Stress Responses and Mechanisms of Phytopathogens Infecting Humans: Threats, Drivers, and Recommendations
Abstract
:1. Introduction
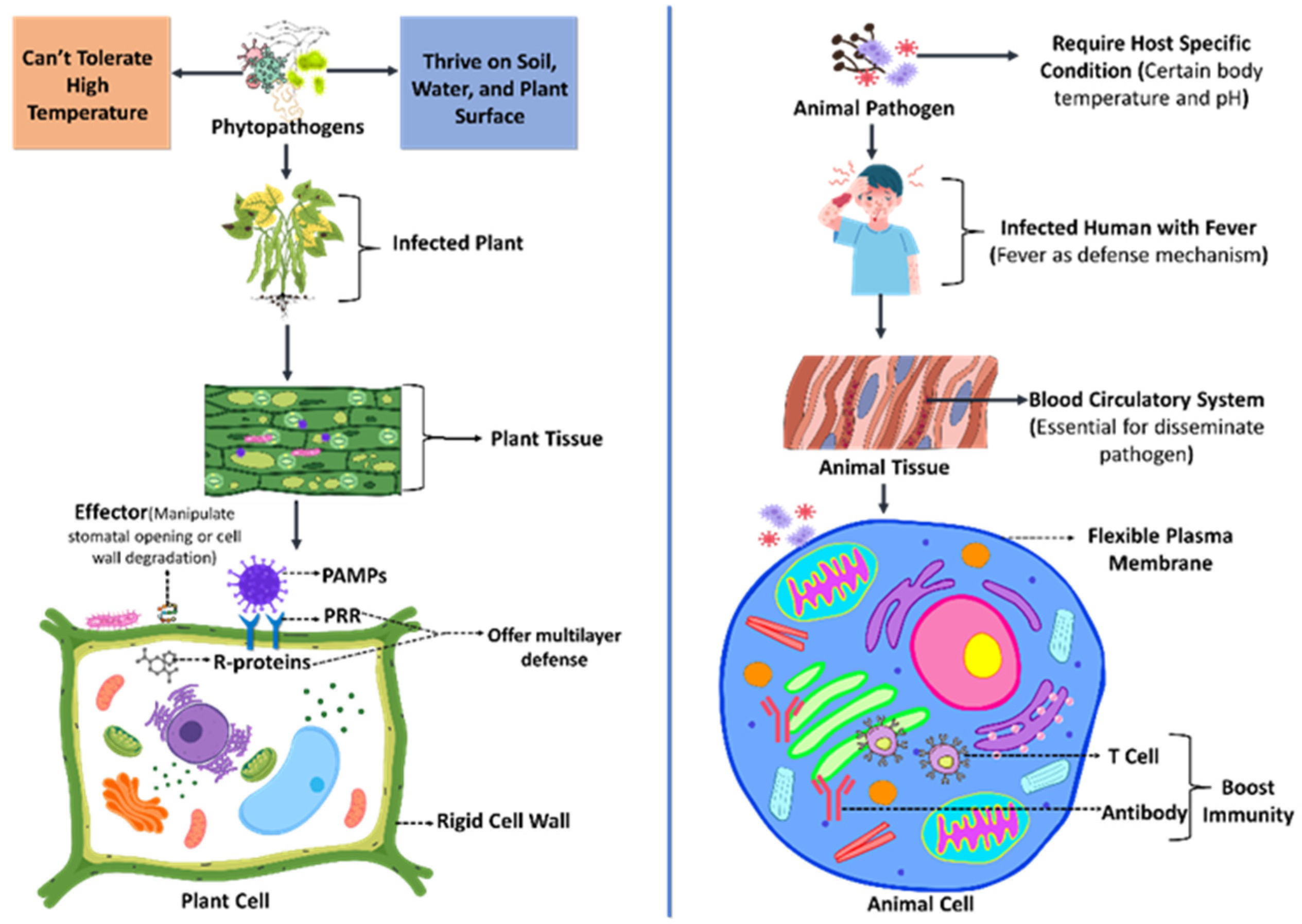
2. Natural Barriers Between Phytopathogens and Animal Pathogens to Prevent Spillover
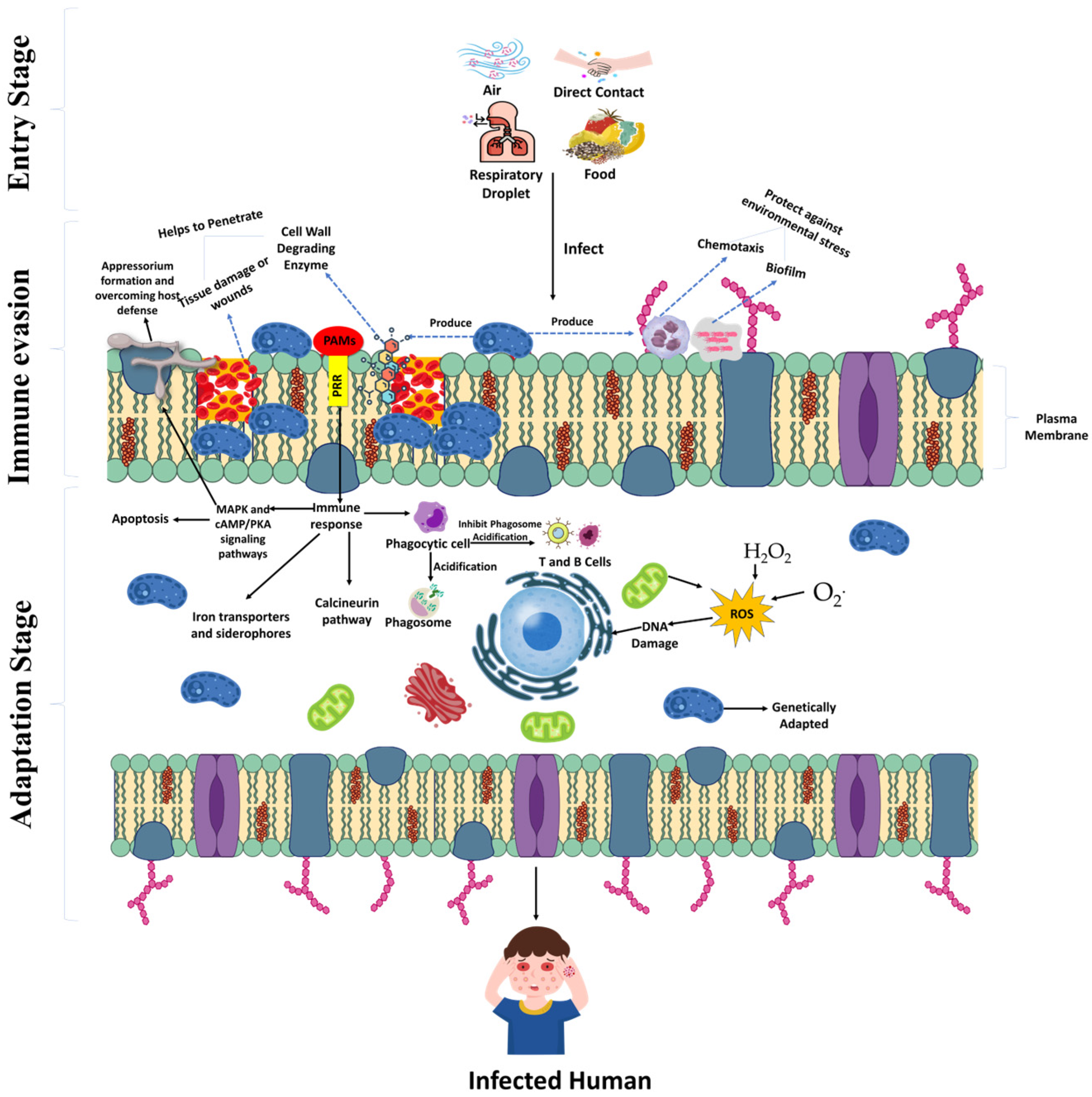
3. Requirements for Cross-Kingdom Pathogenesis
3.1. Breaching the Exterior Physical Barrier
3.2. Conquering the Principal Host Immunological Response
3.3. Oxidative Stress Adaptations
3.4. Nutritional Adaptation
3.5. Thermal Adaptation
3.6. Genetic Adaptations in Cross-Kingdom Infection
3.7. Environmental and Anthropogenic Involvement
4. Cross-Kingdom Pathogenicity and Implications for Health
4.1. Cross-Kingdom Pathogenesis by Fungi and Oomycetes
| Pathogen | Plant Symptoms | Plant Hosts | Clinical Manifestations | References |
|---|---|---|---|---|
| Alternaria infectoria, A. alternata | Blossom blight | Guayule | Phaeohyphomycosis, keratitis | [83,84] |
| Aspergillus fumigatus, A. flavus, A. niger, A. terreus | Ear rot, boll rot, yellow mold, black mold, fruit rot | Corn, cotton, peanut, onion, garlic, grapes, pomegranates, citrus | Pulmonary aspergillosis | [94,95] |
| Bipolaris spicifera, B. hawaiiensis, B. australiensis | Leaf spot, leaf blight, chlorosis, necrotic lesions | Sugarcane, switchgrass, buffalograss, bermudagrass | Surgical site infection, corneal ulcer | [88,90,92] |
| Colletotrichum truncatum | Lesion, blight, necrosis | Strawberry, citrus, cereals | Ophthalmic infection | [113,114] |
| Cladosporium allicinum, C. angustisporum, C. cladosporioides, C. flabelliforme, C. funiculosum, C. halotolerans, C. herbarum, C. macrocarpum, C. perangustum, C. ramotenellum, C. sphaerospermum, C. subinflatum, C. subuliforme | Leaf spot, blossom blight | Dendrobium, Echeveria, strawberry | Respiratory tract superficial fluid infection | [97] |
| Exserohilum rostratum | Leaf spot | Grasses and other monocots | Meningitis | [98,99,100] |
| Fusarium graminearum, F. proliferatum | Head blight, root rot | Tomato, tobacco, legumes, cucurbits | Blood infection | [113,115] |
| Fusarium solani | Foot and/or root rot, sudden death syndrome | Legumes, solanaceous plants | Onychomycosis, keratitis | [116,117] |
| Lasiodiplodia theobromae, L. hormozganensis | Dieback, stem end rot, seed rot, leaf blight, boll rot, basal stem rot | Mango, kenaf, snake plant, grape, papaya, cotton, castor bean | Keratitis, sinusitis, skin infections, phaeohyphomycotic cyst | [48,102,118,119,120,121] |
| Macrophomina phaseolina | Stem and root rot, charcoal rot, and seedling blight | Jute, beans, potato, sorghum, corn, wheat, sun | Endophthalmitis, skin infections, and skin–joint infections | [103,104] |
| Pythium aphanidermatum | Necrosis, rot | Soybean, cucurbits, cotton | Pythiosis | [110,111] |
| Rhizopus arrhizus (syn. oryzae) | Rhizopus rot | Apple, banana, mulberry, sweet potato, tobacco, tomato, forage grass | Mucormycosis | [105,106,107,122,123,124] |
4.2. Cross-Kingdom Pathogenesis by Bacteria
| Pathogen | Plant Disease | Plant Hosts | Clinical Manifestations | References |
| Agrobacterium tumefaciens (Rhizobium radiobacter) | Crown gall disease | Eudicots | Bacteremia, fetal death | [128] |
| Erwinia persinicus Erwinia billingiae | Soft rot, leaf spots, root rot, leaf wilting, fire blight | Perishable vegetables and fruits | Cervical lymphadenitis | [129,130,131,132,133,134,137] |
| Burkholderia cepacian, B. gladioli, B. glumae, B. cenocepacia | Gladiolus corms | Maize, onion, rice, tomato | Septicemia | [138,141,142,155] |
| Pantoea agglomerans Pantoea ananatis | Leaf spots, blotches | Fruit-bearing trees | Septicemia | [77,143,144,145] |
| Pseudomonas aeruginosa | Wilt, rot | Ginseng, wheat, maize, Arabidopsis | Malignant external otitis, endophthalmitis, endocarditis, meningitis, pneumonia, septicemia | [148,149,150,153,156] |
| Ralstonia pickettii | Leaf spot, leaf blight | Bird of Paradise | Bacteremia, neonatal sepsis, endocarditis, meningitis | [147,157] |
4.3. Cross-Kingdom Pathogenesis by Viruses
| Pathogen | Plant Symptoms | Plant Hosts | Clinical Manifestations | References |
|---|---|---|---|---|
| PMMoV | Chlorosis, mottling | Pepper | Fever, abdominal pains | [159,160,161,162,163] |
| TMV | Stunting, yellowing | Tobacco, tomato | Pulmonary diseases | [162,164,166] |
| Xiphinema brevicollum | Root damage (dagger nematode) | Tropical fruits, ornamental plants | Severe abdominal pain | [173,174,175] |
4.4. Cross-Kingdom Pathogenesis by Nematodes
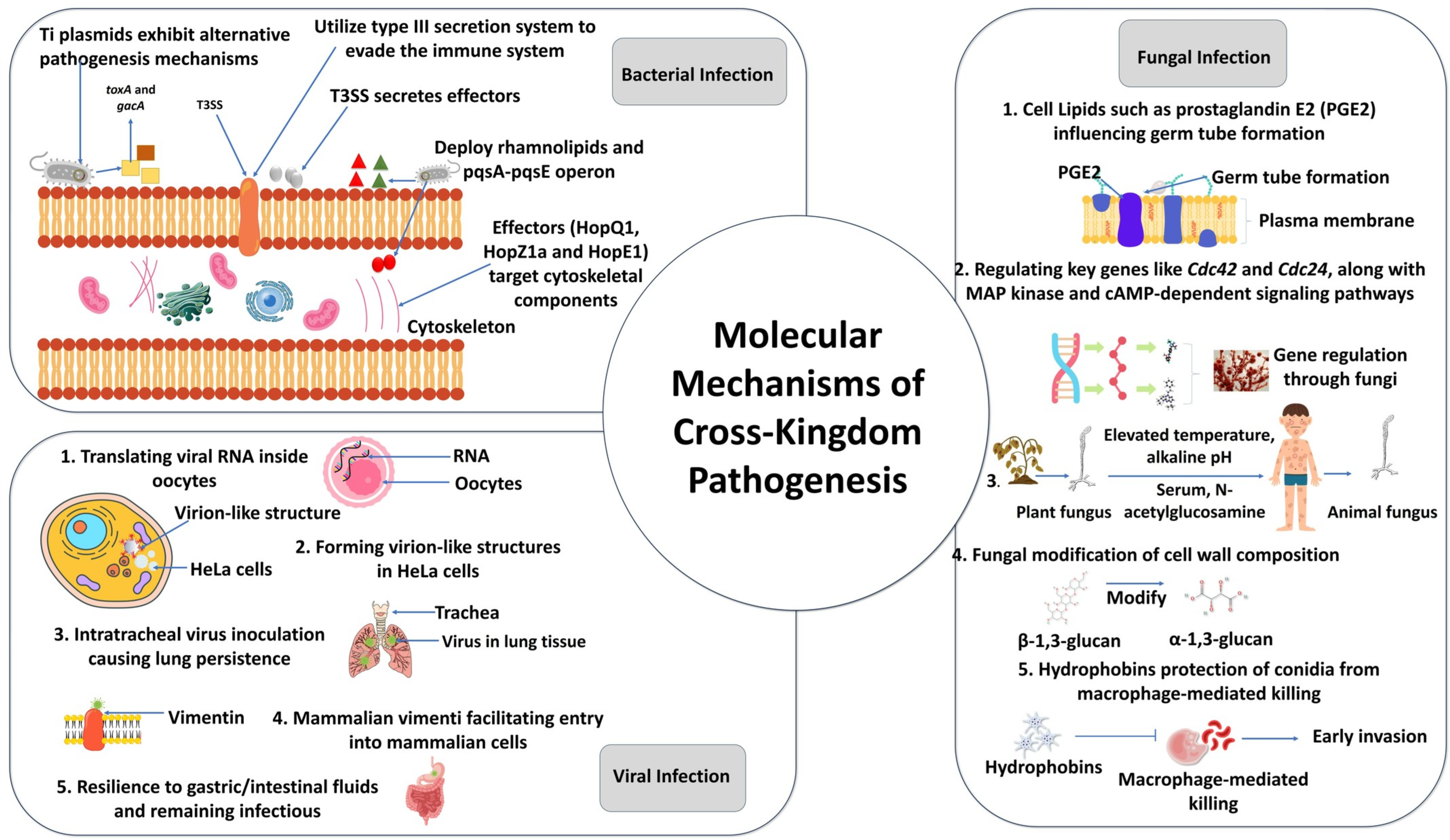
5. Molecular Mechanisms of Cross-Kingdom Pathogenesis
6. Drivers of Cross-Kingdom Pathogenesis by Phytopathogens
6.1. Superseded Immune Systems in Non-Plant Hosts
6.2. Genomic Adaptability of Phytopathogens
6.3. Microorganisms and Hosts in Close Proximity
6.4. Globalization and International Trade
6.5. Global Warming
7. Holistic Approach to Addressing Cross-Kingdom Challenges
7.1. Implementing a One-Health Framework
7.2. Mapping Interspecies Disease Transmission
7.3. Understanding Cross-Kingdom Pathogenesis Determinants
7.4. Horizon Scanning for Emerging Pathogens
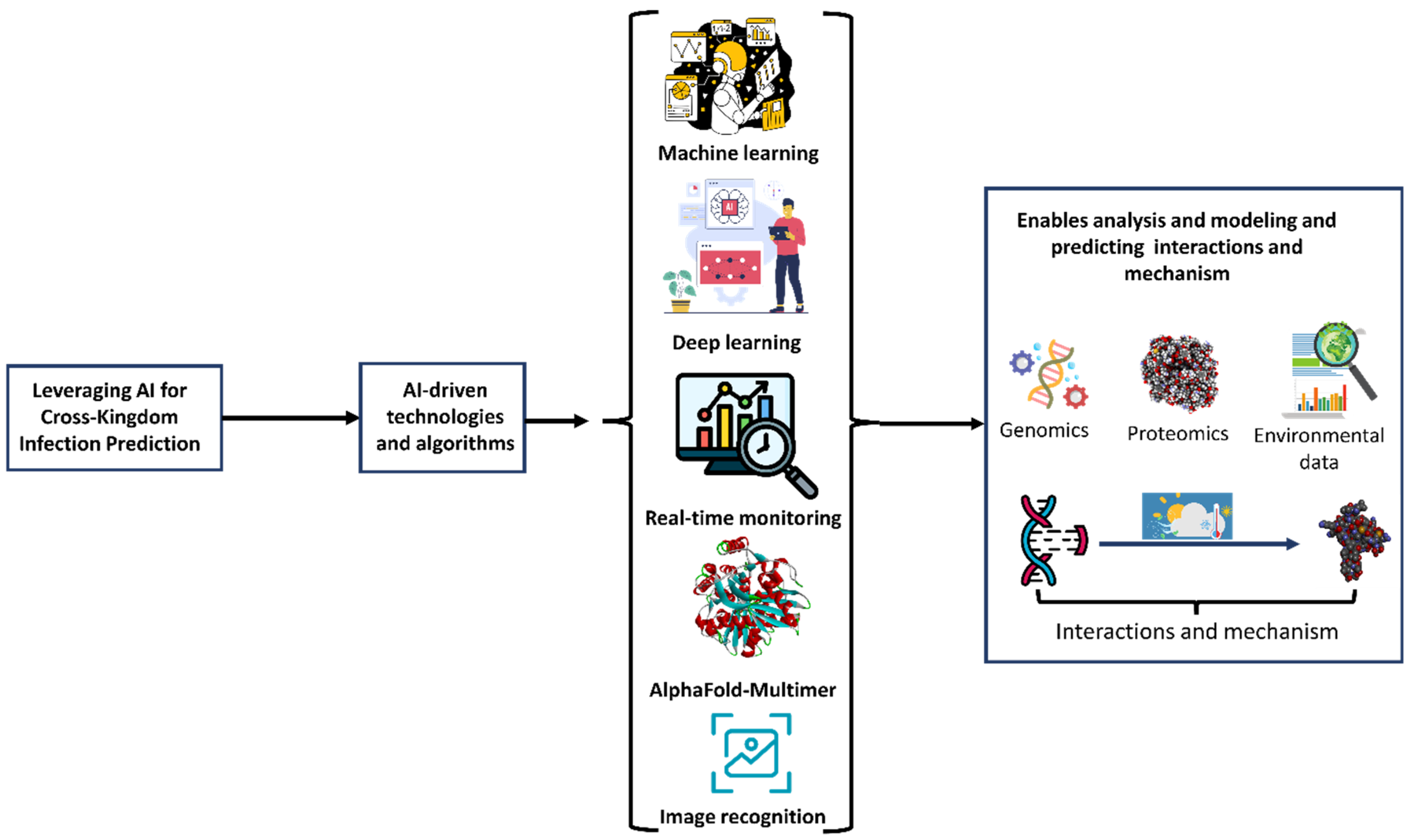
7.5. Leveraging AI for Cross-Kingdom Infection Prediction
7.6. Emergency Measures During Cross-Kingdom Phytopathogen Infections
7.7. Medical Interventions After Cross-Kingdom Phytopathogen Infections
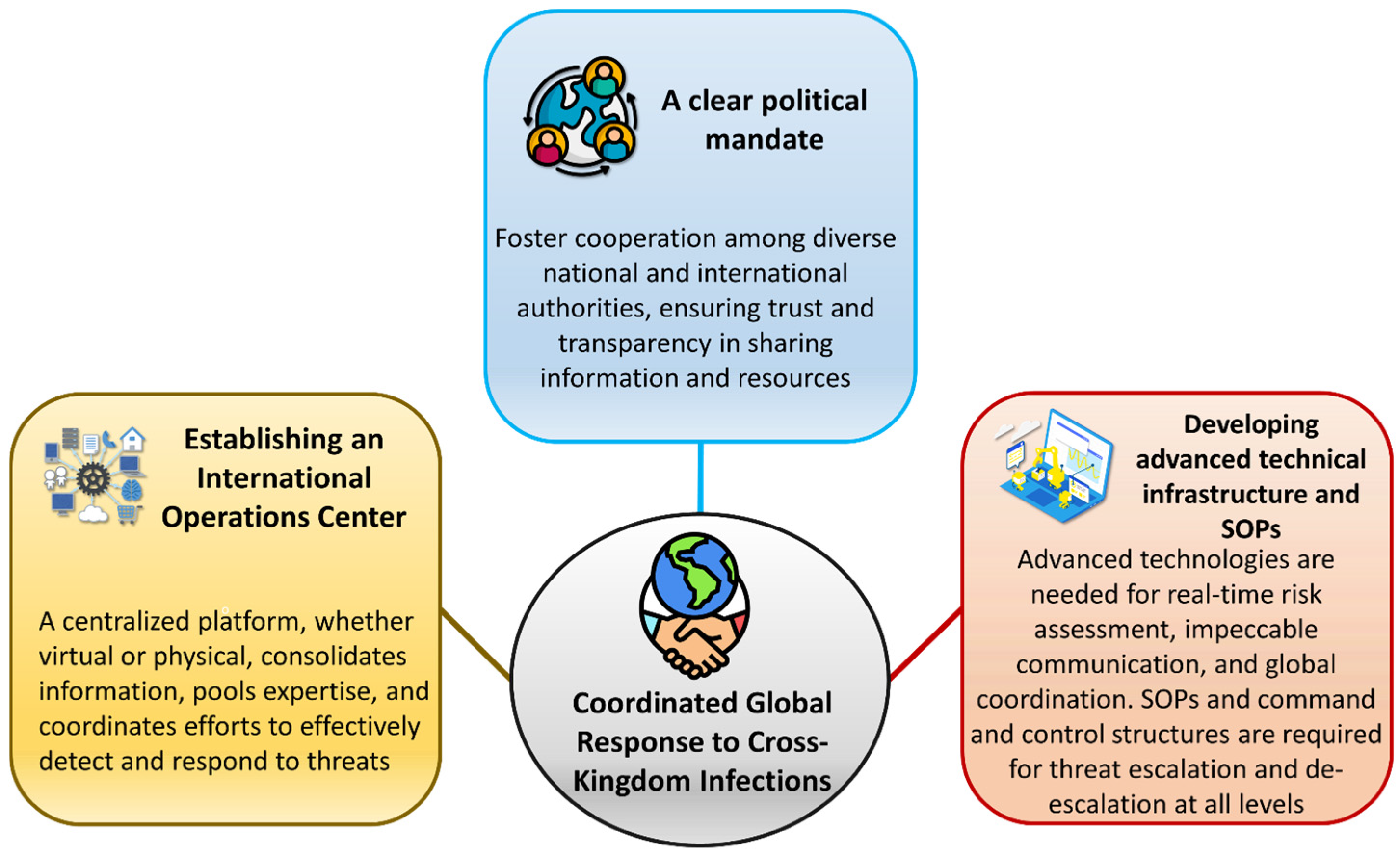
7.8. Coordinated Global Response to Cross-Kingdom Infections
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, G.M.; Keller, N.P. Crossover Fungal Pathogens: The Biology and Pathogenesis of Fungi Capable of Crossing Kingdoms to Infect Plants and Humans. Fungal Genet. Biol. 2013, 61, 146–157. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, S.J.; Park, Y.J.; Kim, S.Y.; Ryu, C.M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. What is a Host? Incorporating the Microbiota into the Damage-Response Framework. Infect. Immun. 2018, 86, e00522-17. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Rahman, M.; Rana, J.A.; Islam, S.S.; Adhikary, S.; Sannal, A.; Hosen, M.A.E.; et al. Plant disease dynamics in a changing climate: Impacts, molecular mechanisms, and climate-informed strategies for sustainable management. Discov. Agric. 2024, 2, 132. [Google Scholar] [CrossRef]
