Assessing the Predatory Effects of Invasive Brown Trout on Native Rio Grande Sucker and Rio Grande Chub in Mountain Streams of New Mexico, USA
Abstract
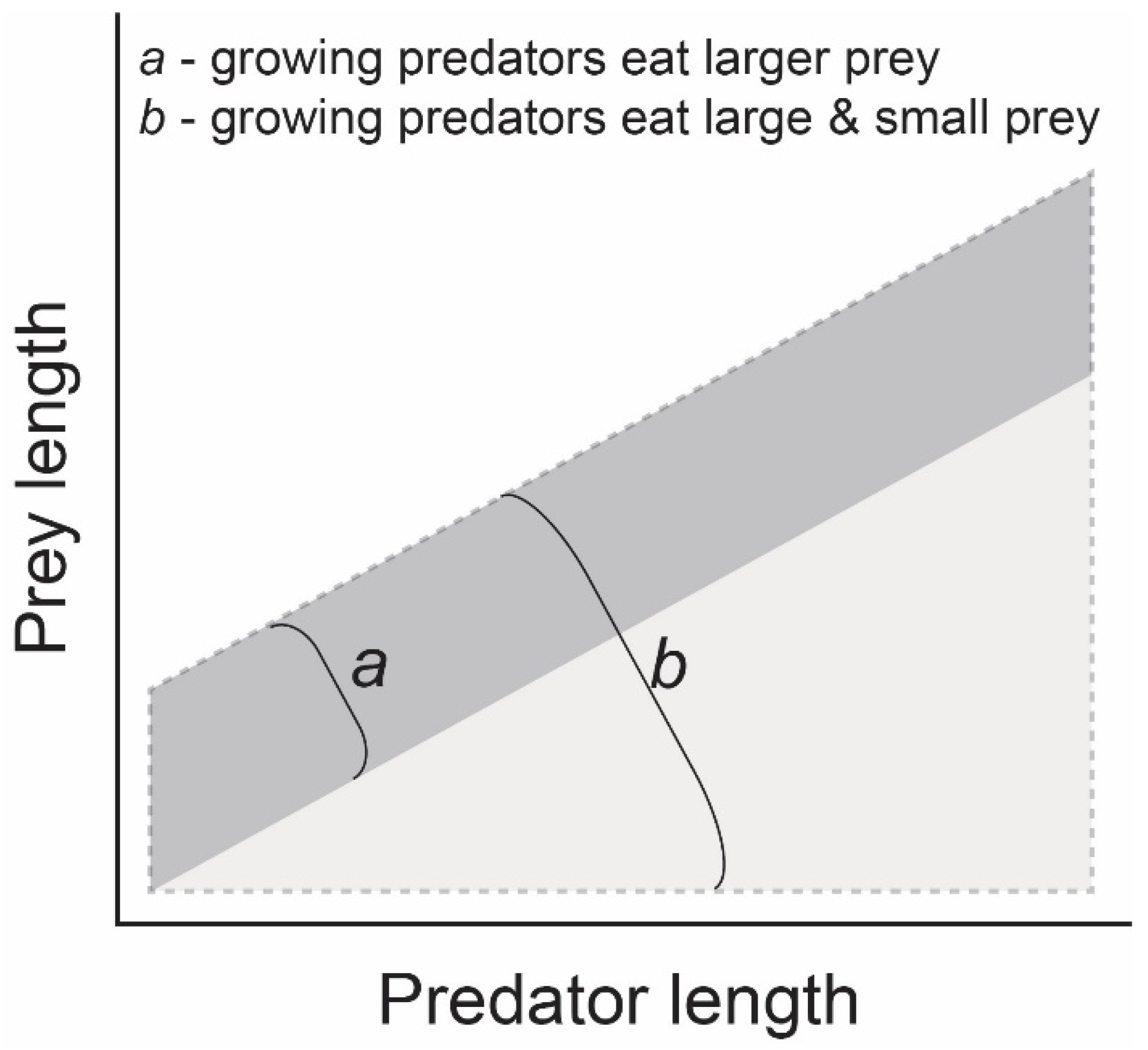
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerfoot, W.C.; Sih, A. Predation: Direct and Indirect Impacts on Aquatic Communities; University Press of New England: Hanover NH, USA, 1987. [Google Scholar]
- Terborgh, J.; Estes, J.A. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Collins, S.F.; Baxter, C.V.; Marcarelli, A.M.; Felicetti, L.; Florin, S.; Wipfli, M.S.; Servheen, G. Reverberating effects of resource exchanges in stream–riparian food webs. Oecologia 2020, 192, 179–189. [Google Scholar] [CrossRef]
- Cohen, J.E.; Pimm, S.L.; Yodzis, P.; Saldaña, J. Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 1993, 62, 67–78. [Google Scholar] [CrossRef]
- Scharf, F.S.; Juanes, F.; Rountree, R.A. Predator size-prey size relationships of marine fish predators: Interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 2000, 208, 229–248. [Google Scholar] [CrossRef]
- Juanes, F.; Buckel, J.A.; Scharf, F.S. Feeding ecology of piscivorous fishes. In Handbook of Fish Biology and Fisheries: Volume 1 Fish Biology; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Publishing: Malden, MA, USA, 2002; pp. 267–283. [Google Scholar]
- Mittelbach, G.G.; Persson, L. The ontogeny of piscivory and its ecological consequences. Can. J. Fish. Aquat. Sci. 1998, 55, 1454–1465. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of Earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Sullivan, C.A. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Cambray, J.A. Impact on indigenous species biodiversity caused by the globalization of alien recreational freshwater fisheries. Hydrobiologia 2003, 500, 217–230. [Google Scholar] [CrossRef]
- Eby, L.A.; Roach, W.J.; Crowder, L.B.; Stanford, J.A. Effects of stocking-up freshwater food webs. Trends Ecol. Evol. 2006, 21, 576–584. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Ormerod, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Harrison, I. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [Green Version]
