Engineering Approach for Production of Arbuscular Mycorrhizal Inoculum Adapted to Saline Soil Management
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical and Meteorological Characteristics of the Studied Soils
2.2. Evaluation of Produced Inoculum
2.2.1. AMF Spore Species Enumeration and Identification
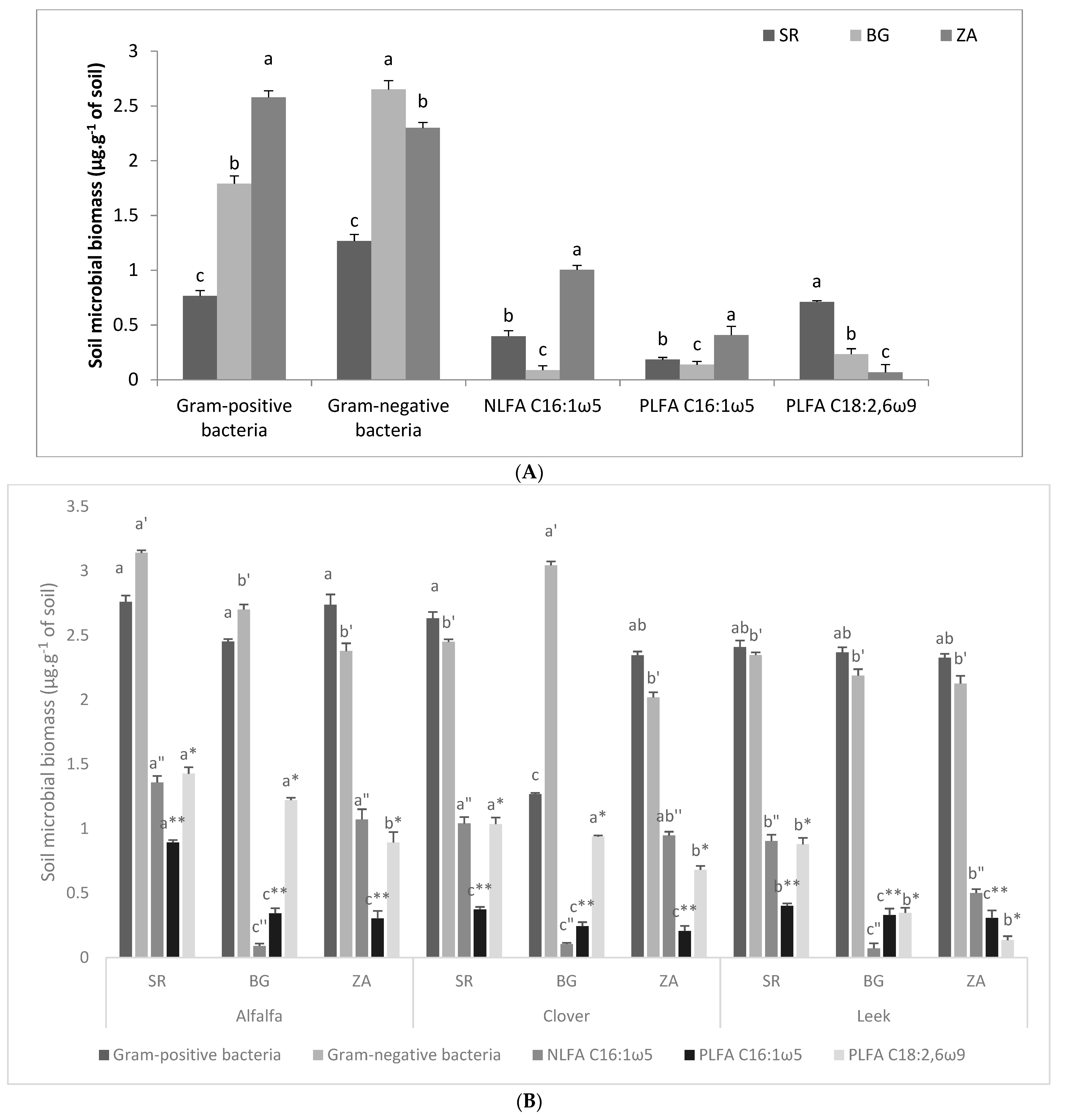
2.2.2. Soil Microbial Biomass
Soil Microbial Biomass before Trap Culture
Soil Microbial Biomass after Trap Culture
2.3. Determination of Mycorrhizal Propagules Using the Most Probable Number (MPN)
2.4. Mycorrhizal Rates
2.5. Global Statistical Analyses
3. Discussion
4. Materials and Methods
4.1. Experimental Sites and Soil Samples
4.2. Description of AMF Native Community
4.3. Experimental Design for AMF Inoculum Production
4.4. Host Plant Total Mycorrhizal Rates
4.4.1. Most Probable Number (MPN)
4.4.2. Soil Microbial Biomass Quantification
4.5. Meteorological Data
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ait-El-Mokhtar, M.; Ben Laouane, R.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi in Saline Soil: Alleviating Date Palm under High-Salt Stress. In Bio-Stimulants for Sustainable Agriculture in Oasis Ecosystem towards Improving date Palm Tolerance to Biotic and Abiotic Stress; Khalifa International Award for Date Palm and Agricultural Innovation: Abu Dhabi, United Arab Emirates, 2021. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.; Hussain, N.; Hanjra, M.A.; Akbar, S. Characterizing soil salinity in irrigated agriculture using a remote sensing approach. Phys. Chem. Earth Parts A/B/C 2013, 55-57, 43–52. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.-G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Haran, M.S.; Thaher, A.Z.T. Effect the single and mixture inoculation with Phosphate solubilizing bacteria, Azotobacter bacteria and P. floresences in growth and production of the corn (Zea mays L.) irrigated with different saline water. Adv. Nat. Appl. Sci. 2019, 13, 22–26. [Google Scholar] [CrossRef]
- Basiru, S.; Mwanza, H.; Hijri, M. Analysis of Arbuscular Mycorrhizal Fungal Inoculant Benchmarks. Microorganisms 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Read, D. Mycorrhizal Symbiosis; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Avila-Salem, M.E.; Montesdeoca, F.; Orellana, M.; Pacheco, K.; Alvarado, S.; Becerra, N.; Marín, C.; Borie, F.; Aguilera, P.; Cornejo, P. Soil Biological Properties and Arbuscular Mycorrhizal Fungal Communities of Representative Crops Established in the Andean Region from Ecuadorian Highlands. J. Soil Sci. Plant Nutr. 2020, 20, 2156–2163. [Google Scholar] [CrossRef]