- Vogt, G. Environmental Adaptation of Genetically Uniform Organisms with the Help of Epigenetic Mechanisms—An Insightful Perspective on Ecoepigenetics. Epigenomes 2022, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Aime, M.C.; Bell, C.D.; Wilson, A.W. Deconstructing the Evolutionary Complexity between Plants and Fungal Pathogens: Coevolutionary Cycles. Annu. Rev. Phytopathol. 2018, 56, 247–266. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function, and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2021, 20, 193–205. [Google Scholar] [CrossRef]
- Venbrux, M.; Crauwels, S.; Rediers, H. Current and emerging trends in techniques for plant pathogen detection. Front. Plant Sci. 2023, 14, 1120968. [Google Scholar] [CrossRef]
- Kumar, R.; Feltrup, T.M.; Kukreja, R.V.; Patel, K.B.; Cai, S.; Singh, B.R. Evolutionary features in the structure and function of bacterial toxins. Toxins 2019, 11, 15. [Google Scholar] [CrossRef]
- Totsline, N.; Kniel, K.E.; Bais, H.P. Microgravity and evasion of plant innate immunity by human bacterial pathogens. npj Microgravity 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Lee, J.; Park, E.; Kim, D.W.; Lee, D.H.; Ryu, C.M.; Choi, D.; Park, J.M. A human pathogenic bacterium Shigella proliferates in plants through adoption of type III effectors for shigellosis. Plant Cell Environ. 2019, 42, 2962–2978. [Google Scholar] [CrossRef]
- Velho, A.C.; Mondino, P.; Stadnik, M.J. Extracellular enzymes of Colletotrichum fructicola isolates associated to Apple bitter rot and Glomerella leaf spot. Mycology 2018, 9, 145–154. [Google Scholar] [CrossRef]
- Tiwari, M.; Mishra, A.K.; Chakrabarty, D. Agrobacterium-mediated gene transfer: Recent advancements and layered immunity in plants. Planta 2022, 256, 37. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef]
- Wan, J.; He, M.; Hou, Q.; Zou, L.; Yang, Y.; Wei, Y.; Chen, X. Cell wall associated immunity in plants. Stress Biol. 2021, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.S.; Cho, J.; Albrecht, T.M.; Ferrell, J.L.; D’Orazio, S.E.F. Egress of Listeria monocytogenes from Mesenteric Lymph Nodes Depends on Intracellular Replication and Cell-to-Cell Spread. Infect. Immun. 2023, 91, 4. [Google Scholar] [CrossRef]
- Worley, M.J. Salmonella bloodstream infections. Trop. Med. Infect. Dis. 2023, 8, 487. [Google Scholar] [CrossRef]
- Mermigka, G.; Amprazi, M.; Mentzelopoulou, A.; Amartolou, A.; Sarris, P.F. Plant and animal innate immunity complexes: Fighting different enemies with similar weapons. Trends Plant Sci. 2020, 25, 80–91. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Magalhães, D.M.; Rodrigues, C.M.; Arena, G.D.; Oliveira, T.S.; Souza-Neto, R.R.; Picchi, S.C.; Martins, P.M.M.; Santos, P.J.C.; Maximo, H.J.; et al. PAMPs, PRRs, effectors and R-genes associated with citrus–pathogen interactions. Ann. Bot. 2016, 119, 749–774. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology, 9th ed.; Garland Science: New York, NY, USA, 2016; ISBN 978-0815345053. [Google Scholar]
- Seong, K.; Krasileva, K.V. Prediction of effector protein structures from fungal phytopathogens enables evolutionary analyses. Nat. Microbiol. 2023, 8, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Hommais, F.; Nasser, W.; Reverchon, S. Plant–phytopathogen interactions: Bacterial responses to environmental and plant stimuli. Environ. Microbiol. 2016, 19, 1689–1716. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, 3. [Google Scholar] [CrossRef]
- Casadevall, A. Thermal restriction as an antimicrobial function of fever. PLoS Pathog. 2016, 12, e1005577. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, M.U. Mechanisms of mycobacterial transmission: How does Mycobacterium tuberculosis enter and escape from the human host. Future Microbiol. 2016, 11, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Merera, O.; Derra, F.; Rahman, M.T.; Hazarika, R. Salmonellosis: A major foodborne disease of global significance. Beverage Food World 2015, 42, 21–24. [Google Scholar]
- Lepetit, M.; Brouquisse, R. Control of the rhizobium–legume symbiosis by the plant nitrogen demand is tightly integrated at the whole plant level and requires inter-organ systemic signaling. Front. Plant Sci. 2023, 14, 1114840. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An immunobiologic process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Pauwels, A.M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, receptors, and signals: Regulation of phagosome maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Queval, C.J.; Song, O.R.; Carralot, J.P.; Saliou, J.M.; Bongiovanni, A.; Deloison, G.; Deboosère, N.; Jouny, S.; Iantomasi, R.; Delorme, V.; et al. Mycobacterium tuberculosis controls phagosomal acidification by targeting CISH-mediated signaling. Cell Rep. 2017, 20, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, W.; Niedźwiedzka-Rystwej, P.; Tokarz-Deptuła, B.; Deptuła, W. Immunological memory cells. Cent. Eur. J. Immunol. 2018, 43, 194–203. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Nelson, K.J.; Knutson, S.T.; Soito, L.; Klomsiri, C.; Poole, L.B.; Fetrow, J.S. Analysis of the peroxiredoxin family: Using active-site structure and sequence information for global classification and residue analysis. Proteins Struct. Funct. Bioinform. 2011, 79, 947–964. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Gruening, N.M.; Krueger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Breitenbach, M.; Weber, M.; Rinnerthaler, M.; Karl, T.; Breitenbach-Koller, L. Oxidative stress in fungi: Its function in signal transduction, interaction with plant hosts, and lignocellulose degradation. Biomolecules 2015, 5, 318–342. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.C.; Gleason, J.E.; Sanchez, H.; Schatzman, S.S.; Culbertson, E.M.; Johnson, C.J.; McNees, C.A.; Coelho, C.; Nett, J.E.; Andes, D.R.; et al. Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis. PLoS Pathog. 2017, 13, e1006763. [Google Scholar] [CrossRef]
- Nordzieke, D.E.; Fernandes, T.R.; El Ghalid, M.; Turrà, D.; Di Pietro, A. NADPH oxidase regulates chemotropic growth of the fungal pathogen Fusarium oxysporum towards the host plant. New Phytol. 2019, 224, 1600–1612. [Google Scholar] [CrossRef]