- Budy, P.; Gaeta, J.W. Brown trout as an invader: A synthesis of problems and perspectives in North America. In Brown Trout: Biology, Ecology, and Management; Lobón-Cerviá, J., Sanz, N., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 525–534. [Google Scholar]
- Sánchez-Hernández, J. Drivers of piscivory in a globally distributed aquatic predator (brown trout): A meta-analysis. Sci. Rep. 2020, 10, 1–10. [Google Scholar]
- MacCrimmon, H.R.; Marshall, T.L. World distribution of Brown Trout, Salmo trutta. J. Fish. Res. Board Can. 1968, 125, 2527–2548. [Google Scholar] [CrossRef]
- Jensen, H.; Kahilainen, K.K.; Amundsen, P.A.; Gjelland, K.Ø.; Tuomaala, A.; Malinen, T.; Bøhn, T. Predation by brown trout (Salmo trutta) along a diversifying prey community gradient. Can. J. Fish. Aquat. Sci. 2008, 65, 1831–1841. [Google Scholar] [CrossRef]
- Jensen, H.; Kiljunen, M.; Amundsen, P.A. Dietary ontogeny and niche shift to piscivory in lacustrine brown trout Salmo trutta revealed by stomach content and stable isotope analyses. J. Fish. Biol. 2012, 80, 2448–2462. [Google Scholar] [CrossRef] [PubMed]
- Bannon, E.; Ringler, N.H. Optimal prey size for stream resident Brown Trout (Salmo trutta): Tests of predictive models. Can. J. Zool. 1986, 64, 704–713. [Google Scholar] [CrossRef]
- Keeley, E.R.; Grant, J.W. Prey size of salmonid fishes in streams, lakes, and oceans. Can. J. Fish. Aquat. Sci. 2001, 58, 1122–1132. [Google Scholar] [CrossRef]
- Cada, G.F.; Loar, J.M.; Cox, D.K. Food and feeding preferences of rainbow and brown trout in southern Appalachian streams. Am. Midl. Nat. 1987, 117, 374–385. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of fish faunas across the United States. Science 2000, 288, 854–856. [Google Scholar] [CrossRef]
- Shemai, B.; Sallenave, R.; Cowley, D.E. Competition between hatchery-raised Rio Grande cutthroat trout and wild brown trout. N. Am. J. Fish. Manag. 2007, 27, 315–325. [Google Scholar] [CrossRef]
- Pritchard, V.L.; Cowley, D.E. Rio Grande Cutthroat Trout (Oncorhynchus clarkii virginalis): A Technical Conservation Assessment. [Online]. USDA Forest Service, Rocky Mountain Region. 2006. Available online: http://www.fs.fed.us/r2/projects/scp/assessments/riograndecutthroattrout.pdf (accessed on 3 December 2021).
