Abstract
According to the climate projections, drought will increase in frequency and severity. Since water stress (WS) impacts a grapevine’s physiology and yield negatively, the evaluation and selection of tolerant genotypes are needed. To analyse the WS effects on the morphology and cell division of three grapevines (Vitis vinifera L.) varieties, “Touriga Franca” (TF), “Touriga Nacional” (TN) and “Viosinho” (VS), in vitro-grown plants were exposed to 10% polyethylene glycol 6000 (PEG) (−0.4 MPa) or 20% PEG (−0.8 MPa), incorporated in the culture medium, for four weeks. Control plants were kept in culture media without PEG. The VS and TN plants showed the highest mean numbers of nodes, shoots and leaves and average mitotic indexes under 20% PEG. The TF and TN plants showed the lowest frequencies of mitotic anomalies under 10% PEG. The VS plant growth was less affected by WS, but TF and TN presented more regular mitosis under moderate WS. Globally, in vitro culture constitutes a cost-effective experimental system for studying grapevine responses to WS and the preliminary selection of resilient genotypes. These approaches could be applied to study plant responses to other abiotic stresses based on additional evaluation techniques (e.g., transcriptional analyses or genome-wide association studies).
1. Introduction
Vitis vinifera L. (grapevine) is a highly economically important fruit species cultivated worldwide, mainly for wine and grape production. It strongly supports the international agricultural sector of viticulture and their related trade [1,2,3]. Viticulture and wine production also have significant historical and socio–cultural roles [3]. However, viticulture faces new challenges related to the climate change response [3]. Beyond innovations regarding practices to improve the efficiency of water use, mitigation of the negative impacts of biotic and abiotic stresses and introduction of new technologies linked to precision viticulture, the improvement or use of varieties more adapted to changing environmental conditions should be pursued [3].
V. vinifera has been the target of several studies focusing on the influence of climate change on its growth, yield and wine quality [1]. Due to the broad area of grapevine cultivation, this fruit crop occupies growing regions with a high climatic diversity, mostly in temperate zones with a Mediterranean climate [1,2]. In a Mediterranean environment, grapevines are exposed to warm and dry summers [1]. Climate change and the forecasted environmental scenarios include a higher frequency of heat waves, drought episodes and erratic precipitation patterns, among other alterations [4]. To minimise yield loss and produce high-quality grapes under stressful conditions, beyond different agronomical practices and short-term measures to mitigate the consequences of abiotic stress, some wine producers have started to apply irrigation at controlled levels [1,3].
The grapevine has been considered tolerant to water scarcity due to an efficient stomatal control that prevents xylem cavitation in response to soil and air dehydration [5]. However, less than 10% of the vineyards in Europe have started to be irrigated to minimise the damage caused by climate change and economic losses [1]. Drought is one of the most relevant environmental factors affecting grapevine productivity and yield, as well as berry and wine quality [6].
Grapevine rootstocks are crucial in plant responses to water deficit by controlling the water and nutrient uptake and their transport to leaves [2,6,7,8]. Rootstocks also participate in stress perception and signalling in the shoot [9]. In recent decades, many studies have focused on various grapevine rootstocks’ differential response to water stress (WS) [2,6,7,8]. Globally, these authors verified that the differential drought tolerance of the grapevine rootstocks depends on the following individual factors and their interaction, namely, the (i) genotype of the rootstock, (ii) genotype of the scion, (iii) rootstock/scion combination and (iv) local environment. Grafting has been a horticultural practice in grapevine since the 19th century to avoid the susceptibility of European varieties to phylloxera [6]. The grafted vines constitute the fusion of the root and shoot systems of two different genotypes, the rootstock and the scion, combining their benefits [8]. Additionally, recent studies have demonstrated that the rootstock’s genotype influences the gene expression patterns in the scion leaves, revealing that the differential responses to drought could vary among tissues, phenological stages and/or regions. Due to the interaction of various factors and incoherence of transcriptional results among different varieties, rootstocks and local environments (that change over time) with distinct irrigation methods, the way in which grafting modulates the phenotypic response to drought or other particular abiotic stress remains an open question [8]. Beyond transcriptomics, physiological and biochemical approaches are among the most used to analyse the drought tolerance of grapevine rootstocks [2,6,7,8]. Studying plant responses to a particular abiotic stress under controlled environments might provide more specific and coherent results. Additionally, contrary to the knowledge about the differential drought tolerance among rootstocks, the WS responses among grapevine varieties used as scions have barely been studied. Considering that most commercial vines are grafted, particular attention should be paid to the differential WS tolerance among the varieties used as scions. Using more resilient genotypes such as elite grapevine scions and drought-tolerant rootstocks would benefit viticulture under the climate change scenario. Therefore, it is essential to test approaches that could allow the fast selection of more resilient grapevine genotypes. Plants grown under greenhouse or in vitro culture conditions were previously used to analyse the tolerance to high temperature or the differential response among grapevine varieties to induced heat stress [10,11,12,13]. This experimental system is cost-effective and allows the analysis of many plants in a small space. Beyond including any stressful agent in the culture medium, changing individual abiotic factors within a growth chamber enables the detection of differential plant responses to the induced abiotic stress among the analysed genotypes [10,11,13]. Like all plants, grapevine has cellular, physiological, metabolic and molecular strategies to deal with adverse environmental conditions [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Although in vitro cultured plants may suffer phenotypic changes, the monitoring of morphological traits related to the vegetative growth of plants under abiotic stress and the evaluation of cytogenetic parameters through comparison with control plants (grown under favourable conditions) may provide insights about the plant’s stress response, and ultimately about its tolerance.