- da Silva Dantas, A.; Patterson, M.J.; Smith, D.A.; MacCallum, D.M.; Erwig, L.P.; Morgan, B.A.; Quinn, J. Thioredoxin regulates multiple hydrogen peroxide-induced signaling pathways in Candida albicans. Mol. Cell. Biol. 2010, 30, 4550–4563. [Google Scholar] [CrossRef]
- Segmüller, N.; Kokkelink, L.; Giesbert, S.; Odinius, D.; van Kan, J.; Tudzynski, P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant-Microbe Interact. 2008, 21, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Paolinelli-Alfonso, M.; Villalobos-Escobedo, J.M.; Rolshausen, P.; Herrera-Estrella, A.; Galindo-Sánchez, C.; López-Hernández, J.F.; Hernandez-Martinez, R. Global transcriptional analysis suggests Lasiodiplodia theobromae pathogenicity factors involved in modulation of grapevine defensive response. BMC Genom. 2016, 17, 615. [Google Scholar] [CrossRef]
- Hamel, L.P.; Nicole, M.C.; Duplessis, S.; Ellis, B.E. Mitogen-activated protein kinase signaling in plant-interacting fungi: Distinct messages from conserved messengers. Plant Cell 2012, 24, 1327–1351. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tan, R.X. Cross-kingdom actions of phytohormones: A functional scaffold exploration. Chem. Rev. 2011, 111, 2734–2760. [Google Scholar] [CrossRef]
- Segorbe, D.; Di Pietro, A.; Pérez-Nadales, E.; Turrà, D. Three Fusarium oxysporum mitogen-activated protein kinases (MAPKs) have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol. Plant Pathol. 2017, 18, 912–924. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Félix, C.; Meneses, R.; Gonçalves, M.F.; Duarte, A.S.; Jorrín-Novo, J.V.; Van De Peer, Y.; Deforce, D.; Van Nieuwerburgh, F.; Alves, A.; Esteves, A.C. Lasiodiplodia hormozganensis causing modulation of pathogenesis-related molecules by temperature. Sci. Total Environ. 2024, 927, 171917. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Lee, S.C.; Heitman, J.; Steinbach, W.J. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2016, 8, 186–197. [Google Scholar] [CrossRef]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef]
- Yan, J.Y.; Zhao, W.S.; Chen, Z.; Xing, Q.K.; Zhang, W.; Chethana, K.W.T.; Xue, M.F. Comparative genome and transcriptome analyses reveal adaptations to opportunistic infections in woody plant degrading pathogens of Botryosphaeriaceae. DNA Res. 2018, 25, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D. Production of toxic metabolites by two strains of Lasiodiplodia theobromae, isolated from a coconut tree and a human patient. Mycologia 2018, 110, 642–653. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Nunes, R.B.; Tilleman, L.; Van de Peer, Y.; Deforce, D.; Van Nieuwerburgh, F.; Esteves, A.C.; Alves, A. Dual RNA sequencing of Vitis vinifera during Lasiodiplodia theobromae infection unveils host-pathogen interactions. Int. J. Mol. Sci. 2019, 20, 6083. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y. Nudix effectors: A common weapon in the arsenal of plant pathogens. PLoS Pathog. 2016, 12, e1005704. [Google Scholar] [CrossRef]
- Tiwari, S.; Thakur, R.; Shankar, J. Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol. Res. Int. 2015, 2015, 132635. [Google Scholar] [CrossRef]
- Fu, M.-S.; De Sordi, L.; Mühlschlegel, F.A. Functional characterization of the small heat shock protein Hsp12p from Candida albicans. PLoS ONE 2012, 7, e42894. [Google Scholar] [CrossRef]
- Ting, W.; Chang, C.; Szonyi, B.; Gizachew, D. Growth and aflatoxin B1, B2, G1, and G2 production by Aspergillus flavus and Aspergillus parasiticus on ground flax seeds (Linum usitatissimum). J. Food Prot. 2020, 83, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.D.A.; Evangelista, A.J.D.J.; Serpa, R.; Marques, F.J.D.F.; Melo, C.V.S.D.; Oliveira, J.S.D.; Franco, J.D.S.; Alencar, L.P.D.; Bandeira, T.D.J.P.G.; Brilhante, R.S.N.; et al. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology 2016, 162, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ortoneda, M.; Guarro, J.; Madrid, M.P.; Caracuel, Z.; Roncero, M.I.G.; Mayayo, E. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immun. 2004, 72, 1760–1766. [Google Scholar] [CrossRef]
- Sáenz, V.; Alvarez-Moreno, C.; Le Pape, P.; Restrepo, S.; Guarro, J.; Celis Ramírez, A.M. A one health perspective to recognize Fusarium as important in clinical practice. J. Fungi 2020, 6, 235. [Google Scholar] [CrossRef]
- Short, D.P.; O’Donnell, K.; Zhang, N.; Juba, J.H.; Geiser, D.M. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 2011, 49, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, N.; da Silva, M.T.N.; van der Heijden, I.M.; Graça, M.G.; de Oliveira, L.M.; Fu, L.; Giudice, M.; de Aquino, M.Z.; Odone-Filho, V.; Marques, H.H.; et al. An outbreak of invasive fusariosis in a children’s cancer hospital. Clin. Microbiol. Infect. 2015, 21, 268.e1. [Google Scholar] [CrossRef]
- Moretti, M.L.; Busso-Lopes, A.F.; Tararam, C.A.; Moraes, R.; Muraosa, Y.; Mikami, Y.; Gonoi, T.; Taguchi, H.; Lyra, L.; Reichert-Lima, F.; et al. Airborne transmission of invasive fusariosis in patients with hematologic malignancies. PLoS ONE 2018, 13, e0196426. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 2003, 5, 719–729. [Google Scholar] [CrossRef]
- Kirzinger, M.W.B.; Nadarasah, G.; Stavrinides, J. Insights into cross-kingdom plant pathogenic bacteria. Genes 2011, 2, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.-M.; Faria-Ramos, I.; Costa Cruz, L.; Pina-Vaz, C.; Rodrigues, A.G. Genesis of azole antifungal resistance from agriculture to clinical settings. J. Agric. Food Chem. 2015, 63, 7463–7468. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.-A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci. Rep. 2017, 7, 45631. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental hotspots for azole resistance selection of Aspergillus fumigatus, the Netherlands. Emerg. Infect. Dis. 2019, 25, 1347. [Google Scholar] [CrossRef]
- Godeau, C.; Reboux, G.; Scherer, E.; Laboissiere, A.; Lechenault-Bergerot, C.; Millon, L.; Rocchi, S. Azole-resistant Aspergillus fumigatus in the hospital: Surveillance from flower beds to corridors. Am. J. Infect. Control 2020, 48, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Buil, J.B.; Hare, R.K.; Zwaan, B.J.; Arendrup, M.C.; Melchers, W.J.G.; Verweij, P.E. The fading boundaries between patient and environmental routes of triazole resistance selection in Aspergillus fumigatus. PLoS Pathog. 2019, 15, e1007858. [Google Scholar] [CrossRef] [PubMed]
