Adnan, M.Y.; Ali, H. Salicylic acid seed priming modulates some biochemical parameters to improve germination and seedling growth of salt stressed wheat (Triticum aestivum L.). Pak. J. Bot. 2019, 51, 385–391. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef] [Green Version]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Reg. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Mahboob, W.; Khan, M.A.; Shirazi, M.U. Characterization of salt tolerant wheat (Triticum aestivum) genotypes on the basis of physiological attributes. Int. J. Agric. Biol. 2017, 19, 726–734. [Google Scholar] [CrossRef]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Afzal, I.; Basra, S.M.; Ali, Z.; Ibrahim, A.M. Sodium exclusion is a reliable trait for the improvement of salinity tolerance in bread wheat. Arch. Agron. Soil Sci. 2018, 64, 272–284. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Chen, Y.; Wang, J.; Wei, M.; Shi, Q. Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci. Hort. 2018, 235, 413–423. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Guo, T. Molecular mechanism of salicylic acid-induced abiotic stress tolerance in higher plants. Acta Physiol. Plant. 2014, 36, 2287–2297. [Google Scholar] [CrossRef]
- Roshdy, A.E.-D.; Alebidi, A.; Almutairi, K.; Al-Obeed, R.; Elsabagh, A. The effect of salicylic acid on the performances of salt stressed strawberry plants, enzymes activity, and salt tolerance index. Agronomy 2021, 11, 775. [Google Scholar] [CrossRef]
- Hasan, M.A.; Al-Taweel, S.K.; Alamrani, H.A.; Al-Naqeeb, M.A.; Al-Baldawwi, M.H.K.; Hamza, J.H. Anatomical and physiological traits of broad bean (Vicia faba L.) seedling affected by salicylic acid and salt stress. Indian J. Agric. Res. 2018, 52, 368–373. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Jain, A.; Saxena, S.C.; Saifi, S.K.; Yadav, S.; Negi, M.; Prasad, R.; Tuteja, N. Salt stress triggers augmented levels of Na+, Ca2+ and ROS and alter stress-responsive gene expression in roots of CBL9 and CIPK23 knockout mutants of Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 265–276. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root respiratory burst oxidase homologue-dependent H2O2 production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure. J. Exp. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; El-Far, M.M.; Koyro, H.-W. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 2015, 118, 67–77. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Rios, J.J.; Martínez-Ballesta, M.C.; Ruiz, J.M.; Blasco, B.; Carvajal, M. Silicon mediated improvement in plant salinity tolerance: The role of aquaporins. Front. Plant Sci. 2017, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [Green Version]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, B.; Rehman, A.; Tanveer, M.; Wang, L.; Park, S.K.; Ali, A. Salt stress in brassica: Effects, tolerance mechanisms, and management. J. Plant Growth Regul. 2021, 41, 781–795. [Google Scholar] [CrossRef]
- Khan, M.; Samrana, S.; Zhang, Y.; Malik, Z.; Khan, M.D.; Zhu, S. Reduced Glutathione Protects Subcellular Compartments from Pb-Induced ROS Injury in Leaves and Roots of Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2020, 11, 412. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Molaei, S.; Soleimani, A.; Rabiei, V.; Razavi, F. Impact of chitosan in combination with potassium sorbate treatment on chilling injury and quality attributes of pomegranate fruit during cold storage. J. Food Biochem. 2021, 45, e13633. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Misra, N.; Misra, R. Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentina leaves grown under salinity stress. Environ. Sci. 2012, 12, 1601–1609. [Google Scholar]
- El-Beltagi, H.S.; Ahmed, S.H.; Namich, A.A.M.; Abdel-Sattar, R.R. Effect of salicylic acid and potassium citrate on cotton plant under salt stress. Fresen. Environ. Bull. 2017, 26, 1091–1100. [Google Scholar]
- Agamy, R.A.; Hafez, E.E.; Taha, T.H. Acquired resistance motivated by salicylic acid applications on salt-stressed tomato (Lycopersicon esculentum Mill.). Am.-Euras. J. Agric. Environ. Sci. 2013, 13, 50–57. [Google Scholar]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Per, T.S.; Fatma, M.; Asgher, M.; Javied, S.; Khan, N.A. Salicylic acid and nutrients interplay in abiotic stress tolerance. In Salicylic Acid: A Multifaceted Hormone; Springer: Singapore, 2017; pp. 221–237. [Google Scholar]