Cytogenetic parameters such as mitotic index (MI) and percentage of dividing cells with anomalies (DCA) have been commonly used to assess the impact of abiotic stress in the growing organs such as leaves and roots, and in the cell cycle regularity [10,11,15]. The leaf mitotic cell cycle, if disturbed due to the occurrence of (natural or induced) abiotic stress, will delay the mitosis and generate abnormal dividing cells, impairing cell differentiation and growth [10,15]. Reducing leaf growth will decrease the photosynthetic area and negatively impact the plant’s productivity [10,15]. The MI parameter is expressed as a percentage and corresponds to the frequency of dividing cells or the rate of cell division, estimated by the ratio between the sum of mitotic cells in prophase, metaphase, anaphase and/or telophase and the total number of scored cells. The total number of scored cells corresponds to the sum of dividing (mitotic) and non-dividing (interphase) cells. The DCA parameter is also expressed in percentage, which results from the ratio between the sum of dividing cells (in the different mitotic phases) with anomalies and the total of scored mitotic cells (normal and irregular).
The Portuguese autochthonous “Touriga Franca” (TF) and “Touriga Nacional” (TN) red wine-producing varieties, as well as the white wine-producing variety “Viosinho” (VS), are officially recommended for ‘Douro’ and/or ‘Porto’ wine production [19,20]. These three varieties are traditionally cultivated in the ‘Douro Demarcated Region’ (DDR) and integrated into the UNESCO World Heritage List. The TN variety, grown from the north to south of Portugal and in the Azores, has good agronomic performance, such as high vigour and fertility and a from medium to high yield [21]. Despite being reported as susceptible to HS and WS [21], some studies based on different approaches have shown its tolerance to these abiotic stresses [10,11,12,13,14,15,16,17,18]. Due to its broader cultivation in Portugal, TN has been the target of various stress studies based on different approaches [10,11,12,13,14,15,16,17,18].
The TF variety presents from medium to high yield, from low to medium fertility, and regular productivity [22]. It is solely reported as resilient to abiotic factors [22]. This assumption is confirmed by its wide cultivation in the DDR, where it faces hot and dry summers and water deficit conditions [20].
The autochthonous VS variety represents only 1% of the Portuguese production area. It has been reported as robust to biotic and abiotic stresses and tolerant to dry and fertile soils and to soils with good drainage [23]. The VS variety has a regular but low productivity and median fertility [23].
Under controlled conditions, the plant stress responses could be more pronounced than in natural environments [13]. This fact could be useful for a preliminary selection of more resilient grapevine genotypes under controlled conditions. Therefore, in this work, we aimed to evaluate the morphological and cytogenetic responses of in vitro-grown plants of the grapevine varieties TF, TN and VS after exposure to a moderate and severe WS, induced by the incorporation of 10% and 20% polyethylene glycol (PEG) solutions, respectively, in the culture media, during four weeks, to extrapolate about their differential WS tolerance. Additionally, we aimed to infer the suitability of in vitro culture as an experimental system for plant stress studies.
2. Results
2.1. Evaluation of the Morphological Traits
Regardless of the variety, the induced WS hampered the rooting of the grapevine plants in the 10% and 20% PEG culture media. Only grapevine plants growing in the control culture medium (0% PEG) were rooted (Figure 1a). The absence of roots in the grapevine plants grown in the PEG-containing culture media impaired the realisation of the statistical test two-way ANOVA, and the number of roots in the control medium was not registered.

Figure 1.
In vitro grapevine plants at four weeks old installed in the (a,b) control culture medium (0% PEG) where one of the plants showed rooting (a), and culture media containing (c) 10% PEG and (d) 20% PEG. In the culture media containing PEG, leaf chlorosis was evident.
Incorporating PEG into the Murashige and Skoog (MS) culture medium [24] avoided its complete polymerisation. To maintain the explants and/or regenerated plants above the medium surface, autoclaved filter papers surrounding them were included in the 10% and 20% PEG media (Figure 1c,d).
After four weeks of WS induction, the number of nodes, shoots, and leaves was registered in plants of all varieties and culture media (Table 1). From this point onwards, different cultural media will be named PEG treatments.

Table 1.
Mean (± standard error, S.E.) values of the morphological traits, number of nodes, number of shoots and number of leaves, determined per grapevine variety (V), PEG treatment (T) and V × T interaction. Different lowercase letters per column represent statistically significant differences (p < 0.05) among V, T and V × T interactions. Notes: TF—“Touriga Franca”; TN—“Touriga Nacional”; VS—“Viosinho”; PEG—Polyethylene glycol.
The VS variety showed the highest mean values of nodes, shoots and leaves, which differed significantly (p ˂ 0.05) from the other varieties (Table 1).
The control culture medium (0% PEG) presented the highest average numbers of nodes, shoots and leaves (Table 1). The mean values of these morphological traits decreased significantly (p ˂ 0.05) in the culture media containing PEG relative to the control medium (Table 1).