- Riat, A.; Plojoux, J.; Gindro, K.; Schrenzel, J.; Sanglard, D. Azole resistance of environmental and clinical Aspergillus fumigatus isolates from Switzerland. Antimicrob. Agents Chemother. 2018, 62, e01128-17. [Google Scholar] [CrossRef] [PubMed]
- Homa, M.; Galgóczy, L.; Manikandan, P.; Narendran, V.; Sinka, R.; Csernetics, Á.; Vágvölgyi, C.; Kredics, L.; Papp, T. South Indian isolates of the Fusarium solani species complex from clinical and environmental samples: Identification, antifungal susceptibilities, and virulence. Front. Microbiol. 2018, 9, 1052. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.-A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Fungicide-driven alterations in azole-resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia 2019, 111, 217–224. [Google Scholar] [CrossRef]
- Bicudo, E.L.; Macedo, V.O.; Carrara, M.A.; Castro, F.F.S.; Rage, R.I. Nosocomial outbreak of Pantoea agglomerans in a pediatric urgent care center. Braz. J. Infect. Dis. 2007, 11, 281–284. [Google Scholar] [CrossRef]
- Cruz, A.T.; Cazacu, A.C.; Allen, C.H. Pantoea agglomerans, a plant pathogen causing human disease. J. Clin. Microbiol. 2007, 45, 1989–1992. [Google Scholar] [CrossRef]
- Kratz, A.; Greenberg, D.; Barki, Y.; Cohen, E.; Lifshitz, M. Pantoea agglomerans as a cause of septic arthritis after palm tree thorn injury; case report and literature review. Arch. Dis. Child. 2003, 88, 542–544. [Google Scholar] [CrossRef]
- Hossain, M.M. Diseases of Lablab. In Handbook of Vegetable and Herb Diseases, Handbook of Plant Disease Management; Elmer, W.H., McGrath, M., McGovern, R.J., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Islam, S.S.; Adhikary, S.; Mostafa, M.; Hossain, M. Vegetable Beans: Comprehensive insights into diversity, production, nutritional benefits, sustainable cultivation, and future prospects. Online J. Biol. Sci. 2024, 24, 477–494. [Google Scholar] [CrossRef]
- Al-Ahmad, M.; Jusufovic, E.; Arifhodzic, N.; Rodriguez, T.; Nurkic, J. Association of Molds and Metrological Parameters to Frequency of Severe Asthma Exacerbation. Allergy Asthma Clin. Immunol. 2019, 15, 29. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, G.; Barber, D.; Tome-Amat, J.; Garrido-Arandia, M.; Diaz-Perales, A. Alternaria as an Inducer of Allergic Sensitization. J. Fungi 2008, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- NaPier, E.M.; Redd, T.K. Alternaria fungus growing on top of cyanoacrylate glue in a patient with perforated corneal ulcer. Am. J. Ophthalmol. Case Rep. 2022, 28, 101717. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Aguilar, C.; Bougnoux, M.; Noël, N.; Charlier, C.; Denis, B.; Lecuit, M.; Lortholary, O. Success of posaconazole therapy in a heart transplanted patient with Alternaria infectoria cutaneous infection. Med. Mycol. 2012, 50, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Hipolito, E.; Faria, E.; Alves, A.F.; Hoog, G.S.; Anjos, J.; Gonçalves, T.; Morais, P.V.; Estevão, H. Alternaria infectoria brain abscess in a child with chronic granulomatous disease. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 28, 377–380. [Google Scholar] [CrossRef]
- Vu, A.L.; Dee, M.M.; Gualandi, R.J., Jr.; Huff, S.; Zale, J.; Gwinn, K.D.; Ownley, B.H. First report of leaf spot caused by Bipolaris spicifera on switchgrass in the United States. Plant Dis. 2011, 95, 1191. [Google Scholar] [CrossRef]
- Amaradasa, B.S.; Amundsen, K. First Report of Curvularia inaequalis and Bipolaris spicifera Causing Leaf Blight of Buffalograss in Nebraska. Plant Dis. 2014, 98, 279. [Google Scholar] [CrossRef]
- Mahadevakumar, S.; Mahesh, M.; Maharachchikumbura, S.S.N.; Lavanya, S.N.; Rajashekara, H.; Prakash, G.; Vikas, K.; Tarasatyavati, C.; Chandranayaka, S. First report of Curvularia spicifera (≡ Bipolaris spicifera) causing spathe blight and leaf spot disease of pearl millet (Cenchrus americanus) in India. Plant Dis. 2024, 108, 3193. [Google Scholar] [CrossRef]
- Pham, C.D.; Purfield, A.E.; Fader, R.; Pascoe, N.; Lockhart, S.R. Development of a multilocus sequence typing system for medically relevant Bipolaris species. J. Clin. Microbiol. 2015, 53, 3239–3246. [Google Scholar] [CrossRef]
- Manamgoda, D.; Rossman, A.; Castlebury, L.; Crous, P.; Madrid, H.; Chukeatirote, E.; Hyde, K. The genus Bipolaris. Stud. Mycol. 2014, 79, 221–288. [Google Scholar] [CrossRef]
- Pai, H.V.; Jamal, E.; Yegneswaran, P.P. Corneal ulcer due to a rare pleosporalean member of the genus Bipolaris following cow tail injury to the eye: A case report and review of literature. Indian J. Ophthalmol. 2017, 65, 403–405. [Google Scholar] [CrossRef]
- Cighir, A.; Mare, A.D.; Vultur, F.; Cighir, T.; Pop, S.D.; Horvath, K.; Man, A. Fusarium spp. in Human Disease: Exploring the Boundaries between Commensalism and Pathogenesis. Life 2023, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L. An Overview of Aspergillus Species Associated with Plant Diseases. Pathogens 2024, 13, 813. [Google Scholar] [CrossRef]
- Fukuda, Y.; Homma, T.; Suzuki, S.; Takuma, T.; Tanaka, A.; Yokoe, T.; Ohnishi, T.; Niki, Y.; Sagara, H. High burden of Aspergillus fumigatus infection among chronic respiratory diseases. Chronic Respir. Dis. 2018, 15, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.B.; Casadevall, A.; Taylor, J.W.; Heitman, J.; Cowen, L. One Health: Fungal Pathogens of Humans, Animals, and Plants; American Society for Microbiology: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gene, J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef]
- Jia, J.; Nan, L.; Song, Z.; Chen, X.; Xia, J.; Cheng, L.; Zhang, B.; Mu, F. Cross-species transmission of a novel bisegmented orfanplasmovirus in the phytopathogenic fungus Exserohilum rostratum. Front. Microbiol. 2024, 15, 1409677. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Goss, E.M.; Dickstein, E.R.; Smith, M.E.; Johnson, J.A.; Southwick, F.S.; van Bruggen, A.H.C. Exserohilum rostratum: Characterization of a Cross-Kingdom Pathogen of Plants and Humans. PLoS ONE 2014, 9, e108691. [Google Scholar] [CrossRef]
- CDC. Multistate Outbreak of Fungal Meningitis and Other Infections. 2013. Available online: https://archive.cdc.gov/www_cdc_gov/hai/outbreaks/laboratory/index.html (accessed on 10 January 2025).