- Hamayun, M.; Sohn, E.Y.; Khan, S.A.; Shinwari, Z.K.; Khan, A.L.; Lee, I.J. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pak. J. Bot. 2010, 42, 1713–1722. [Google Scholar]
- Qiong, Y.; Guo, Y.; Zhixia, X.; Ke, S.; Jin, X.; Ting, Y.; Xiaojing, L. Effects of salt stress on tillering nodes to the growth of winter wheat (Triticum aestivum L.). Pak. J. Bot. 2016, 48, 1775–1782. [Google Scholar]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signaling and tolerance. Plant Growth Reg. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Kaleem, F.; Shabir, G.; Aslam, K.; Rasul, S.; Manzoor, H.; Shah, S.M.; Khan, A.R. An overview of the genetics of plant response to salt stress: Present status and the way forward. Appl Biochem. Biotechnol. 2018, 186, 306–334. [Google Scholar] [CrossRef]
- El Sebai, T.N.; Abdallah, M.M.S.; El-Bassiouny, H.M.S.; Ibrahim, F.M. Amelioration of the adverse effects of salinity stress by using compost, Nigella sativa extract or ascorbic acid in quinoa plants. Int. J. PharmTech Res. 2016, 9, 127–144. [Google Scholar]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestic, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Noreen, S.; Fatima, K.; Athar, H.U.R.; Ahmad, S.; Hussain, K. Enhancement of physio-biochemical parameters of wheat through exogenous application of salicylic acid under drought stress. J. Anim. Plant Sci. 2017, 27, 153–163. [Google Scholar]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Strnad, M.; Ayaz, F.A. The Effects of exogenous salicylic acid on endogenous phytohormone status in Hordeum vulgare L. under salt stress. Plants 2022, 11, 618. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M.; Sohrab, S.S.; Ansari, M.K.A. Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric. Food Secur. 2018, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Bleiholder, H.; Weber, E.; Lancashire, P.D.; Feller, C.; Buhr, L.; Hess, M.; Wicke, H.; Hack, H.; Meier, U.; Klose, R.; et al. Growth Stages of Mono- and Dicotyledonous Plants–BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001. [Google Scholar]
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 1765. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzyme in isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Pro-tective roles of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, J.H. Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalase and peroxidase. Methods Enzymol. 1955, 2, 764–817. [Google Scholar]
- Spitz, D.R.; Oberly, L.W. Measurement of MnSOD and CuZnSOD activity in mammalian tissue homogenates. Curr. Protoc. Toxicol. 2001, 8, 751–758. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhri, M.A. Implications for water stress-induced changes in the level of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Yoshida, S.D.; Forno, J.C.; Gomez, K. Determination of sugars and starch in plant tissue. Laboratory manual for physiological studies of rice. Philipp. Int. Rice Res. Inst. 1976, 3, 46–49. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- McCue, P.; Zheng, Z.; Pinkham, J.L.; Shetty, K. A model for enhanced pea seedling vigour following low pH and salicylic acid treatments. Process. Biochem. 2000, 35, 603–613. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Kwon, S.H.; Torrie, J.H. Heritability and interrelationship of traits of two soybean populations. Crop Sci. 1964, 4, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics—A Biometrical Approach, 3rd ed.; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Saman, R.U.; Rehman, A.; Naz, N.; Akram, M.; Haider, F.U. Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress. Stresses 2023, 3, 372-386. https://doi.org/10.3390/stresses3010027
Maqsood MF, Shahbaz M, Zulfiqar U, Saman RU, Rehman A, Naz N, Akram M, Haider FU. Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress. Stresses. 2023; 3(1):372-386. https://doi.org/10.3390/stresses3010027
Chicago/Turabian StyleMaqsood, Muhammad Faisal, Muhammad Shahbaz, Usman Zulfiqar, Rafia Urooj Saman, Abdul Rehman, Nargis Naz, Muhammad Akram, and Fasih Ullah Haider. 2023. "Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress" Stresses 3, no. 1: 372-386. https://doi.org/10.3390/stresses3010027
APA StyleMaqsood, M. F., Shahbaz, M., Zulfiqar, U., Saman, R. U., Rehman, A., Naz, N., Akram, M., & Haider, F. U. (2023). Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress. Stresses, 3(1), 372-386. https://doi.org/10.3390/stresses3010027