- Coelho, I.R.; Pedone-Bonfim, M.V.L.; Silva, F.; Maia, L.C. Optimization of the production of mycorrhizal inoculum on substrate with organic fertilizer. Braz. J. Microbiol. 2014, 45, 1173–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, A.T.; Zhu, Y.; Chen, Y.-L.; Ren, H.-X.; Li, J.-Y.; Abbott, L.K.; Xiong, Y.-C. Arbusculat mycorrhizal gungus alters root-sourced signal (abscisic acid) for better drought acclimataion in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Vymazal, J.; Chen, Z. Application of arbuscular mycorrhizal fungi for pharmaceuticals and personal care productions removal in constructed wetlands with different substrate. J. Clean. Prod. 2022, 339, 130760. [Google Scholar] [CrossRef]
- Moreira, B.C.; Junior, P.P.; Jordão, T.C.; Silva, M.D.C.S.D.; Ribeiro, A.P.F.; Stürmer, S.L.; Salomão, L.C.C.; Otoni, W.C.; Kasuya, M.C.M. Effect of Inoculation of Pineapple Plantlets with Arbuscular Mycorrhizal Fungi Obtained from Different Inoculum Sources Multiplied by the On-Farm Method. Rev. Bras. Ciência Solo 2019, 43, e0180148. [Google Scholar] [CrossRef] [Green Version]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Trejo-Aguilar, D.; Banuelos, J. Isolation and Culture of Arbuscular Mycorrhizal Fungi from Field Samples. In Arbuscular Mycorrhizal Fungi Methods and Protocols, Methods in Molecular Biology; Ferrol, N., Lanfranco, L., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 2146. [Google Scholar] [CrossRef]
- Bencherif, K.; Boutekrabt, A.; Dalpé, Y.; Lounes Hadj-Sahraoui, A. Soil and seasons affect arbuscular mycorrhizal fungi associated with Tamarix rhizosphere in arid and semi-arid steppes. Appl. Soil Ecol. 2016, 107, 182–190. [Google Scholar] [CrossRef]
- Basiru, S.; Hijri, M. Does Commercial Inoculation Promote Arbuscular Mycorrhizal Fungi Invasion? Microorganisms 2022, 10, 404. [Google Scholar] [CrossRef]
- Salomon, M.J.; Watts-Williams, S.J.; McLaughlin, M.J.; Bücking, H.; Singh, B.K.; Hutter, I.; Schneider, C.; Martin, F.M.; Vosatka, M.; Guo, L.; et al. Establishing a quality management framework for commercial inoculants containing arbuscular mycorrhizal fungi. iScience 2022, 25, 104636. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Azcón, R. Viability and infectivity of mycorrhizal spores after long term storage in soils with different water potentials. Appl. Soil Ecol. 1996, 3, 183–186. [Google Scholar] [CrossRef]
- Dietrich, P.; Roscher, C.; Clark, A.T.; Eisenhauer, N.; Schmid, B.; Wagg, C. Diverse plant mixtures sustain a greater arbuscular mycorrhizal fungi spore viability than monocultures after 12 years. J. Plant Ecol. 2020, 13, 478–488. [Google Scholar] [CrossRef]
- Wiseman, P.E.; Colvin, K.H.; Wells, C.E. Performance of Mycorrhizal Products Marketed for Woody Landscape Plants. J. Environ. Hortic. 2009, 27, 41–50. [Google Scholar] [CrossRef]