- Coutinho, I.B.L.; Freire, F.C.O.; Lima, C.S.; Lima, J.S.; Gonçalves, F.J.T.; Machado, A.R.; Silva, A.M.S.; Cardoso, J.E. Diversity of genus Lasiodiplodia associated with perennial tropical fruit plants in northeastern Brazil. Plant Pathol. 2017, 66, 90–104. [Google Scholar] [CrossRef]
- Maurya, A.K.; Kumari, S.; Behera, G.; Bhadade, A.; Tadepalli, K. Rhino sinusitis caused by Lasiodiplodia theobromae in a diabetic patient. Med. Mycol. Case Rep. 2023, 40, 22–24. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Toumasis, P.; Vrioni, G.; Gardeli, I.; Michelaki, A.; Exindari, M.; Orfanidou, M. Macrophomina phaseolina: A Phytopathogen Associated with Human Ocular Infections—A Case Report of Endophthalmitis and Systematic Review of Human Infections. J. Clin. Med. 2025, 14, 430. [Google Scholar] [CrossRef]
- Wang, R.Y.; Gao, B.; Chen, S.L.; Ma, J.; Li, X.H.; Xue, C.X. First report of Rhizopus oryzae causing soft rot on storage roots of sweetpotato in China. Plant Dis. 2017, 101, 1039. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhang, M.; Zhang, C.Q.; Chen, X.J.; Tan, Q.Q.; Ma, J.; Li, Z. Pathogen detection, carbon metabolic phenotype analysis of Rhizopus oryzae from tobacco and its sensitivity to seven fungicides. J. Pestic. Sci. 2018, 20, 743–748. [Google Scholar] [CrossRef]
- Dai, M.; Tan, X.; Chen, X.; Cai, K.; Zhong, Y.; Ye, Z.; Kong, D. Green control for inhibiting Rhizopus oryzae growth by stress factors in forage grass factory. Front. Microbiol. 2024, 15, 1437799. [Google Scholar] [CrossRef]
- Osaigbovo, I.I.; Ekeng, B.E.; Davies, A.A.; Oladele, R.O. Mucormycosis in Africa: Epidemiology, diagnosis and treatment outcomes. Mycoses 2023, 66, 13581. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rahman, M.M.; Ahasan, M.T.; Sarkar, N.; Akash, S.; Islam, M.; Islam, F.; Aktar, M.N.; Saeed, M.; Rashid, H.O.; et al. The impact of mucormycosis (black fungus) on SARS-CoV-2-infected patients: At a glance. Environ. Sci. Pollut. Res. 2022, 29, 69341–69366. [Google Scholar] [CrossRef]
- Calvano, T.P.; Blatz, P.J.; Vento, T.J.; Wickes, B.L.; Sutton, D.A.; Thompson, E.H.; White, C.E.; Renz, E.M.; Hospenthal, D.R. Pythium aphanidermatum Infection Following Combat Trauma. J. Clin. Microbiol. 2011, 49, 3710–3713. [Google Scholar] [CrossRef]
- Thongsuk, P.; Plongla, R.; Thammahong, A.; Tiewsurin, J.; Worasilchai, N.; Chindamporn, A.; Suankratay, C. Vascular pythiosis caused by Pythium aphanidermatum: The first case report in Asia. Eur. J. Med. Res. 2021, 26, 1. [Google Scholar] [CrossRef]
- Vilela, R.; Taylor, J.W.; Walker, E.D.; Mendoza, L. Lagenidium giganteum Pathogenicity in mammals. Emerg. Infect. Dis. 2015, 21, 290–297. [Google Scholar] [CrossRef]
- Shivaprakash, M.R.; Appannanavar, S.B.; Dhaliwal, M.; Gupta, A.; Gupta, S.; Gupta, A.; Chakrabarti, A. Colletotrichum truncatum: An unusual pathogen causing mycotic keratitis and endophthalmitis. J. Clin. Microbiol. 2011, 49, 2894–2898. [Google Scholar] [CrossRef]
- Rao, S.; Sharda, S.; Oddi, V.; Nandineni, M.R. The landscape of repetitive elements in the refined genome of chilli anthracnose fungus Colletotrichum truncatum. Front. Microbiol. 2018, 9, 2367. [Google Scholar] [CrossRef]
- Palmore, T.N.; Shea, Y.R.; Childs, R.W.; Sherry, R.M.; Walsh, T.J. Fusarium proliferatum soft tissue infection at the site of a puncture by a plant: Recovery, isolation, and direct molecular identification. J. Clin. Microbiol. 2010, 48, 338–342. [Google Scholar] [CrossRef]
- Muhammed, M.; Anagnostou, T.; Desalermos, A.; Kourkoumpetis, T.K.; Carneiro, H.A.; Glavis-Bloom, J.; Coleman, J.J.; Mylonakis, E. Fusarium infection. Medicine 2013, 92, 305–316. [Google Scholar] [CrossRef]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.W.; Lima, N.B.; De Morais, M.A.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Assunção, I.P.; Lima, G.S.A.; Marques, M.W.; Lima, W.G.; Monteiro, J.H.A.; De Queiroz Balbino, V.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with papaya stem-end rot in Brazil. Fungal Divers. 2014, 67, 127–141. [Google Scholar] [CrossRef]
- Correia, K.C.; Silva, M.A.; De Morais, M.A.; Armengol, J.; Phillips, A.J.L.; Câmara, M.P.S.; Michereff, S.J. Phylogeny, Distribution and Pathogenicity of Lasiodiplodia Species Associated with Dieback of Table Grape in the Main Brazilian Exporting Region. Plant Pathol. 2015, 65, 92–103. [Google Scholar] [CrossRef]
- Custódio, F.A.; Machado, A.R.; Soares, D.J.; Pereira, O.L. Lasiodiplodia hormozganensis Causing Basal Stem Rot on Ricinus communis in Brazil. Australas. Plant Dis. Notes 2018, 13, 25. [Google Scholar] [CrossRef]
- Kwon, J.; Ryu, J.; Chi, T.T.P.; Shen, S.; Choi, O. Soft Rot of Rhizopus oryzae as a Postharvest Pathogen of Banana Fruit in Korea. Mycobiology 2012, 40, 214–216. [Google Scholar] [CrossRef]
- Khokhar, I.; Wang, J.; Jia, Y.; Yan, Y. First Report of Rhizopus Soft Rot on Tomato (Lycopersicon esculentum) Caused by Rhizopus oryzae in China. Plant Dis. 2019, 103, 1041. [Google Scholar] [CrossRef]
- Gnanesh, B.; Tejaswi, A.; Arunakumar, G.; Supriya, M.; Manojkumar, H.; Tewary, P. Molecular phylogeny, identification and pathogenicity of Rhizopus oryzae associated with root rot of mulberry in India. J. Appl. Microbiol. 2020, 131, 360–374. [Google Scholar] [CrossRef]
- Holmes, J.E.; Sanghera, H.; Punja, Z.K. Crown gall development on cannabis (Cannabis sativa L., marijuana) plants caused by Agrobacterium tumefaciens species-complex. Can. J. Plant Pathol. 2023, 45, 433–445. [Google Scholar] [CrossRef]
- Tiwari, S.; Beriha, S.S. Primary Bacteremia Caused by Rhizobium radiobacter in Neonate: A Rare Case Report. J. Clin. Diagn. Res. 2015, 9, DD01–DD02. [Google Scholar] [CrossRef]
- İyigün, F. Rhizobium radiobacter infection in a preterm infant and review of the literature. Ümraniye Pediatri Dergisi J. Umra. Pediatr. 2024, 47, 47–52. [Google Scholar] [CrossRef]
- Fenner, B.J.; Kumar, A.; Tan, N.Y.Q.; Ang, M. Case of Isolated Rhizobium radiobacter Contact Lens-Related Infectious Keratitis: A Plant Microbe Now Emerging as a Human Pathogen. Am. J. Ophthalmol. Case Rep. 2019, 15, 100476. [Google Scholar] [CrossRef]
- Cho, H.; Park, J.Y.; Kim, Y.K.; Sohn, S.-H.; Park, D.S.; Kwon, Y.-S.; Kim, C.-W.; Back, C.-G. Whole-Genome Sequence of Erwinia persicina B64, Which Causes Pink Soft Rot in Onions. Microbiol. Resour. Announc. 2019, 8, e01302-18. [Google Scholar] [CrossRef] [PubMed]
- Canik Orel, D. Erwinia persicina as the New Causal Agent of Lettuce Soft Rot. Eur. J. Plant Pathol. 2020, 158, 223–235. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Abe, D.; Saito, T.; Nogata, Y.; Nomiyama, K.; Kohyama, N.; Takahashi, A.; Yoshioka, T.; Ishikawa, N.; Tomioka, K. Pink seed of barley caused by Erwinia persicina. J. Gen. Plant Pathol. 2021, 87, 106–109. [Google Scholar] [CrossRef]
- Wasendorf, C.; Schmitz-Esser, S.; Eischeid, C.J.; Leyhe, M.J.; Nelson, E.N.; Rahic-Seggerman, F.M.; Sullivan, K.E.; Peters, N.T. Genome analysis of Erwinia persicina reveals implications for soft rot pathogenicity in plants. Front. Microbiol. 2022, 13, 1001139. [Google Scholar] [CrossRef]
- González, A.J.; Tello, J.C.; Rodicio, M.R. Erwinia persicina causing chlorosis and necrotic spots in leaves and tendrils of Pisum sativum in southeastern Spain. Plant Dis. 2007, 91, 460. [Google Scholar] [CrossRef]
- Zhang, Z.; Nan, Z. Erwinia persicina, a possible new necrosis and wilt threat to forage or grain legumes production. Eur. J. Plant Pathol. 2014, 139, 349–358. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Steigerwalt, A.G.; Hill, B.C.; Miller, J.M.; Brenner, D.J. First report of a human isolate of Erwinia persicinus. J. Clin. Microbiol. 1998, 36, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Mohamaden, W.I.; Zhen-Fen, Z.; Hegab, I.M.; Shang-Li, S. Experimental infection in mice with Erwinia persicina. Microb. Pathog. 2019, 130, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Prod’homme, M.; Micol, L.A.; Weitsch, S.; Gassend, J.L.; Martinet, O.; Bellini, C. Cutaneous infection and bacteremia caused by Erwinia billingiae: A case report. New Microbes New Infect. 2017, 19, 134–136. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Mahenthiralingam, E.; Baldwin, A.; Dowson, C.G. Burkholderia cepacia complex bacteria: Opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008, 104, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Leigh, M.; Ribeiro, C.; Yankaskas, J.; Burns, K.; Gilligan, P.; Sokol, P.; Boucher, R. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 2002, 70, 4547–4555. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, S.; Kakar, K.U.; Xie, G.; Li, B.; Chen, G.; Zhu, B. Genome Sequence and Adaptation Analysis of the Human and Rice Pathogenic Strain Burkholderia glumae AU6208. Pathogens 2021, 10, 87. [Google Scholar] [CrossRef]
- Rajendraprasad, S.; Creech, Z.A.; Truong, G.T.D.; Nguyen, T.; Addula, M.; Mendoza, N.; Velagapudi, M. Fatal case of Burkholderia gladioli pneumonia in a patient with COVID-19. Ochsner J. 2022, 22, 349–352. [Google Scholar] [CrossRef]
- Yablon, B.R.; Dantes, R.; Tsai, V.; Lim, R.; Moulton-Meissner, H.; Arduino, M.; Jensen, B.; Patel, M.T.; Vernon, M.O.; Grant-Greene, Y.; et al. Outbreak of Pantoea agglomerans bloodstream infections at an oncology clinic-Illinois, 2012–2013. Infect. Control Hosp. Epidemiol. 2017, 38, 314–319. [Google Scholar] [CrossRef]