- Meredith, C.S.; Budy, P.; Thiede, G.P. Predation on native sculpin by exotic Brown Trout exceeds that by native Cutthroat Trout within a mountain watershed (Logan, UT, USA). Ecol. Freshw. Fish 2015, 24, 133–147. [Google Scholar] [CrossRef]
- Flynn, L. Susceptibility of Rio Grande Cutthroat Trout to Displacement by Non-native Brown Trout. Master’s Thesis, New Mexico State University, Las Cruces, NM, USA, 2020. [Google Scholar]
- Benjamin, J.R.; Baxter, C.V. Is a trout a trout? A range-wide comparison shows nonnative Brook Trout exhibit greater density, biomass, and production than native inland Cutthroat Trout. Biol. Invasions 2012, 14, 1865–1879. [Google Scholar] [CrossRef]
- Bestgen, K.R.; Compton, R.I.; Zelasko, K.A.; Alves, J.E. Distribution and status of Rio Grande chub in Colorado. Larval Fish Laboratory Contribution 135. Fort Collins, CO: Larval Fish Laboratory, Department of Fishery and Wildlife Biology, Colorado State University. 2003. Available online: https://meridian.allenpress.com/jfwm/article-supplement/209670/pdf/022016-jfwm-018_s2 (accessed on 3 December 2021).
- Platania, S.P. Fishes of the Rio Chama and upper Rio Grande, New Mexico, with preliminary comments on their longitudinal distribution. Southwest. Nat. 1991, 36, 186–193. [Google Scholar] [CrossRef]
- Calamusso, B.; Rinne, J.N. Native montane fishes of the middle Rio Grande ecosystem: Status, threats, and conservation. In Rio Grande Ecosystems: Linking Land, Water, and People: Toward a Sustainable Future for the Middle Rio Grande Basin; Finch, D.M., Whitney, J.C., Kelly, J.F., Loftin, S.R., Eds.; Proc. RMRS-P-7: Albuquerque, NM, USA; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 1999; pp. 237–321. [Google Scholar]
- Cope, E.D.; Yarrow, H.C. Report upon the Collections of Fishes Made in Portions of Nevada, Utah, California, Colorado, New Mexico, and Arizona: During the Years 1871, 1872, 1873, and 1874; United States Engineer Office, Geographical Explorations and Surveys West of the One Hundredth Meridian: Washington, DC, USA, 1875; Volume 5. Available online: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101689201-bk (accessed on 10 November 2021).
- Rees, D.E.; Carr, R.J.; Miller, W.J. Rio Grande Chub (Gila pandora): A Technical Conservation Assessment. USDA Forest Service, Rocky Mountain Region. 2005. Available online: http://www.fs.fed.us/r2/projects/scp/assessments/riograndechub.pdf (accessed on 15 February 2022).
- New Mexico Department of Game and Fish. State Wildlife Action Plan for New Mexico; New Mexico Department of Game and Fish: Santa Fe, NM, USA, 2016. [Google Scholar]
- Langlois, D.; Alves, J.; Apker, J. Rio Grande Sucker Recovery Plan; Colorado Division of Wildlife: Montrose, CO, USA, 1994. [Google Scholar]
- Texas Parks and Wildlife Department. Texas Conservation Action Plan 2012–2016: Overview; Connally, W., Ed.; Texas Conservation Action Plan Coordinator: Austin, TX, USA, 2012. Available online: https://tpwd.texas.gov/landwater/land/tcap/ (accessed on 10 November 2021).
- WildEarth Guardians. Petition to List the Rio Grande Chub (Gila Pandora) under the Endangered Species Act; Petition Submitted to the US Secretary of the Interior Acting through the US Fish and Wildlife Service. 2013. Available online: https://pdf.wildearthguardians.org/site/DocServer/Rio_Grande_Chub_WG.pdf (accessed on 10 November 2021).
- WildEarth Guardians. Petition to List the Rio Grande Sucker (Catostomus Plebeius) under the Endangered Species Act; Petition Submitted to the US Secretary of the Interior Acting through the US Fish and Wildlife Service. 2014. Available online: https://ecos.fws.gov/docs/tess/petition/746.pdf (accessed on 10 November 2021).