- Faye, A.; Dalpé, Y.; Ndung’U-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of commercial arbuscular mycorrhizal inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Porter, W. The ‘most probable number’ method for enumerating infective propagules of vesicular arbuscular mycorrhizal fungi in soil. Soil Res. 1979, 17, 515–519. [Google Scholar] [CrossRef]
- Norris, C.E.; Swallow, M.J.; Liptzin, D.; Cope, M.; Mac Bean, G.; Cappellazzi, S.B.; Greub, K.L.; Rieke, E.L.; Tracy, P.W.; Morgan, C.L.; et al. Use of phospholipid fatty acid analysis as phenotypic biomarkers for soil health and the influence of management practices. Appl. Soil Ecol. 2023, 185, 104793. [Google Scholar] [CrossRef]
- Deluca, T.H.; Pingree, M.R.A.; Gao, H. Assessing soil biological health in forest soils. Developments in Soil Science. Dev. Soil Sci. 2019, 36, 397–426. [Google Scholar] [CrossRef]
- Islam, M.N.; Germida, J.J.; Walley, F.L. Survival of a commercial AM fungal inoculant and its impact on indigenous AM fungal communities in field soils. Appl. Soil Ecol. 2021, 166, 103979. [Google Scholar] [CrossRef]
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2018, 273, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; Alatalo, J.M.; Al-Quraishy, S.; Andriyanova, E.; Anslan, S.; et al. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Nagahashi, G.; Pfeffer, P.E.; Kayser, W.M.; Reider, C. On-farm production and utilization of arbuscular mycorrhizal fungus inoculum. Can. J. Plant Sci. 2005, 85, 15–21. [Google Scholar] [CrossRef]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Ben Jeddi, F.; Sahraoui, A.L.-H. Mycorrhizal biofertilization improves grain yield and quality of hulless Barley (Hordeum vulgare ssp. nudum L.) under water stress conditions. J. Cereal Sci. 2022, 104, 103436. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Struble, J.E.; Skipper, H.D. Vesicular-arbuscular mycorrhizal fungal spore production as influenced by plant species. Plant Soil 1988, 109, 277–280. [Google Scholar] [CrossRef]
- Dalpé, Y.; Monreal, M. Arbuscular Mycorrhiza Inoculum to Support Sustainable Cropping Systems. Crop. Manag. 2004, 3, 1–11. [Google Scholar] [CrossRef]
- dos Santos, R.S.; Ferreira, J.S.; Scoriza, R.N. Inoculum production of arbuscular mycorrhizal fungi native to soils under different forest covers. Rev. Ceres Viçosa 2017, 64, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Rena, W.; Lia, Y.; Xua, Y.F.; Tenga, Y.; Christiea, P.; Luoa, Y. Nontargeted metabolomic analysis to unravel the impact of di (2-ethylhexyl) phthalate stress on root exudates of alfalfa (Medicago sativa). Sci. Total Environ. 2019, 646, 212–219. [Google Scholar] [CrossRef]