- De Baere, T.; Verhelst, R.; Labit, C.; Verschraegen, G.; Wauters, G.; Claeys, G.; Vaneechoutte, M. Bacteremic Infection with Pantoea ananatis. J. Clin. Microbiol. 2004, 42, 4393–4395. [Google Scholar] [CrossRef]
- Habsah, H.; Zeehaida, M.; Van Rostenberghe, H.; Noraida, R.; Wan Pauzi, W.I.; Fatimah, I.; Rosliza, A.; Sharimah, N.N.; Maimunah, H. An outbreak of Pantoea spp. in a neonatal intensive care unit secondary to contaminated parenteral nutrition. J. Hosp. Infect. 2005, 61, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.R.; Rabiey, M.; Friman, V.; Jackson, R.W. Seeing the forest for the trees: Use of phages to treat bacterial tree diseases. Plant Pathol. 2021, 70, 1987–2004. [Google Scholar] [CrossRef]
- Moradi, F.; Behbahani, M.R.; Gorginpour, J.; Dezhkam, A.; Hadi, N. Ralstonia pickettii bloodstream infection in the patient with Guillain-Barre syndrome under plasmapheresis. New Microbes New Infect. 2024, 57, 101218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Buch, A. Pseudomonas aeruginosa predominates as multifaceted rhizospheric bacteria with combined abilities of P-solubilization and biocontrol. J. Pure Appl. Microbiol. 2019, 13, 319–328. [Google Scholar] [CrossRef]
- Tiwari, P.; Singh, J.S. A plant growth promoting rhizospheric Pseudomonas aeruginosa strain inhibits seed germination in Triticum aestivum (L) and Zea mays (L). Microbiol. Res. 2017, 8, 7233. [Google Scholar] [CrossRef]
- Ambreetha, S.; Marimuthu, P.; Mathee, K.; Balachandar, D. Plant-Associated Pseudomonas aeruginosa Strains Harbor Multiple Virulence Traits Critical for Human Infection. J. Med. Microbiol. 2022, 71. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.O.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Høiby, N. Pseudomonas aeruginosa Biofilms in the Respiratory Tract of Cystic Fibrosis Patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef]
- Wilson, M.G.; Pandey, S. Pseudomonas aeruginosa. [Updated 8 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557831/ (accessed on 16 December 2024).
- Kirienko, N.V.; Kirienko, D.R.; Larkins-Ford, J.; Wählby, C.; Ruvkun, G.; Ausubel, F.M. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe 2013, 13, 406–416. [Google Scholar] [CrossRef]
- Coutinho, C.P.; de Carvalho, C.C.C.R.; Madeira, A.; Pinto-de-Oliveira, A.; Sá-Correia, I. Burkholderia cenocepacia phenotypic clonal variation during a 3.5-year colonization in the lungs of a cystic fibrosis patient. Infect. Immun. 2011, 79, 2950–2960. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Wang, C.W.; Lu, B.H. First report of bacterial root rot of ginseng caused by Pseudomonas aeruginosa in China. Plant Dis. 2014, 98, 1577. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.; Sahu, R.; Owen, D.R.; Dennis, V.A. Pseudomonas aeruginosa reference strains PAO1 and PA14: A genomic, phenotypic, and therapeutic review. Front. Microbiol. 2022, 13, 1023523. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. The novel coronavirus–a snapshot of current knowledge. Microb. Biotechnol. 2020, 13, 607–612. [Google Scholar] [CrossRef]
- Balique, F.; Lecoq, H.; Raoult, D.; Colson, P. Can Plant Viruses Cross the Kingdom Border and Be Pathogenic to Humans? Viruses 2015, 7, 2074–2098. [Google Scholar] [CrossRef]
- Zhang, T.; Breitbart, M.; Lee, W.H.; Run, J.Q.; Wei, C.L.; Soh, S.W.; Hibberd, M.L.; Liu, E.T.; Rohwer, F.; Ruan, Y. RNA viral community in human feces: Prevalence of plant pathogenic viruses. PLoS Biol. 2006, 4, e3. [Google Scholar] [CrossRef]
- Jiwaji, M.; Matcher, G.F.; de Bruyn, M.M.; Awando, J.A.; Moodley, H.; Waterworth, D.; Jarvie, R.A.; Dorrington, R.A. Providence virus: An animal virus that replicates in plants or a plant virus that infects and replicates in animal cells? PLoS ONE 2019, 14, e0217494. [Google Scholar] [CrossRef]
- Bousbia, S.; Papazian, L.; La Scola, B.; Raoult, D. Detection of Plant DNA in the Bronchoalveolar Lavage of Patients with Ventilator-Associated Pneumonia. PLoS ONE 2010, 5, e11298. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Richet, H.; Desnues, C.; Balique, F.; Moal, V.; Grob, J.J.; Berbis, P.; Lecoq, H.; Harlé, J.-R.; Berland, Y.; et al. Pepper Mild Mottle Virus, a Plant Virus Associated with Specific Immune Responses, Fever, Abdominal Pains, and Pruritus in Humans. PLoS ONE 2010, 5, e10041. [Google Scholar] [CrossRef]
- Liu, R.; Vaishnav, R.A.; Roberts, A.M.; Friedland, R.P. Humans have antibodies against a plant virus: Evidence from tobacco mosaic virus. PLoS ONE 2013, 8, e60621. [Google Scholar] [CrossRef]
- Knowland, J. Protein synthesis directed by the RNA from a plant virus in a normal animal cell. Genetics 1974, 78, 383–394. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Xiao, R.; Zhu, G.; Li, Y.; Liu, C.; Yang, R.; Tang, Z.; Li, J.; Huang, W.; et al. The invasion of tobacco mosaic virus RNA induces endoplasmic reticulum stress-related autophagy in HeLa cells. Biosci. Rep. 2012, 32, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, K.J.; Destito, G.; Plummer, E.M.; Trauger, S.A.; Siuzdak, G.; Manchester, M. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog. 2009, 5, e1000417. [Google Scholar] [CrossRef]
- Li, L.; Victoria, J.G.; Wang, C.; Jones, M.; Fellers, G.M.; Kunz, T.H.; Delwart, E. Bat guano virome: Predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 2010, 84, 6955–6965. [Google Scholar] [CrossRef]
- Phan, T.G.; Kapusinszky, B.; Wang, C.; Rose, R.K.; Lipton, H.L.; Delwart, E.L. The fecal viral flora of wild rodents. PLoS Pathog. 2011, 7, e1002218. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, O.A.; Solovyova, Y.V.; Solovyov, A.V. Results of algae viruses search in human clinical material. Ukr. Bioorganica Acta 2011, 2, 53–56. [Google Scholar]
- Yolken, R.H.; Jones-Brando, L.; Dunigan, D.D.; Kannan, G.; Dickerson, F.; Severance, E.; Sabunciyan, S.; Talbot, C.C., Jr.; Prandovszky, E.; Gurnon, J.R.; et al. Chlorovirus ATCV-1 is part of the human oropharyngeal virome and is associated with changes in cognitive functions in humans and mice. Proc. Natl. Acad. Sci. USA 2014, 111, 16106–16111. [Google Scholar] [CrossRef]
- Garcia-Lopez, R.; Perez-Brocal, V.; Moya, A. Beyond cells—The virome in the human holobiont. Microb. Cell 2019, 6, 373–396. [Google Scholar] [CrossRef]
- Magunacelaya, J.C. Concepts in Management of Tree Crops Nematodes in Fruit Production Systems. In Integrated Management of Fruit Crops Nematodes; Ciancio, A., Mukerji, K., Eds.; Integrated Management of Plant Pests and Diseases; Springer: Dordrecht, The Netherland, 2009; Volume 4. [Google Scholar] [CrossRef]
- Li, Q.; Liang, W.; Zhang, X.; Mahamood, M. Nematode genera and species description along the transect. In Soil Nematodes of Grasslands in Northern China; Academic Press: London, UK; Zhejiang University Press: Hangzhou, China, 2017; Volume 45, p. 228. [Google Scholar]
- Haouchine, D.; Mantelet, S.; Marteau, A.; Brun, S.; Dabi, P.; Abbas, M.; Izri, A.; Akhoundi, M. First evidence of human infection by Xiphinema brevicollum (Nematoda: Longidoridae). Int. J. Infect. Dis. 2022, 122, 609–611. [Google Scholar] [CrossRef]
- Jasmer, D.P.; Goverse, A.; Smant, G. Parasitic nematode interactions with mammals and plants. Annu. Rev. Phytopathol. 2003, 41, 245–270. [Google Scholar] [CrossRef]
- Stepek, G.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. Human gastrointestinal nematode infections: Are new control methods required? Int. J. Exp. Pathol. 2006, 87, 325–341. [Google Scholar] [CrossRef]
- Robinson, M.W.; Dalton, J.P. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2763–2776. [Google Scholar] [CrossRef] [PubMed]
- Kantor, M.; Vieira, P.; Handoo, Z. First Report of the Barley Root-Knot Nematode, Meloidogyne naasi, from Pennsylvania, with New Host Report. Plant Dis. 2024, 108, 2583. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, R.S.; Speare, R. Passage of Meloidogyne Eggs in Human Stool: Forgotten, but Not Gone. J. Clin. Microbiol. 2015, 53, 1458–1459. [Google Scholar] [CrossRef] [PubMed]
- Petrunia, I.V.; Frolova, O.Y.; Komarova, T.V.; Kiselev, S.L.; Citovsky, V.; Dorokhov, Y.L. Agrobacterium tumefaciens-induced bacteremia does not lead to reporter gene expression in mouse organs. PLoS ONE 2008, 3, e2352. [Google Scholar] [CrossRef]
- Diggle, S.P.; Lumjiaktase, P.; Dipilato, F.; Winzer, K.; Kunakorn, M.; Barrett, D.A.; Chhabra, S.R.; Cámara, M.; Williams, P. Functional Genetic Analysis Reveals a 2-Alkyl-4-Quinolone Signaling System in the Human Pathogen Burkholderia pseudomallei and Related Bacteria. Chem. Biol. 2006, 13, 701–710. [Google Scholar] [CrossRef]
- Kang, M.; Lee, D.; Mannaa, M.; Han, G.; Choi, H.; Lee, S.; Lim, G.; Kim, S.; Kim, T.; Seo, Y. Impact of quorum sensing on the virulence and survival traits of Burkholderia plantarii. Plants 2024, 13, 2657. [Google Scholar] [CrossRef]
- Bzdyl, N.M.; Moran, C.L.; Bendo, J.; Sarkar-Tyson, M. Pathogenicity and Virulence of Burkholderia pseudomallei. Virulence 2022, 13, 1945–1965. [Google Scholar] [CrossRef]