Statistically significant differences (p ˂ 0.05) among the ‘variety (V) × PEG treatment (T)’ interactions were also found. The highest average numbers of nodes, shoots and leaves were detected in the ‘Viosinho × Control’ interaction, which showed statistically significant differences (p ˂ 0.05) relative to the remaining V × T interactions (Table 1). Except for the ‘TN × T’ interactions, the control medium’s mean number of nodes, shoots and leaves showed a significant (p ˂ 0.05) reduction in the culture media containing PEG (Table 1). In the particular case of the ‘TN × 20% PEG’ interaction, the mean numbers of nodes, shoots and leaves were significantly higher (p ˂ 0.05) than those shown by the ‘TN × Control’ interaction (Table 1). The morphological results achieved in the ‘V × T’ interactions revealed that the PEG-induced WS negatively impacted the vegetative growth and development of the TF and VS varieties. However, the mean values of the three morphological traits determined for the ‘VS × 20% PEG’ and ‘TN × 20% PEG’ interactions did not differ statistically (p > 0.05) (Table 1).
The WS induction experiment was interrupted after four weeks due to the high frequency of regenerated leaves with chlorosis and the death of almost 50% of the plants from all varieties installed in the culture media containing 20% PEG.
2.2. Analysis of the Leaf Mitotic Cell Cycle
The cytogenetic evaluation of the leaf cell cycle was based on a variable number of cells scored in at least 50 fields microscopically observed per mitotic preparation. The number of counted cells was highly dependent on the quality of the mesophyll cell suspensions and mitotic preparations. Therefore, the cytogenetic data resulted from scoring a total of 5290, 4607 and 4129 cells (interphase and mitotic) in the TF, TN and VS varieties, respectively. Additionally, 5171, 4115 and 4740 cells (interphase and mitotic) were scored in the 0% PEG (control), 10% PEG and 20% PEG treatments.
Based on the cell scoring, the cytogenetic parameters, mitotic index (MI) and dividing cells with anomalies (DCA) were determined (Table 2).

Table 2.
Mean (± S.E.) percentage values of the cytogenetic parameters, MI and DCA, determined per grapevine variety (V), PEG treatment (T) and V × T interaction. Different lowercase letters per column represent statistically significant differences (p < 0.05) among V, T and V × T interactions. Notes: TF—“Touriga Franca”; TN—“Touriga Nacional”; VS—“Viosinho”; MI—mitotic index; DCA—dividing cells with anomalies; PEG—Polyethylene glycol.
The average percentage values of mitotic index (MI) and dividing cells with anomalies (DCAs) showed statistically significant differences (p ˂ 0.05) among the three grapevine varieties (V), PEG treatments (T) and their interaction (V × T) (Table 2).
In plant stress studies based on a cytogenetic approach, the interpretation of the MI results should be performed in parallel with those of the DCA parameter since, for the MI calculation, the number of irregular dividing cells is considered (see Equation (1)). Therefore, a high average MI could correspond to a high DCA mean value, constituting the percentage of irregular mitotic cells in the total number of scored mitotic cells (see Equation (2)).
The high mean value of MI found in the VS variety differed significantly (p ˂ 0.05) from the other two varieties (Table 1). Nonetheless, the average DCA values of the TN and VS varieties were significantly (p ˂ 0.05) lower than those of the TF (Table 1).
Concerning the PEG treatments, the highest mean values of MI were detected in the 10% PEG and 20% PEG treatments, which showed statistically significant differences (p ˂ 0.05) relative to the control (Table 2). As expected, the highest mean DCA value was registered in the 20% PEG treatment (severe WS), which showed statistically significant differences (p ˂ 0.05) relative to the control (Table 2). The lowest average values of DCA were registered in the control (0% PEG) and 10% PEG treatments without statistical significance (p > 0.05) between them (Table 2). Mitotic irregularities in the grapevine plants kept in the control medium (0% PEG) could be explained by the mother plants’ internal stresses or the explant’s adaptive response to the in vitro installation.
Considering the ‘V × T’ interactions, the highest average values of MI were shown by the ‘VS × 20% PEG’ and ‘VS × 10% PEG’ interactions (Table 2). The ‘VS × 20% PEG’ interaction showed the highest DCA value, which differed significantly (p ˂ 0.05) from the remaining ‘V × T’ interactions (Table 2). The lowest average values of DCA were determined for the ‘VS × Control’, ‘TN × 10% PEG’ and ‘TF × 10% PEG’ interactions and did not show statistically significant differences (p > 0.05) among them (Table 2).
Regardless of the grapevine variety and PEG treatment, the silver-stained interphases showed one to two nucleoli per nucleus, as demonstrated in Figure 2a. No irregular interphase cells were found.