- Catford, J.; Staehelin, C.; Lerat, S.; Piché, Y.; Vierheilig, H. Suppression of arbuscular mycorrhizal colonization and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with Nod factors. J. Exp. Bot. 2003, 54, 1481–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Avio, L.; Sbrana, C. Functional significance of anastomosis in arbuscular mycorrhizal networks. In Mycorrhizal Networks; Horton, T., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 224, pp. 41–67. [Google Scholar]
- Moreira, M.; Baretta, D.; Tsai, S.; Gomes-da-Costa, S.M.; Nogueira Cardoso, E.J.B. Biodiversity and distribution of arbuscular mycorrhizal fungi in Araucaria angustifolia Forest. Sci. Agric. 2007, 64, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Rabouh, A. Modélisation ET Mise en éVidence Des Structures Diapirique AU Nord Sahara Algérien (Cas du Rocher de Sel-Djelfa ET El Outaya). Master’s Thesis, University of Ouragla, Ouragla, Algeria, 2018. [Google Scholar]
- Torrecillas, E.; Alguacil, M.D.M.; Roldán, A.; Díaz, G.; Montesinos-Navarro, A.; Torres, M.P. Modularity Reveals the Tendency of Arbuscular Mycorrhizal Fungi To Interact Differently with Generalist and Specialist Plant Species in Gypsum Soils. Appl. Environ. Microbiol. 2014, 80, 5457–5466. [Google Scholar] [CrossRef] [Green Version]
- Palacio, S.; Johnson, D.; Escudero, A.; Montserrat-Martí, G. Root colonisation by AM fungi differs between gypsum specialist and non-specialist plants: Links to the gypsophile behaviour. J. Arid. Environ. 2012, 76, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Okkon, J.; Okkon, I.; Embong, E.; Grace, D.O. Mitigation of salt induced stress via arbuscular mycorrhizal fungi (Rhizophagus irregularis) inoculation in Cucurbita maxima Duch. Int. J. Mol. Biol. Open Access 2019, 4, 30–36. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Chwat, G.; Symanczik, S.; Góralska, A. Dominikia duoreactiva sp. nov. and Dominikia difficilevidera sp. nov., two new species in the Glomeromycota. Botany 2015, 93, 389–396. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Millner, P.D. Biodiversity of Arbuscular Mycorrhizal Fungi in Agroecosystems in Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Paoletti, M.G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Miranda, J.C.C. Cerrado: Micorriza Arbuscular-Ocorrência Emanejo; Embrapa Cerrados: Planaltina, Brazil, 2008; p. 169. [Google Scholar]
- Schreiner, R.P. Effects of native and nonnative arbuscular mycorrhizal fungi on growth and nutriente uptake of ‘Pinot noir’ (Vitis vinifera L.) in two soils with contrasting levels of phosphorus. Appl. Soil Ecol. 2007, 36, 2005–2015. [Google Scholar]
- Chantelot, E. L’activité biologique des sols. In Méthodes D’évaluation; Institut Technique de l’Agriculture Biologique: Paris, France, 2003; Available online: www.itab.asso.fr (accessed on 15 October 2003).