- RGC and RGS Conservation Team. Conservation Agreement for Rio Grande Chub and Rio Grande Sucker; New Mexico Department of Game and Fish: Santa Fe, NM, USA, 2018; p. 33. [Google Scholar]
- Budy, P.; Thiede, G.P.; Lobón-Cerviá, J.; Fernandez, G.G.; McHugh, P.; McIntosh, A.; Vøllestad, L.A.; Becares, E.; Jellyman, P. Limitation and facilitation of one of the world’s most invasive fish: An intercontinental comparison. Ecology 2013, 94, 356–367. [Google Scholar] [CrossRef]
- Goff, F. Valles Caldera: A Geologic History; UNM Press: Albuquerque, NM, USA, 2009. [Google Scholar]
- Milewski, C.L.; Brown, M.L. Proposed Standard Weight (Ws) Equation and length-categorization standards for stream-dwelling Brown Trout (Salmo trutta). J. Freshwater Ecol. 1994, 9, 111–116. [Google Scholar] [CrossRef]
- Cade, B.S.; Noon, B.R. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 2003, 1, 412–420. [Google Scholar] [CrossRef]
- Scharf, F.S.; Juanes, F.; Sutherland, M. Inferring ecological relationships from the edges of scatter diagrams: Comparison of regression techniques. Ecology 1998, 79, 448–460. [Google Scholar] [CrossRef]
- Jonsson, B.; Sandlund, O.T. Environmental factors and life histories of isolated river stocks of brown trout (Salmo trutta m. fario) in Søre Osa river system, Norway. Environ. Biol. Fish 1979, 4, 43–54. [Google Scholar] [CrossRef]
- Johnson, R.L.; Blumenshine, S.C.; Coghlan, S.M. A bioenergetic analysis of factors limiting brown trout growth in an Ozark tailwater river. Environ. Biol. Fish 2006, 77, 121–132. [Google Scholar] [CrossRef]
- Vik, J.O.; Borgstrøm, R.; Skaala, Ø. Cannibalism governing mortality of juvenile brown trout, Salmo trutta, in a regulated stream. Regul. Rivers Res. Manag. 2001, 17, 583–594. [Google Scholar] [CrossRef]
- De Sostoa, A.; Lobon-Cervia, J. Observations on feeding relationships between fish predators and fish assemblages in a Mediterranean stream. Regul. Rivers Res. Manag. 1989, 4, 157–163. [Google Scholar] [CrossRef]
- Rinne, J.N. Reproductive biology of the Rio Grande sucker, Catostomus plebeius (Cypriniformes), in a montane stream, New Mexico. Southwest. Nat. 1995, 40, 237–241. [Google Scholar]
- Rinne, J.N. Reproductive biology of the Rio Grande Chub, Gila pandora (Teleostomi: Cypriniformes), in a montane stream, New Mexico. Southwest. Nat. 1995, 40, 107–110. [Google Scholar]
- McPhee, M.V. Age, growth, and life history comparisons between the invasive White Sucker (Catastomus commersoni) and native Rio Grande Sucker (C. plebeius). Southwest. Nat. 2007, 52, 15–25. [Google Scholar] [CrossRef]
- Bellmore, J.R.; Baxter, C.V.; Martens, K.; Connolly, P.J. The floodplain food web mosaic: A study of its importance to salmon and steelhead with implications for their recovery. Ecol. Appl. 2013, 23, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.F.; Baxter, C.V.; Marcarelli, A.M.; Wipfli, M.S. Effects of experimentally added salmon subsidies on resident fishes via direct and indirect pathways. Ecosphere 2016, 7, e01248. [Google Scholar] [CrossRef]
- Layman, C.A.; Rypel, A.L. Secondary production is an underutilized metric to assess restoration initiatives. Food Webs 2020, 25, e00174. [Google Scholar] [CrossRef]
- Rypel, A.L.; Saffarinia, P.; Vaughn, C.C.; Nesper, L.; O’Reilly, K.; Parisek, C.A.; Ayers, D. Goodbye to “Rough Fish”: Paradigm Shift in the Conservation of Native Fishes. Fisheries 2021, 46, 605–616. [Google Scholar] [CrossRef]