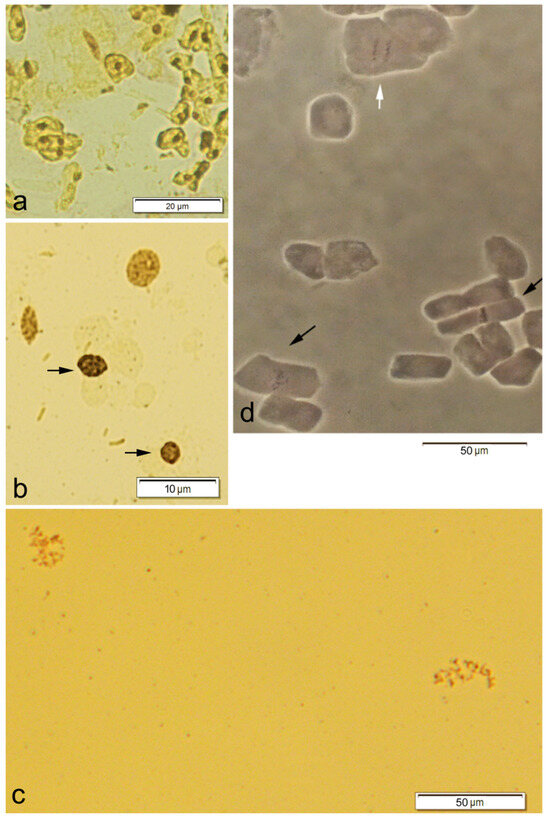
Figure 2.
Interphase nuclei (a) and dividing cells in different mitotic phases (b–d) of grapevine (2n = 2x = 38) after silver nitrate staining (a–c) or observed on the phase contrast microscope (d), showing (a) one to two nucleoli per interphase nucleus; (b) four prophase nuclei, two of which have chromatin stickiness (arrows); (c) one irregular anaphase cell with chromosomal misorientation and laggard chromosomes (on the left) and a C-mitosis, (on the right); (d) a normal anaphase (white arrow) and two metaphase cells (black arrows), one of which exhibits chromosomal misalignment (black arrow on the left).
Most normal and irregular mitotic cells were in prophase (Figure 2b; Table 3 and Table 4). Normal and irregular metaphase and/or anaphase cells were also observed (Figure 2c,d; Table 3 and Table 4). In this work, no normal or irregular telophase cells were observed.

Table 3.
Mean (± S.E.) number of normal dividing cells in different mitotic phases, determined per grapevine variety (V), PEG treatment (T) and V × T interaction. Different lowercase letters per column represent statistically significant differences (p < 0.05) among V, T and V × T interactions. Notes: TF—“Touriga Franca”; TN—“Touriga Nacional”; VS—“Viosinho”; PEG—Polyethylene glycol.

Table 4.
Mean (± S.E.) number of irregular dividing cells in different mitotic phases, determined per grapevine variety (V), PEG treatment (T) and V × T interaction. Different lowercase letters per column represent statistically significant differences (p < 0.05) among V, T and V × T interactions. Notes: TF—“Touriga Franca”; TN—“Touriga Nacional”; VS—“Viosinho”; PEG—Polyethylene glycol.
The irregular prophase cells showed a single type of anomaly, namely, sticky and highly condensed chromatin, which was easily identified by its smaller size and higher staining than that detected in the normal ones (Figure 2b).
The irregular metaphase cells presented anomalies such as C-mitosis (Figure 2c) or chromosomal misalignment (Figure 2d). The irregular anaphase cells showed chromosomal misorientation and laggard chromosomes (Figure 2c).
The mean numbers of normal prophase, metaphase and/or anaphase cells differed significantly (p ˂ 0.05) among varieties, PEG treatments and their interactions (Table 3).
The highest average values of normal prophase were detected in the VS and TF varieties, which differed significantly (p ˂ 0.05) from TN (Table 3). Normal anaphase cell frequency was reduced in the TN variety and 10% PEG treatment (Table 3). Consequently, the mean number of normal anaphase cells showed statistically significant differences (p ˂ 0.05) among varieties, PEG treatments and V × T interactions (Table 3).
Table 4 presents the mean number of irregular mitotic cells determined for each grapevine variety, PEG treatment and V × T interaction.
Regardless of the mitotic phase, the mean number of irregular dividing cells (Table 4) was higher than that of the normal dividing cells (Table 3). The average number of irregular prophase and metaphase cells showed statistically significant differences (p ˂ 0.05) among varieties, PEG treatments and their interactions (Table 4). The mean number of irregular anaphase cells presented significant differences (p ˂ 0.05) among the PEG treatments and ‘V × T’ interactions (Table 4).
The two highest mean numbers of irregular prophase cells were determined for the TF and VS varieties, control and 20% PEG treatments, and ‘TN × Control’ and ‘TF× 20% PEG’ interactions (Table 4). For each variety, PEG treatment and V × T interaction, the mean value of irregular metaphase cells was higher than that of irregular anaphase cells (Table 4). The highest mean numbers of irregular metaphase cells were registered in the TN variety, 20% PEG treatment and ‘TN × 20% PEG’ interaction (Table 4).
Except for the ‘TF × 10% PEG’ and ‘VS × 10% PEG’ interactions, irregular anaphase cells were observed in all grapevine varieties and PEG treatments (Table 4). The mean values of irregular anaphase cells showed statistically significant differences (p ˂ 0.05) among varieties (V), treatments (T) and V × T interactions (Table 4).
Considering the absence of telophase cells in all grapevine varieties and PEG treatments, the detection of anaphase cells represented the highest progression in the leaf mitotic cell cycle. Hence, the highest average numbers of irregular anaphase cells were detected in the TN variety, control (0% PEG) treatment, ‘TF × 20% PEG’ and ‘TN × 20% PEG’ interactions (Table 4).
3. Discussion
This work aimed to evaluate the morphological and cytogenetic responses of in vitro-grown plants from the grapevine varieties TN, TF and VS to the induced moderate and severe WS and to infer the feasibility of in vitro culture as an experimental system for plant stress studies.