- USDA. Soil Classification: A Comprehensives System (Prepared by) Soil Survey Staff; USDA: Washington, DC, USA, 2013. [Google Scholar]
- CEEAEQ. Détérmination de la Matière Organique Par Dosage du Carbone Organique Dans Les Sols Agricoles: Method Walkley-Black Mo-Difiée, Ma.101–Wb. 1.0; Ministère de l’environnement du Québéc: Quebec, QC, Canada, 2003; p. 10. [Google Scholar]
- Petard, J. Les Méthodes D’analyse Du Sol; Tome I. L’Institut Français De recherche scientfou pour le développement en coopération (ORSTOM); IRD. Edition; Centre de Nouméa: Nouméa, Nouvelle-Calédonie, 1993. [Google Scholar]
- AFNOR. Contrôle de la qualité des analyses du sol. In Méthodes D’analyses Officielles Tome 1. AFNOR- DGCCRF; Association Française de Normalisation: Paris, France, 1995. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; La, D. Estimation of Available Phosphorus by Extraction with Sodium Bicarbonate (Ciecular39); USDA: Washington, DC, USA, 1954. [Google Scholar]
- Nathan, M.V.; Stecker, J.A.; Sun, Y. Soil Testing in Missouri A Guide for Conducting Soil Tests in Missouri; University of Missouri: Columbia, SC, USA, 2012. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Blaszkowski, J. Glomeromycota; IB Publisher Polish Academy of Sciences: Kraków, Poland, 2012. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular rnycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Gianinazzi-Pearson, V.; Gianinazzi, S.; Trouvelot, A. Evaluation of the infectivity and effectiveness of indigenous vesicular–arbuscular fungal populations in some agricultural soils in Burgundy. Can. J. Bot. 1985, 63, 1521–1524. [Google Scholar] [CrossRef]
- Cochran, W.G. Estimation of Bacterial Densities by Means of the “Most Probable Number”. Biometrics 1950, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Frostegård, A.; Tunlid, A.; Baath, E. Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biol. Biochem. 1996, 28, 55–63. [Google Scholar] [CrossRef]
- Idder-Ighili, H.; Idder, M.A.; Doumandji-Mitiche, B.; Chenchouni, H. Modeling the effects of climate on date palm scale (Parlatoria blanchardi) population dynamics during different phenological stages of life history under hot arid conditions. Int. J. Biometeorol. 2015, 59, 1425–1436. [Google Scholar] [CrossRef]