- Clarkson, R.W.; Marsh, P.C.; Stefferud, S.E.; Stefferud, J.A. Conflicts between native fish and nonnative sport fish management in the southwestern United States. Fisheries 2005, 30, 20–27. [Google Scholar] [CrossRef]
- Carey, M.P.; Sanderson, B.L.; Friesen, T.A.; Barnas, K.A.; Olden, J.D. Smallmouth bass in the Pacific Northwest: A threat to native species; a benefit for anglers. Rev. Fish. Sci. 2011, 19, 305–315. [Google Scholar] [CrossRef]
- Lintermans, M.; Raadik, T. Local eradication of trout from streams using rotenone: The Australian experience. In Managing Invasive Freshwater Fish in New Zealand; Hicks, B.J., Ed.; Department of Conservation: Wellington, New Zealand, 2001; pp. 10–12. [Google Scholar]
- Budy, P.; Walsworth, T.; Thiede, G.P.; Thompson, P.D.; McKell, M.D.; Holden, P.B.; Chase, P.D.; Saunders, W.C. Remarkably rapid recovery of native trout following removal of a dominant non-native trout sub-population: Evidence of resilience and conservation potential. Conserv. Biol. Conserv. Sci. Pract. 2021, 3, e325. [Google Scholar] [CrossRef]




| Stream | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|
| East Fork Jemez River | -- | 13.0 | 3.9 | 0.0 | 12.5 | 0.0 |
| San Antonio | 0.0 | 2.9 | 3.0 | 40.0 | 35.3 | 0.0 |
| Jemez River | -- | 16.7 | 0.0 | 0.0 | 50.0 | 0.0 |
| Rio de las Vacas | 2.8 | 11.1 | 0.0 | 0.0 | 28.6 | 0.0 |
| Rio Cebolla | 1.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Guadalupe | 2.8 | 0.0 | 8.3 | 9.1 | 58.3 | 36.4 |
| Quantile | Term | Estimate | SE | p-Value | L95% | U95% |
|---|---|---|---|---|---|---|
| 0.1 | intercept | 19.23 | 23.6 | 0.416 | −27.14 | 65.61 |
| predator length | 0.03 | 0.1 | 0.711 | −0.16 | 0.24 | |
| 0.3 | intercept | 9.17 | 13.1 | 0.486 | −16.63 | 34.98 |
| predator length | 0.12 | 0.05 | 0.038 | 0.007 | 0.23 | |
| 0.5 | intercept | 13.02 | 15.8 | 0.412 | −18.10 | 44.15 |
| predator length | 0.12 | 0.07 | 0.069 | −0.01 | 0.26 | |
| 0.7 | intercept | −16.27 | 33.5 | 0.628 | −82.06 | 49.51 |
| predator length | 0.32 | 0.1 | 0.027 | 0.03 | 0.61 | |
| 0.9 | intercept | −38.53 | 17.5 | 0.029 | −73.02 | −4.05 |
| predator length | 0.50 | 0.07 | <0.001 | 0.35 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivie, J.; George, O.; Collins, S.F. Assessing the Predatory Effects of Invasive Brown Trout on Native Rio Grande Sucker and Rio Grande Chub in Mountain Streams of New Mexico, USA. Conservation 2022, 2, 514-525. https://doi.org/10.3390/conservation2030035
Ivie J, George O, Collins SF. Assessing the Predatory Effects of Invasive Brown Trout on Native Rio Grande Sucker and Rio Grande Chub in Mountain Streams of New Mexico, USA. Conservation. 2022; 2(3):514-525. https://doi.org/10.3390/conservation2030035
Chicago/Turabian StyleIvie, Jansen, Owen George, and Scott F. Collins. 2022. "Assessing the Predatory Effects of Invasive Brown Trout on Native Rio Grande Sucker and Rio Grande Chub in Mountain Streams of New Mexico, USA" Conservation 2, no. 3: 514-525. https://doi.org/10.3390/conservation2030035
APA StyleIvie, J., George, O., & Collins, S. F. (2022). Assessing the Predatory Effects of Invasive Brown Trout on Native Rio Grande Sucker and Rio Grande Chub in Mountain Streams of New Mexico, USA. Conservation, 2(3), 514-525. https://doi.org/10.3390/conservation2030035