To induce moderate and severe WS, 10% PEG and 20% PEG solutions were incorporated into the culture medium. After four weeks of WS induction, the plants were evaluated at the morphological and cytogenetic levels.
Both morphological and cytogenetic data evidenced a differential response to the PEG-induced WS among the analysed grapevine varieties. By comparison with the control plants, the number of nodes, shoots and leaves decreased significantly with the increase in PEG concentration in the culture medium. A reduction in plant growth and development under water deficit is expected for grapevines and other plant species.
The highest mean values of each morphological trait were mainly detected in the ‘VS × 20% PEG’, ‘VS × 10% PEG’ and ‘TN × 20% PEG’, suggesting that the moderate and/or severe WS did not negatively affect the vegetative growth of VS or TN.
Under severe and prolonged WS, the control of the photosynthetic rate by the stomata loses effectiveness, and the mesophyll metabolism is affected [5]. The decreased synthesis and activity of the ribulose biphosphate (Rubisco) enzyme reduces the photosynthetic capacity and plant productivity [5]. Also, reducing the leaf number and area in response to water scarcity negatively affects the photosynthetic capacity. This work showed that the PEG-induced WS reduced the mean number of leaves, nodes and shoots relative to the control. Despite the in-depth understanding of the WS consequences at the physiological and biochemical levels, less attention has been paid to the cellular effects. Considering that plant growth and development are highly correlated with cell division, this study was expected to determine higher leaf MI values in the varieties with the highest vegetative growth. The highest mean values of MI were registered in the ‘VS × 20% PEG’, ‘VS × 10% PEG’ and ‘TN × 20% PEG’ interactions. Hence, the MI indexes achieved in VS and TN corroborated their morphological results. Nevertheless, the lowest mean values of DCA were determined for the ‘TN × 10% PEG’ and ‘TF × 10% PEG’ interactions, suggesting a lesser impact of the moderate WS on the regularity of the leaf mitotic cell cycle. The TF and TN varieties are widely cultivated in the DDR, evidencing tolerance to extreme climatic conditions, particularly during the summer [15,16,17]. As demonstrated earlier, the leaf cell cycle of various grapevine varieties is negatively affected by induced or naturally occurring stresses [10,15]. Oxidative stress is a physiological consequence of any abiotic stress, and it causes the increase and accumulation of reactive oxygen species (ROS) [15]. The DNA damage caused by oxidative stress generates breaks in the DNA strands, leading to chromosomal anomalies [15]. Some of those anomalies are irreversible, such as the chromatin stickiness [15], which was detected in this work in prophase cells of the three analysed grapevine varieties. Under stressful conditions, the leaf cell division is delayed due to the activation of cell cycle checkpoints [10,15]. During this checking, the cell division could be arrested in prophase or metaphase [10,15]. The highest mean values of prophase and metaphase cells confirmed this assumption. Regardless of the grapevine variety or PEG treatment, no normal or irregular telophase cells were observed, suggesting cell cycle arresting in anaphase. This result may be due to internal stresses occurring in the mother plants sampled in the vineyard or an adaptive response to the in vitro culture.
The hampering of leaf cell cycle progression and its reduced regularity under WS justifies the reduced number of nodes, shoots and leaves in the PEG treatments relative to the control. In addition, plants of all varieties exposed to PEG did not develop roots, impairing the nutrient uptake from the medium culture and leading to a decrease in plant growth, development and photosynthetic activity.
Despite the difficulty in simulating the abiotic factors interacting in natural environments [25] and the continuous change in climate conditions, researchers attempt to mimic and induce individual or combined abiotic stresses to evaluate the plant responses under controlled conditions using different approaches [10,11,12,18]. Resilient genotypes assessed by experimental systems developed under controlled conditions would certainly withstand extreme abiotic factors in open field conditions and have the potential to be genetically improved [12]. One proof of this assumption is the fact that the previous evaluation of the leaf mitotic cell cycle in TF and TN plants growing in the DDR under summer stress (combination of high temperature and radiation, and water deficit) revealed results similar to the ones achieved in this work [15]. Under stressful summer conditions, TF and TN plants growing in the DDR showed cell cycle arrest in prophase and metaphase, as well as chromosomal and cell cycle anomalies [15]. Based on cytogenetic, biochemical, and molecular analyses related to cell cycle regulation and antioxidant response, it was verified that, under natural conditions, TN is more tolerant to stressful summer conditions than TF [15]. The present morphological and cytogenetic results reinforced the previously reported tolerance of the TN variety to water deficit [14,18]. Additionally, such coincidence of results supports the reliability of in vitro culture as an experimental system for plant stress studies.
The VS variety was studied less regarding abiotic stress responses than the TN and TF varieties. The present results revealed that the induced WS did not significantly affect the VS plant growth. However, this variety showed higher frequencies of cellular anomalies under moderate WS than the TF and TN varieties.