- Ambreetha, S.; Marimuthu, P.; Mathee, K.; Balachandar, D. Rhizospheric and Endophytic Pseudomonas aeruginosa in Edible Vegetable Plants Share Molecular and Metabolic Traits with Clinical Isolates. J. Appl. Microbiol. 2021, 132, 3226–3248. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Strateva, T.; Mitov, I. Contribution of an arsenal of virulence factors to pathogenesis of Pseudomonas aeruginosa infections. Ann. Microbiol. 2011, 61, 717–732. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Rezzonico, F.; Orfei, B.; Cortese, C.; Moreno-Pérez, A.; Van Den Burg, H.A.; Onofri, A.; Firrao, G.; Ramos, C.; Smits, T.H.M.; et al. Synergistic interaction between the type III secretion system of the endophytic bacterium Pantoea agglomerans DAPP-PG 734 and the virulence of the causal agent of olive knot Pseudomonas savastanoi pv. savastanoi DAPP-PG 722. Mol. Plant Pathol. 2021, 22, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Mardaneh, J.; Pouresmaeil, O. Pantoea agglomerans: An emerging pathogen in hospitals and foods, A narrative review. Rev. Clin. Med. 2024, 11, 4–12. [Google Scholar] [CrossRef]
- Chang, X.; Heene, E.; Qiao, F.; Nick, P. The Phytoalexin Resveratrol Regulates the Initiation of Hypersensitive Cell Death in Vitis Cell. PLoS ONE 2011, 6, e26405. [Google Scholar] [CrossRef]
- Chang, X.; Riemann, M.; Liu, Q.; Nick, P. Actin as Deathly Switch? How Auxin Can Suppress Cell-Death-Related Defence. PLoS ONE 2015, 10, e0125498. [Google Scholar] [CrossRef] [PubMed]
- Rougerie, P.; Miskolci, V.; Cox, D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: Role and regulation of the actin cytoskeleton. Immunol. Rev. 2013, 256, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Park, Y.J.; Kim, J.S.; Lee, S.; Lee, S.H.; Choi, S.; Min, J.; Choi, I.; Ryu, C. Pseudomonas syringae evades phagocytosis by animal cells via type III effector-mediated regulation of actin filament plasticity. Environ. Microbiol. 2018, 20, 3980–3991. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kim, P.; Li, G.; Elowsky, C.G.; Alfano, J.R. A bacterial effector co-opts calmodulin to target the plant microtubule network. Cell Host Microbe 2016, 19, 67–78. [Google Scholar] [CrossRef]
- Doona, C.J.; Feeherry, F.E.; Setlow, P.; Kustin, K. Quasi-chemical Germination Kinetics (QCGK) modeling of HPP germination of bacterial spores and the effects of lowering water activity by nonelectrolytic humectants. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 130–140. [Google Scholar] [CrossRef]
- Sexton, A.C.; Howlett, B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef]
- Kornitzer, D. Regulation of Candida albicans Hyphal Morphogenesis by Endogenous Signals. J. Fungi 2019, 5, 21. [Google Scholar] [CrossRef]
- D’Enfert, C.; Kaune, A.; Alaban, L.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2020, 45, 3. [Google Scholar] [CrossRef]
- Araújo, D.S.; Pereira, M.; Portis, I.G.; De Castro Moreira Dos Santos, A.; Junior, F.W.; De Sousa, M.V.; Assunção, L.D.P.; Baeza, L.C.; Bailão, A.M.; Ricart, C.A.O.; et al. Metabolic Peculiarities of Paracoccidioides brasiliensis Dimorphism as Demonstrated by iTRAQ Labeling Proteomics. Front. Microbiol. 2019, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Carrion, S.; Leal, S.M.; Ghannoum, M.A.; Aimanianda, V.; Latgé, J.; Pearlman, E. The RodA Hydrophobin on Aspergillus fumigatus Spores Masks Dectin-1– and Dectin-2–Dependent Responses and Enhances Fungal Survival In Vivo. J. Immunol. 2013, 191, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2014, 37, 141–152. [Google Scholar] [CrossRef]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Balloy, V.; Hennequin, C. Bronchial Epithelial Cells on the Front Line to Fight Lung Infection-Causing Aspergillus fumigatus. Front. Immunol. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Dash, S.; Duraivelan, K.; Samanta, D. Cadherin-mediated host–pathogen interactions. Cell. Microbiol. 2021, 23, 13316. [Google Scholar] [CrossRef]
- Balique, F.; Colson, P.; Barry, A.O.; Nappez, C.; Ferretti, A.; Moussawi, K.A.; Ngounga, T.; Lepidi, H.; Ghigo, E.; Mege, J.; et al. Tobacco Mosaic Virus in the Lungs of Mice following Intra-Tracheal Inoculation. PLoS ONE 2013, 8, e54993. [Google Scholar] [CrossRef]
- Omole, A.O.; De Oliveira, J.F.A.; Sutorus, L.; Karan, S.; Zhao, Z.; Neun, B.W.; Cedrone, E.; Clogston, J.D.; Xu, J.; Sierk, M.; et al. Cellular fate of a plant virus immunotherapy candidate. Commun. Biol. 2024, 7, 1. [Google Scholar] [CrossRef]
- Zhou, Q.; Verne, G.N. Intestinal hyperpermeability: A gateway to multi-organ failure? J. Clin. Investig. 2018, 128, 4764–4766. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Steinmetz, N.F. The pharmacology of plant virus nanoparticles. Virology 2021, 556, 39–61. [Google Scholar] [CrossRef]
- De Medeiros, R.B.; Figueiredo, J.; Resende, R.D.O.; De Avila, A.C. Expression of a viral polymerase-bound host factor turns human cell lines permissive to a plant- and insect-infecting virus. Proc. Natl. Acad. Sci. USA 2005, 102, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, E.; Bastidas-Quintero, A.M.; Weidl, D.; Full, F. Pioneer factors in viral infection. Front. Immunol. 2023, 14, 1286617. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Molecular Detection of Human Bacterial Pathogens; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-1239-6. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Filamentous fungal human pathogens from food emphasising Aspergillus, Fusarium and Mucor. Microorganisms 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Morogovsky, A.; Handelman, M.; Abou Kandil, A.; Shadkchan, Y.; Osherov, N. Horizontal gene transfer of triazole resistance in Aspergillus fumigatus. Microbiol. Spectr. 2022, 10, e01112-22. [Google Scholar] [CrossRef]
- Husain, S.; Alexander, B.D.; Munoz, P.; Avery, R.K.; Houston, S.; Pruett, T.; Jacobs, R.; Dominguez, E.A.; Tollemar, J.G.; Baumgarten, K.; et al. Opportunistic mycelial fungal infections in organ transplant recipients: Emerging importance of non-Aspergillus mycelial fungi. Clin. Infect. Dis. 2003, 37, 221–229. [Google Scholar] [CrossRef]
- Nucci, F.; Nouer, S.A.; Capone, D.; Anaissie, E.; Nucci, M. Fusariosis. Semin. Respir. Crit. Care Med. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Lainhart, W. Fusarium spp., a genus of common plant pathogens that can cause devastating, opportunistic human disease. Clin. Microbiol. Newsl. 2018, 40, 1–5. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Li, L.; Zhou, M. Septic shock caused by Rhizobium radiobacter in an elderly woman. Medicine 2019, 98, e18267. [Google Scholar] [CrossRef]
- Yan, Q.; Rogan, C.J.; Pang, Y.; Davis, E.W.; Anderson, J.C. Ancient co-option of an amino acid ABC transporter locus in Pseudomonas syringae for host signal-dependent virulence gene regulation. PLoS Pathog. 2020, 16, e1008680. [Google Scholar] [CrossRef]
- Mallet, L.V.; Becq, J.; Deschavanne, P. Whole genome evaluation of horizontal transfers in the pathogenic fungus Aspergillus fumigatus. BMC Genom. 2010, 11, 171. [Google Scholar] [CrossRef]
- Mehrabi, R.; Bahkali, A.H.; Abd-Elsalam, K.A.; Moslem, M.; Ben M’Barek, S.; Gohari, A.M.; Jashni, M.K.; Stergiopoulos, I.; Kema, G.H.; de Wit, P.J. Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS Microbiol. Rev. 2011, 35, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, M.; Shintani, M. Microbial evolution through horizontal gene transfer by mobile genetic elements. Microb. Biotechnol. 2024, 17, e14408. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Stukenbrock, E.H. Evolution and genome architecture in fungal plant pathogens. Nat. Rev. Microbiol. 2017, 15, 756–771. [Google Scholar] [CrossRef]
- Tsushima, A.; Gan, P.; Kumakura, N.; Narusaka, M.; Takano, Y.; Narusaka, Y.; Shirasu, K. Genomic plasticity mediated by transposable elements in the plant pathogenic fungus Colletotrichum higginsianum. Genome Biol. Evol. 2019, 11, 1487–1500. [Google Scholar] [CrossRef]
- Bloom, A.L.M.; Jin, R.M.; Leipheimer, J.; Bard, J.E.; Yergeau, D.; Wohlfert, E.A.; Panepinto, J.C. Thermotolerance in the pathogen Cryptococcus neoformans is linked to antigen masking via mRNA decay-dependent reprogramming. Nat. Commun. 2019, 10, 12907. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Schrecker, J. Fusarium spp.: Infections and intoxications. GMS Infect. Dis. 2024, 12, Doc04. [Google Scholar] [CrossRef]
- Heitman, J. Sexual reproduction and the evolution of microbial pathogens. Curr. Biol. 2006, 16, R711–R725. [Google Scholar] [CrossRef]
- Latgé, J.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant, and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Guo, Y.; Zhai, Y.; Zhang, Z.; Li, D.; Wang, Z.; Li, J.; He, Z.; Hu, S.; Kang, Y.; Gao, Z. Complete genomic analysis of a kingdom-crossing Klebsiella variicola isolate. Front. Microbiol. 2018, 9, 2428. [Google Scholar] [CrossRef]
- Ramsay, J.; Colombi, E.; Terpolilli, J.; Ronson, C. Symbiosis Islands. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Klockgether, J.; Cramer, N.; Wiehlmann, L.; Davenport, C.F.; Tümmler, B. Pseudomonas aeruginosa Genomic Structure and Diversity. Front. Microbiol. 2011, 2, 150. [Google Scholar] [CrossRef]
- Jurgensen, C.W.; Madsen, A.M. Exposure to the airborne mould Botrytis and its health effects. Ann. Agric. Environ. Med. 2009, 16, 183–196. [Google Scholar] [PubMed]
- Jabeen, R.; Kizhisseri, M.I.; Mayanaik, S.N.; Mohamed, M.M. Bioaerosol assessment in indoor and outdoor environments: A case study from India. Sci. Rep. 2023, 13, 18066. [Google Scholar] [CrossRef] [PubMed]
- CDC. Exserohilum Rostratum. Center for Disease Control and Prevention. 2012. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6141a4.htm (accessed on 10 January 2025).