- Wilczewski, E.; Skinder, Z.; Szczepanek, M. Effects of weather conditions on yield of tansy placelia and common sunflower grown at stubble catch crop. Pol. J. Environ. Stud. 2012, 21, 1053–1060. [Google Scholar]
- De Martonne, E. Traité de Géographie Physique: Tome 3, 4th ed.; Chevalier, A., Cuénot, L., Eds.; A. Colin: Paris, France, 1925; p. 1519. [Google Scholar]
- Mc Cunn, B.; Mefford, M.J. Pc-Ord: Multivariate Analysis of Ecological Data; MjM Software: Gleneden Beach, OR, USA, 2011; p. 22. [Google Scholar]
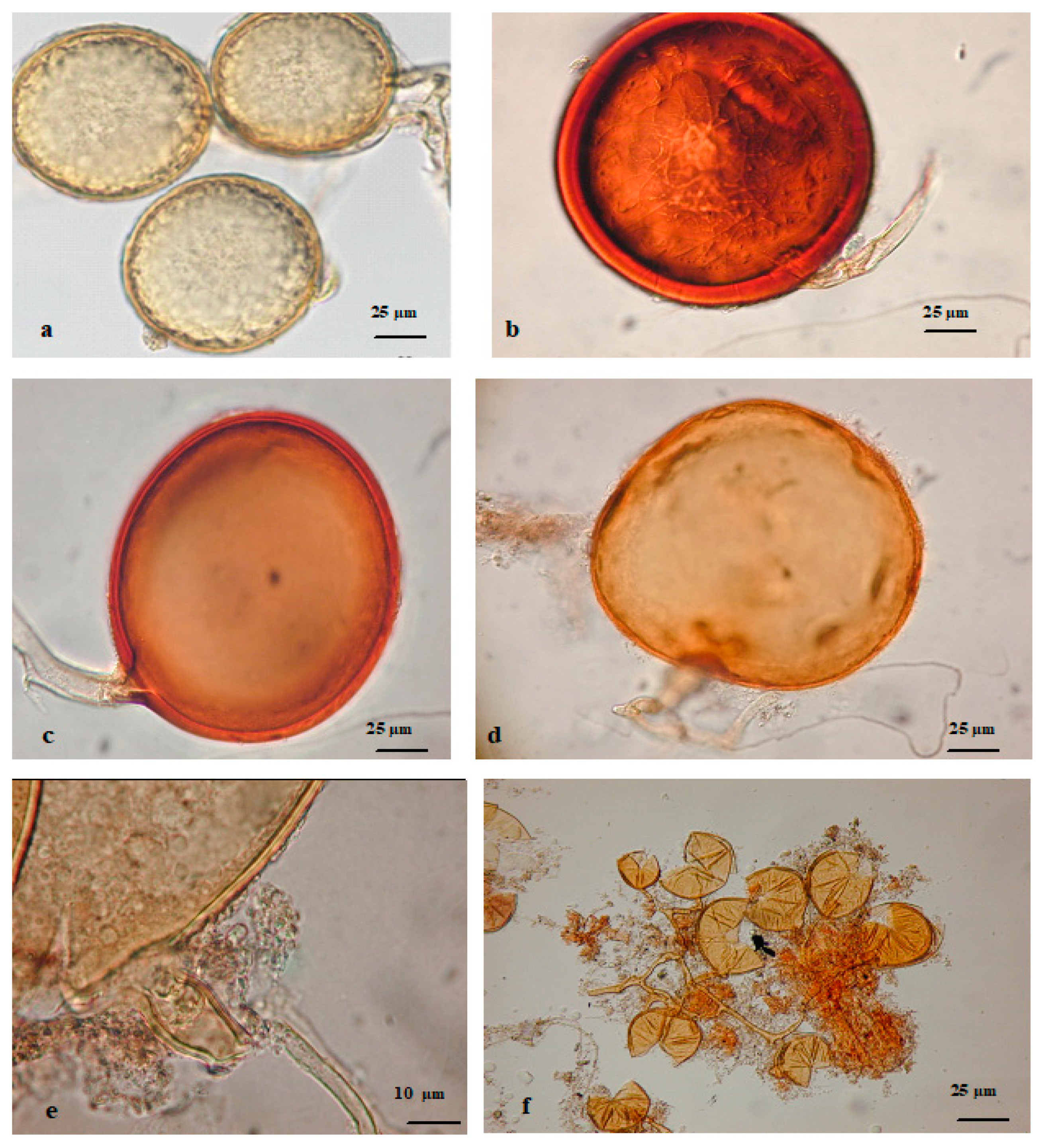




| SITES | |||
|---|---|---|---|
| Soil Parameters | Salt Rocket (SR) | Zaafrane (ZA) | Boughzoul (BG) |
| M (°C) | 35.5 | 36.3 | 34.5 |
| m (°C) | 1.2 | 1.8 | 0.86 |
| R (mm/year) | 279 | 245.23 | 950 |
| Altitude (m) | 1083 | 950 | 635 |
| Emberger pluvio-thermic quotient (Q3) | 27.9 | 24.37 | 21.43 |
| Bioclimatic stage (Emberger classification) | Medium semi-arid with cold winter | Semi-arid lower with mild winter | High arid with mild winter |
| pH water | 5.95 | 9.5 | 7.48 |
| EC (dS·m−1) | 8.5 | 9.93 | 4.15 |
| Salinity (g·L−1) | 4.94 | 5.75 | 3.24 |
| Available phosphorus (mg·g−1) | 0.027 | 0.1 | 0.21 |
| Moisture (%) | 13.85 | 23.8 | 4.94 |
| Nitrogen (mg·g−1) | 0.2 | 0.1 | 0.42 |
| Carbone (mg·g−1) | 8.9 | 3.18 | 8.3 |
| C/N | 44.5 | 31.8 | 19.76 |
| Mg (meq 100 g−1) | 31 | 15.2 | 38.4 |
| K (mg·g−1) | 0.12 | 0.14 | 30.01 |
| Na (meq.100 g−1) | 22 | 21 | 11 |
| OM (%) | 1.53 | 0.54 | 1.43 |
| Total calcareous (%) | 7.46 | 9.8 | 12.62 |
| Clay (%) | 2.90 | 13.5 | 9.5 |
| Silt (%) | 75.69 | 25.1 | 19.95 |
| Sand | 21.90 | 61.1 | 70.5 |
| Texture | Loamy–sandy | Sandy–loamy | Sandy–loamy |
| Number of AMF Spore Species/10 g of Soil | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Plantation | After Plantation with | |||||||||||
| Alfalfa | Clover | Leek | ||||||||||
| SR | ZA | BG | SR | ZA | BG | SR | ZA | BG | SR | ZA | BG | |
| Claroideoglomus etunicatum | 0 | 30 | 10 | 10 | 50 | 60 | 3 | 25 | 52 | 2 | 22 | 10 |