The approaches used in this work are versatile since they could be applied to other grapevine varieties or plant species or used to study plant responses to different individual or combined abiotic stresses. Considering the grapevine responses to WS, the approaches used in this work could constitute complementary or preliminary methods to the ones developed under field conditions since they enable the faster analysis of a higher number of plants or varieties in a smaller space and are not dependent on the season or phenological stage of the plant. Additionally, the morphological and cytogenetic evaluation of in vitro-grown plants under induced WS can provide additional data on the stress response not pursued in ecophysiological studies performed under open-field conditions. However, future studies involving in vitro-grown grapevine plants towards the in-depth knowledge of WS responses for a further selection of resilient genotypes might include (i) a known WS-sensible grapevine genotype; (ii) a higher number of in vitro-grown plants under induced WS through the use of PEG solutions with additional concentrations and collection of plant material in different time points through the experiment, to enable (iii) physiological and biochemical characterisations related to the membrane stability index, lipid peroxidation, antioxidant defence, osmoregulation; (iv) molecular analyses of WS-responsive genes by quantitative real-time PCR or RNASeq; the (v) integration of molecular and phenotypic data using genome-wide association studies (GWAS).
With the fast advance of climate change and its adverse impacts on viticulture, which constitutes an essential economic activity worldwide, a quick selection of more resilient genotypes is demanded. The induction of WS or other abiotic stress to in vitro-grown plants could produce more exacerbated responses than under natural conditions. Detecting differential stress responses among grapevine varieties using the in vitro experimental system could provide helpful information for nurseries, vine producers, or plant breeders. Using WS-tolerant grapevine varieties as elite scions in WS-tolerant rootstocks might allow high agronomic performance in the dryer and warmer growing regions.
4. Materials and Methods
4.1. Sampling of Plant Material
The analysed grapevine varieties, TF, TN and VS, are cultivated at the “Quinta N. Srª. de Lurdes”, located within the UTAD Campus (41°17′18.13″ N; −7°44′21.94″ W), in the North of Portugal. The vines are grafted into the 1103 Paulsen (Vitis berlianderi × Vitis rupestris) rootstock.
During the ecodormancy period, 40 cm-long canes were cut from vines that were 20 years old. Three canes corresponding to three different grapevine varieties were analysed per grapevine variety.
The pruned canes were stored at 4 °C for three months to break bud dormancy.
4.2. Shoot Forcing, Culture Media Preparation and Explant Installation for WS Induction
The canes were segmented into cuttings containing six nodes and placed in distilled water at room temperature for four weeks to force the shoot development (Figure 3a).

Figure 3.
(a) Grapevine canes after shoot development forced in distilled water at room temperature; (b) shoots installed in MS medium; (c) cutting with a single stem node installed in MS medium without PEG (control medium).
In this work, three culture media were prepared. The basal MS culture medium [24], without phytohormones, supplied with 2% (w/v) sucrose and 7.5 g.L−1 of agar, was common to the three media. The MS medium without PEG (0% PEG) was used as a control. Two filtered aqueous solutions of 10% (w/v) or 20% (w/v) polyethylene glycol (PEG) 6000, corresponding to the water potentials of −0.4 MPa (−4 bar, moderate WS) or −0.8 MPa (−8 bar, severe WS), respectively, were added to the solid basal MS medium. The pH of the three-culture media was set to 5.6. All the culture media were autoclaved at 121 °C for 15 min at 1 bar. Before completely cooling the culture media, within the laminar flow cabinet, around 15 mL of each culture medium were distributed through glass flasks that were previously sterilised at 170 °C for 1 h 30 min.
The newly developed shoots were disinfected by washing them in running tap water for 15 min, followed by incubation in 70% ethanol for 1 min. Then, in the laminar flow cabinet, the explants were immersed in an aqueous solution of 40% sodium hypochlorite (bleach) for 20 min and washed three times in autoclaved distilled water for 15 min. During the disinfection step, the shoots developed from different pruned canes (corresponding to different plants) were maintained separately.
Within the laminar flow cabinet, the disinfected shoots were installed in solid basal MS culture medium, without phytohormones or PEG, and placed within a growth chamber, the Fitoclima ‘Walk-in’ Model 20000 E (Aralab), with a 16 h photoperiod and a light intensity of 300 µmol.m−2.s−1, for one month (Figure 3b).
After this period, the shoots were excised to obtain cuttings with a single stem node (Figure 3c) and transferred to the MS culture media containing 10% or 20% PEG and to the control medium (0% PEG).
Ninety explants per grapevine variety were used. The 90 explants were distributed by the three solid culture media: MS without PEG (control medium, 0% PEG), MS + 10% PEG and MS + 20% PEG. Hence, 30 explants of each variety were installed per culture medium. These 30 explants per variety correspond to three plants (three biological replicates) and 10 technical replicates (n = 10).
The TF, TN and VS explants, installed in the three culture media, were placed within a growth chamber with a 16 h photoperiod and a light intensity of 300 µmol.m−2.s−1 for four weeks.
4.3. Monitoring of the Morphological Traits
In all grapevine varieties and PEG treatments, the morphological traits, the number of nodes, the number of shoots and the number of leaves were registered weekly. The morphological characteristics were registered within the growth chamber without removing the explants from the glass flasks to avoid contamination. This analysis ended after four weeks of exposure to PEG due to extensive leaf chlorosis and death of almost 50% of the explants in the culture media with 20% PEG, imposing the end of the experiment.
4.4. Analysis of the Leaf Cell Cycle
Due to the absence of rooting in all grapevine varieties cultured in the media supplied with 10% PEG and 20% PEG after four weeks of WS induction, the cell cycle was analysed in the newly regenerated leaves.