- Casadevall, A.; Pirofski, L.A. Exserohilum rostratum fungal meningitis associated with methylprednisolone injections. Future Microbiol. 2013, 8, 135–137. [Google Scholar] [CrossRef] [PubMed]
- CDC. Fusarium Keratitis—Multiple States, 2006. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 400–401. [Google Scholar]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W. Outbreak of Intestinal Infection Due to Rhizopus microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef]
- Sheyman, A.T.; Cohen, B.Z.; Friedman, A.H.; Ackert, J.M. An outbreak of fungal endophthalmitis after intravitreal injection of compounded combined bevacizumab and triamcinolone. JAMA Ophthalmol. 2013, 131, 864–869. [Google Scholar] [CrossRef]
- Yu, J.; Yang, M.; Han, J.; Pang, X. Fungal and mycotoxin occurrence, affecting factors, and prevention in herbal medicines: A review. Toxin Rev. 2021, 41, 976–994. [Google Scholar] [CrossRef]
- Ahmed, M.A.E.G.E.S.; Abbas, H.S.; Kotakonda, M. Fungal Diseases Caused by Serious Contamination of Pharmaceuticals and Medical Devices, and Rapid Fungal Detection Using Nano-Diagnostic Tools: A Critical Review. Curr. Microbiol. 2024, 81, 10. [Google Scholar] [CrossRef]
- Parke, J.L.; Gurian-Sherman, D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 2001, 39, 225–258. [Google Scholar] [CrossRef]
- Rasheed, M.U.; Thajuddin, N.; Ahamed, P.; Teklemariam, Z.; Jamil, K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev. Inst. Med. Trop. São Paulo 2014, 56, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Adley, C.C. Ralstonia spp.: Emerging global opportunistic pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 291–304. [Google Scholar] [CrossRef]
- Banks, N.C.; Paini, D.R.; Bayliss, K.L.; Hodda, M. The role of global trade and transport network topology in the human-mediated dispersal of alien species. Ecol. Lett. 2015, 18, 188–199. [Google Scholar] [CrossRef]
- Eschen, R.; Grégoire, J.C.; Hengeveld, G.M.; de Hoop, B.M.; Rigaux, L.; Potting, R.P. Trade patterns of the tree nursery industry in Europe and changes following findings of citrus longhorn beetle, Anoplophora chinensis Forster. NeoBiota 2015, 26, 1–20. [Google Scholar] [CrossRef]
- Magyar, D.; Tischner, Z.; Páldy, A.; Kocsubé, S.; Dancsházy, Z.; Halász, Á.; Kredics, L. Impact of global megatrends on the spread of microscopic fungi in the Pannonian Biogeographical Region. Fungal Biol. Rev. 2021, 37, 71–88. [Google Scholar] [CrossRef]
- Burgess, T.I.; Crous, C.J.; Slippers, B.; Hantula, J.; Wingfield, M.J. Tree invasions and biosecurity: Eco-evolutionary dynamics of hitchhiking fungi. AoB Plants. 2016, 8, plw076. [Google Scholar] [CrossRef]
- Triest, D.; Hendrickx, M. Postharvest disease of banana caused by Fusarium musae: A public health concern? PLoS Pathog. 2016, 12, e1005940. [Google Scholar] [CrossRef]
- Alkhalifah, D.H.M.; Damra, E.; Beni Melhem, M.; Hozzein, W.N. Fungus under a changing climate: Modeling the current and future global distribution of Fusarium oxysporum using geographical information system data. Microorganisms 2023, 11, 468. [Google Scholar] [CrossRef]
- Levine, J.M.; Vilà, M.; Antonio, C.M.D.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef]
- Robert, V.A.; Casadevall, A. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 2009, 200, 1623–1626. [Google Scholar] [CrossRef]
- Araujo, R.; Rodrigues, A.G. Variability of germinative potential among pathogenic species of Aspergillus. J. Clin. Microbiol. 2004, 42, 4335–4337. [Google Scholar] [CrossRef] [PubMed]
- Petzold, E.; Wills, E.; Himmelreich, U.; Mylonakis, E.; Rude, T.; Toffaletti, D.; Cox, G.M.; Miller, J.L.; Perfect, J.R. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 2006, 74, 5877–5887. [Google Scholar] [CrossRef]
- Jung, W.H.; Sham, A.; White, R.; Kronstad, J.W. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 2006, 4, e410. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- Thompson, R.N.; Cobb, R.C.; Gilligan, C.A.; Cunniffe, N.J. Management of invading pathogens should be informed by epidemiology rather than administrative boundaries. Ecol. Model. 2016, 324, 28–32. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Webber, J.F. Tree pests and diseases: The threat to biodiversity and the delivery of ecosystem services. Biodivers. Conserv. 2017, 26, 3167–3181. [Google Scholar] [CrossRef]
- Wylezich, C.; Papa, A.; Beer, M.; Hoper, D. A versatile sample processing workflow for metagenomic pathogen detection. Sci. Rep. 2018, 8, 13108. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef]
- Tan, C.C.S.; Van Dorp, L.; Balloux, F. The evolutionary drivers and correlates of viral host jumps. Nat. Ecol. Evol. 2024, 8, 960–971. [Google Scholar] [CrossRef]
- Li, Y.; Teng, Z.; Zhao, D. Plant-derived cross-kingdom gene regulation benefits human health. Trends Plant Sci. 2023, 28, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vass, M.; Shi, D.; Abualrous, E.; Chambers, J.M.; Chopra, N.; Higgs, C.; Kasavajhala, K.; Li, H.; Nandekar, P.; et al. Benchmarking refined and unrefined AlphaFold2 structures for hit discovery. J. Chem. Inf. Model. 2023, 63, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Lin, L.; Alqarihi, A.; Luo, G.; Youssef, E.G.; Alkhazraji, S.; Yount, N.Y.; Ibrahim, B.A.; Bolaris, M.A.; Edwards, J.E., Jr.; et al. The Hyr1 protein from the fungus Candida albicans is a cross kingdom immunotherapeutic target for Acinetobacter bacterial infection. PLoS Pathog. 2018, 14, e1007056. [Google Scholar] [CrossRef]
- Wong-Bajracharya, J.; Singan, V.R.; Monti, R.; Plett, K.L.; Ng, V.; Grigoriev, I.V.; Martin, F.M.; Anderson, I.C.; Plett, J.M. The ectomycorrhizal fungus Pisolithus microcarpus encodes a microRNA involved in cross-kingdom gene silencing during symbiosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2103527119. [Google Scholar] [CrossRef]
- González-Rodríguez, V.E.; Izquierdo-Bueno, I.; Cantoral, J.M.; Carbú, M.; Garrido, C. Artificial Intelligence: A Promising Tool for Application in Phytopathology. Horticulturae 2024, 10, 197. [Google Scholar] [CrossRef]
- Homma, F.; Huang, J.; van der Hoorn, R.A.L. AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface. Nat. Commun. 2023, 14, 6040. [Google Scholar] [CrossRef]
- Bermas, A.; Geddes-McAlister, J. Combatting the evolution of anti-fungal resistance in Cryptococcus neoformans. Mol. Microbiol. 2020, 114, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.; Ball, B.; Krieger, J.R.; Geddes-McAlister, J. Cross-Kingdom Infection of Macrophages Reveals Pathogen- and Immune-Specific Global Reprogramming and Adaptation. mBio 2022, 13, e01687-22. [Google Scholar] [CrossRef]
- Johansen, H.K.; Gøtzsche, P.C. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database Syst. Rev. 2014, 2014, CD000239. [Google Scholar] [CrossRef]
- Sam, Q.H.; Yew, W.S.; Seneviratne, C.J.; Chang, M.W.; Chai, L.Y.A. Immunomodulation as Therapy for Fungal Infection: Are We Closer? Front. Microbiol. 2018, 9, 1612. [Google Scholar] [CrossRef]
- Umbrello, M.; Marini, J.J.; Formenti, P. Metabolic Support in Acute Respiratory Distress Syndrome: A Narrative Review. J. Clin. Med. 2023, 12, 3216. [Google Scholar] [CrossRef] [PubMed]
- Said, D.G.; Rallis, K.I.; Al-Aqaba, M.A.; Ting, D.S.J.; Dua, H.S. Surgical management of infectious keratitis. Ocul. Surf. 2023, 28, 401–412. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Kundu, M.; Adhikary, S.; Akter, N.; Saha, A.; Sabbir, M.A.A. Stress Responses and Mechanisms of Phytopathogens Infecting Humans: Threats, Drivers, and Recommendations. Stresses 2025, 5, 28. https://doi.org/10.3390/stresses5020028
Hossain MM, Sultana F, Mostafa M, Ferdus H, Kundu M, Adhikary S, Akter N, Saha A, Sabbir MAA. Stress Responses and Mechanisms of Phytopathogens Infecting Humans: Threats, Drivers, and Recommendations. Stresses. 2025; 5(2):28. https://doi.org/10.3390/stresses5020028
Chicago/Turabian StyleHossain, Md. Motaher, Farjana Sultana, Mahabuba Mostafa, Humayra Ferdus, Mrinmoy Kundu, Shanta Adhikary, Nabela Akter, Ankita Saha, and Md. Abdullah Al Sabbir. 2025. "Stress Responses and Mechanisms of Phytopathogens Infecting Humans: Threats, Drivers, and Recommendations" Stresses 5, no. 2: 28. https://doi.org/10.3390/stresses5020028
APA StyleHossain, M. M., Sultana, F., Mostafa, M., Ferdus, H., Kundu, M., Adhikary, S., Akter, N., Saha, A., & Sabbir, M. A. A. (2025). Stress Responses and Mechanisms of Phytopathogens Infecting Humans: Threats, Drivers, and Recommendations. Stresses, 5(2), 28. https://doi.org/10.3390/stresses5020028