| Dominikia sp. | 80 | 10 | 74 | 120 | 8 | 98 | 95 | 54 | 90 | 90 | 50 | 88 |
| Funnelifromis coronatus | 16 | 0 | 47 | 30 | 8 | 52 | 25 | 0 | 36 | 10 | 0 | 0 |
| Funneliformis mosseae | 15 | 3 | 10 | 50 | 20 | 45 | 35 | 14 | 28 | 23 | 11 | 20 |
| Glomus deseerticola (Septoglomus deserticola) | 50 | 3 | 2 | 60 | 6 | 12 | 42 | 5 | 10 | 34 | 0 | 3 |
| Gigaspora gigantea | 2 | 0 | 0 | 8 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Gigaspora margarita | 3 | 0 | 2 | 20 | 0 | 7 | 4 | 0 | 1 | 1 | 0 | 0 |
| Glomus macrocarpum | 10 | 1 | 2 | 75 | 28 | 30 | 44 | 25 | 35 | 44 | 14 | 21 |
| Innospora (Paraglomus majewskii) | 13 | 8 | 7 | 60 | 28 | 70 | 23 | 24 | 32 | 22 | 12 | 15 |
| Microkamienskia sp. | 2 | 0 | 0 | 20 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 |
| Rhizophagus fasciculatum | 0 | 2 | 15 | 10 | 4 | 11 | 1 | 0 | 10 | 0 | 0 | 8 |
| Rhizophagus irregularis | 2 | 70 | 70 | 120 | 88 | 98 | 60 | 45 | 100 | 21 | 25 | 15 |
| Septoglomus constrictum | 2 | 20 | 60 | 52 | 50 | 80 | 2 | 20 | 60 | 2 | 20 | 60 |
| Total spore number/10 g of soil | 195 | 147 | 299 | 635 | 290 | 563 | 344 | 212 | 454 | 249 | 154 | 240 |
| Species richness (S) | 11 | 9 | 11 | 13 | 10 | 11 | 13 | 8 | 11 | 10 | 7 | 9 |
| Shannon diversity index (H’) | 1.70 | 1.54 | 1.86 | 2.28 | 1.94 | 2.17 | 2.04 | 1.91 | 2.11 | 1.81 | 1.80 | 1.78 |
| Studied Parameters | Alfalfa | Clover | Leek | |||
|---|---|---|---|---|---|---|
| Pearson r | p Value | Pearson r | p Value | Pearson r | p Value | |
| Mycorrhizal rate × soil salinity | −0.5 * | 0.05 | −0.7 * | 0.05 | −0.9 ** | 0.01 |
| Shannon index × Mycorrhizal rate | 0.9 * | <0.0001 | 0.9 *** | <0.02 | 0.8 * | 0.01 |
| AMF species richness × Mycorrhizal rate | 0.7 * | <0.05 | 0.7 * | 0.05 | 0.9 * | 0.01 |
| MPN × Mycorrhizal rate | 0.9 * | <0.001 | 0.9 ** | 0.2 | 0.3 ns | 0.01 |
| NLFA C16:1ω5 × Mycorrhizal rate | 0.2 ns | 0.7222 | 0.9 ** | 0.5 | 0.4 * | 0.01 |
| PLFA C16:1ω5 × Mycorrhizal rate | 0.6 | <0.0001 | 0.7 * | 0.5 | 0.3 * | 0.01 |
| Gram-positive bacterial biomass × Mycorrhizal rate | 0.7 * | 0.6 | 0.3 ns | 0.0 | 0.6 * | 0.01 |
| Gram-negative bacterial biomass × Mycorrhizal rate | 0.9 * | 0.0 | 0.2 ns | 0.1 | 0.5 * | 0.01 |