Within the laminar flow cabinet, the explants were placed on autoclaved papers, and the leaves were collected and immediately fixed in absolute ethanol and acetic acid solution in the proportion of 3:1 (v/v). The fixed leaves were maintained at −20 °C till the preparation of mesophyll cell suspensions.
The fixed leaves were enzymatically digested to prepare suspensions of dividing mesophyll cells following [10]. A volume of 30 µL of each cell suspension was dropped on an ethanol-cleaned glass slide that was air-dried in a horizontal position and aged for 2 h at 60 °C. The mitotic preparations were treated in 2× standard saline buffer (SSC) for 5 min at 55 °C and then stained with 100% silver nitrate for 8 min at 60 °C as described earlier [10].
Cell scoring was performed using an optical microscope. At least 50 microscopic fields were observed per preparation and used for cell scoring. Since the number of cells is variable per microscopic field and depends on the cell suspensions and quality of the mitotic preparations, a variable number of interphase and dividing cells per grapevine and PEG treatment were scored.
The mitotic phases and anomalies were identified. These data were used for the determination of the cytogenetic parameters, mitotic index (MI) (Equation (1)) and dividing cells with anomalies (DCAs) (Equation (2)), both expressed as percentages:
The total number of scored cells in Equation (1) corresponds to the sum of interphase and mitotic (dividing) cells.
The cell images were captured on an epifluorescence microscope Olympus BX41 (Olympus America, Inc., Hauppauge, NY, USA) coupled with a digital XC10 charge-coupled device (CCD) camera (Olympus America, Inc., Hauppauge, NY, USA), using bright field, a magnification of 2000× (resulting from the use of the 100× objective, a 10× eyepiece and a magnifier set to 2×), and the cellSens ver. 1.18 imaging software (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
4.5. Statistical Analyses
The morphological and cytogenetic data are presented as mean ± standard error values. The mean values arose from three replicates (n = 3) that constituted, simultaneously, biological and technical replicates.
The three culture media will be presented as PEG treatments throughout the manuscript. Therefore, to analyse the individual and interacting influence of the grapevine variety (V) and PEG treatment (T) variables on the morphological and cytogenetic results, two-way analyses of variance (ANOVA), the post hoc Fisher’s protected least significant difference (PLSD) test and the equality of variances F test were performed.
To compare the mean values achieved among the V × T interactions, the One Sample t-test was performed. All statistical tests were performed with the software StatView 5.0 (SAS Institute, Inc., Copyright ©1992–1998, Cary, NC, USA) and the significance level was established for a probability lower than 5% (p < 0.05).
5. Conclusions
In conclusion, the results achieved in this work demonstrated that VS plant growth was less affected by the induced moderate and severe WS. However, the TF and TN varieties withstand better with moderate and severe WS induction than VS at the leaf cell division. The detection of the lowest frequency of mitotic anomalies in the TF and TN varieties implies that the induced WS did not negatively affect the leaf growth. The negative impacts of abiotic stresses on leaf growth directly influence the photosynthetic area and, ultimately, plant yield. Overall, among the three analysed grapevine varieties, based on the morphological and cytogenetic evaluations performed, the TN variety was demonstrated to be the most tolerant to the induced WS. Furthermore, this work demonstrated the feasibility of using in vitro culture as a cost-effective and experimental system for studying the effects of induced WS on the plant morphology and leaf cell division of grapevine. Furthermore, this experimental system could allow a fast and preliminary selection of grapevine genotypes that are more tolerant to WS to be used in the field or as a target of genetic breeding programs.
In vitro culture constitutes a versatile experimental system since it could be adapted to the study of plant responses to other abiotic stresses based on different techniques, such as transcriptional analyses of stress-responsive genes by quantitative real-time PCR, RNASeq or GWAS to integrate the data gathered by the phenotype and genotype evaluations.
Author Contributions
Conceptualization, A.C. and J.L.-B.; methodology, A.C., F.L., C.C., A.C. and J.L.-B.; investigation, A.C. and J.L.-B.; resources, A.C., F.L. and J.L.-B.; writing—original draft preparation, A.C. and C.C.; writing—review and editing, all authors; supervision, A.C. and J.L.-B.; funding acquisition, A.C., F.L. and J.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds provided by Portugal 2020 under the project INNOVINE&WINE—Vineyard and Wine Innovation Platform (NORTE-01-0145-FEDER-000038, research line Viticulture) and postdoctoral grant BPD/UTAD/INNOVINE&WINE/593/2016 (attributed to author A.C.); and by national funds provided by the Portuguese Foundation for Science and Technology (“Fundação para a Ciência e a Tecnologia”—FCT) to CITAB under the project UIDB/04033/2020 (https://doi.org/10.54499/UIDB/04033/2020).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The author Ana Carvalho thanks the funding attributed by the Portuguese Foundation for Science and Technology (“Fundação para a Ciência e a Tecnologia”—FCT) with the reference DL 57/2016/CP1378/CT0003 (doi:10.54499/DL57/2016/CP1378/CT0003), which allowed her to be hired by UTAD as a doctorate researcher in the scope of the D.L. no. 57/2016 and Law no. 57/2017.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Bonarota, M.-S.; Toups, H.S.; Bristow, S.T.; Santos, P.; Jackson, L.E.; Cramer, G.R.; Barrios-Masias, F.H. Drought response and recovery mechanisms of grapevine rootstocks grafted to a common Vitis vinifera scion. Plant Stress 2024, 11, 100346. [Google Scholar] [CrossRef]
- Jordão, A.M. Introductory Chapter: New challenges and innovations in grape and wine production. In Recent Advances in Grapes and Wine Production—New Perspectives for Quality Improvement; Jordão, A.M., Botelho, R., Miljić, U., Eds.; IntechOpen: Rijeka, Croatia, 2023; ISBN 978-1-80356-325-1. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) 2022; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; 3056p. [Google Scholar]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Bianchi, D.; Ricciardi, V.; Pozzoli, C.; Grossi, D.; Caramanico, L.; Pindo, M.; Stefani, E.; Cestaro, A.; Brancadoro, L.; De Lorenzis, G. Physiological and transcriptomic evaluation of drought effect on own-rooted and grafted grapevine rootstock (1103P and 101-14MGt). Plants 2023, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Simeoni, F.; Galbiati, M.; Meggio, F.; Tonelli, C.; Scienza, A.; Espen, L. Grapevine rootstocks differently affect physiological and molecular responses of the scion under water deficit condition. Agronomy 2021, 11, 289. [Google Scholar] [CrossRef]
- Harris, Z.N.; Pratt, J.E.; Kovacs, L.G.; Klein, L.L.; Kwasniewski, M.T.; Londo, J.P.; Wu, A.S.; Miller, A.J. Grapevine scion gene expression is driven by rootstock and environment interaction. BMC Plant Biol. 2023, 23, 211. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.; Amâncio, S. Cutting the Gordian knot of abiotic stress in grapevine: From the test tube to climate change adaptation. Physiol. Plant. 2018, 165, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Leal, F.; Matos, M.; Lima-Brito, J. Effects of heat stress in the leaf mitotic cell cycle and chromosomes of four wine-producing grapevine varieties. Protoplasma 2018, 255, 1725–1740. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Leal, F.; Matos, M.; Lima-Brito, J. Heat stress tolerance assayed in four wine-producing grapevine varieties using a cytogenetic approach. Ciencia Tec. Vitiv. 2019, 34, 61–70. [Google Scholar] [CrossRef]
- Nogales, A.; Ribeiro, H.; Nogales Bueno, J.; Hansen, L.D.; Gonçalves, E.F.; Coito, J.L.; Rato, A.E.; Peixe, A.; Viegas, W.; Cardoso, H. Response of mycorrhizal “Touriga Nacional” variety grapevines to high temperatures measured by calorespirometry and near-infrared spectroscopy. Plants 2020, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Crisóstomo, C.; Leal, F.; Lima-Brito, J. Selection of reference genes and HSP17.9A expression profiling in heat-stressed grapevine varieties. Genes 2024, 15, 1283. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Ramos, M.J.N.; Faísca-Silva, D.; van der Kellen, D.; Fernandes, J.C.; Egipto, R.; Lopes, C.M.; Amâncio, S. Developmental Regulation of transcription in Touriga Nacional berries under deficit irrigation. Plants 2022, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Dinis, L.-T.; Luzio, A.; Bernardo, S.; Moutinho-Pereira, J.; Lima-Brito, J. Cytogenetic and molecular effects of kaolin’s foliar application in grapevine (Vitis vinifera L.) under summer’s stressful growing conditions. Genes 2024, 15, 747. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field. Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Moutinho-Pereira, J.M.; Correia, C.M.; Gonçalves, B.M.; Bacelar, E.A.; Torres-Pereira, J.M. Leaf gas exchange and water relations of grapevines grown in three different conditions. Photosynthetica 2004, 42, 81–86. [Google Scholar] [CrossRef]
- Cabral, I.L.; Teixeira, A.; Lanoue, A.; Unlubayir, M.; Munsch, T.; Valente, J.; Alves, F.; da Costa, P.L.; Rogerson, F.S.; Carvalho, S.M.P.; et al. Impact of deficit irrigation on grapevine cv. ‘Touriga Nacional’ during three seasons in Douro region: An agronomical and metabolomics approach. Plants 2022, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Portaria no. 383/2017, Diário da República, 1.ª série–N.º 243–20 de Dezembro de 2017; Ministério da Agricultura, Florestas e Desenvolvimento Rural: Lisboa, Portugal, 2017; pp. 6659–6660. (In Portuguese)
- Portaria no. 380/2012, Diário da República, 1a Série—N.º 226—22 de Novembro de 2012; Ministério da Agricultura, do Mar, do Ambiente e do Ordenamento do Território: Lisboa, Portugal, 2012; pp. 6712–6715. (In Portuguese)
- Touriga Nacional—Vine and Wine Cluster, COLAB Vines & Wines, 7p. Available online: https://www.advid.pt/uploads/TourigaNacional_Final_25.10.pdf (accessed on 27 May 2024). (In Portuguese).
- Touriga Franca—Vine and Wine Cluster, COLAB Vines & Wines, 6p. Available online: https://www.advid.pt/uploads/TourigaFranca_Final_25.10.pdf (accessed on 27 May 2024). (In Portuguese).
- Viosinho—Vine and Wine Cluster, COLAB Vines & Wines, 5p. Available online: https://www.advid.pt/uploads/Viosinho_Final_25.08.pdf (accessed on 10 July 2024). (In Portuguese).
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).