| Soil organic matter × Mycorrhizal rate | 0.38 * | 0.05 | 0.52 * | 0.05 | 0.5 * | 0.05 |
| Soil nitrogen × Mycorrhizal rate | 0.5 * | 0.04 | 0.41 * | 0.05 | 0.48 * | 0.05 |
| Soil phosphorus × Mycorrhizal rate | 0.51 ** | 0.01 | 0.51 * | 0.05 | 0.2 ns | 0.654 |
| Soil humidity × Mycorrhizal rate | −0.04 ns | 0.878 | 0.21 ns | 0.78 | 0.6 ns | 0.542 |
| Host Plant Species | Soil Salinity | Climate | Host Plant × Soil Salinity | Host Plant × Climate | Soil Salinity × Climate | Host Plant × Soil Salinity × Climate | |
|---|---|---|---|---|---|---|---|
| F (value) Mycorrhizal colonization | 9.14 * | 7.06 ** | 1.31 * | 6.39 * | 2.85 ns | 3.61 * | 8.54 * |
| F (Value) Soil microbial biomass | 2.33 ** | 4.54 *** | 1.32 ns | 2.25 ** | 5.36 * | 1.52 ns | 2.3 ** |
| F (Value) AMF diversity index | 8.69 *** | 5.64 ** | 6.5 * | 1.36 * | 2.36 * | 2.52 * | 8.45 * |
| F (Value) Mycorrhizal potential | 3.17 * | 2.15 ** | 1.31 ns | 6.39 * | 1.23 ns | 1.15 ns | 1.14 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencherif, K.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Lounés-Hadj Sahraoui, A. Engineering Approach for Production of Arbuscular Mycorrhizal Inoculum Adapted to Saline Soil Management. Stresses 2023, 3, 404-423. https://doi.org/10.3390/stresses3020030
Bencherif K, Laruelle F, Tisserant B, Dalpé Y, Lounés-Hadj Sahraoui A. Engineering Approach for Production of Arbuscular Mycorrhizal Inoculum Adapted to Saline Soil Management. Stresses. 2023; 3(2):404-423. https://doi.org/10.3390/stresses3020030
Chicago/Turabian StyleBencherif, Karima, Frederic Laruelle, Benoit Tisserant, Yolande Dalpé, and Anissa Lounés-Hadj Sahraoui. 2023. "Engineering Approach for Production of Arbuscular Mycorrhizal Inoculum Adapted to Saline Soil Management" Stresses 3, no. 2: 404-423. https://doi.org/10.3390/stresses3020030
APA StyleBencherif, K., Laruelle, F., Tisserant, B., Dalpé, Y., & Lounés-Hadj Sahraoui, A. (2023). Engineering Approach for Production of Arbuscular Mycorrhizal Inoculum Adapted to Saline Soil Management. Stresses, 3(2), 404-423. https://doi.org/10.3390/stresses3020030








