Fruticose Lichen Communities at the Edge: Distribution and Diversity in a Desert Sky Island on the Colorado Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Selection, Field Methods, and Bulk Sampling
2.2. Molecular Laboratory Methods
2.3. Short-Read Data Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Calder, W.J.; Shuman, B. Extensive wildfires, climate change, and an abrupt state change in subalpine ribbon forests, Colorado. Ecology 2017, 98, 2585–2600. [Google Scholar] [CrossRef] [PubMed]
- Yanahan, A.D.; Moore, W. Impacts of 21st-century climate change on montane habitat in the Madrean Sky Island Archipelago. Divers. Distrib. 2019, 25, 1625–1638. [Google Scholar] [CrossRef]
- Higuera, P.E.; Shuman, B.N.; Wolf, K.D. Rocky Mountain subalpine forests now burning more than any time in recent millennia. Proc. Natl. Acad. Sci. USA 2021, 118, e2103135118. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Rumann, C.S.; Kemp, K.B.; Higuera, P.E.; Harvey, B.J.; Rother, M.T.; Donato, D.C.; Morgan, P.; Veblen, T.T. Evidence for declining forest resilience to wildfires under climate change. Ecol. Lett. 2018, 21, 243–252. [Google Scholar] [CrossRef]
- Hoecker, T.J.; Hansen, W.D.; Turner, M.G. Topographic position amplifies consequences of short-interval stand-replacing fires on postfire tree establishment in subalpine conifer forests. For. Ecol. Manag. 2020, 478, 118523. [Google Scholar] [CrossRef]
- Atkins, K.E.; Travis, J.M.J. Local adaptation and the evolution of species’ ranges under climate change. J. Theor. Biol. 2010, 266, 449–457. [Google Scholar] [CrossRef]
- Comer, P.J.; Hak, J.C.; Reid, M.S.; Auer, S.L.; Schulz, K.A.; Hamilton, H.H.; Smyth, R.L.; Kling, M.M. Habitat Climate Change Vulnerability Index Applied to Major Vegetation Types of the Western Interior United States. Land 2019, 8, 108. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Lumbsch, H.T. A data-driven evaluation of lichen climate change indicators in Central Europe. Biodivers Conserv. 2020, 29, 3959–3971. [Google Scholar] [CrossRef]
- Kalwij, J.M.; Wagner, H.H.; Scheidegger, C. Effects of stand-level disturbances on the spatial distribution of a lichen indicator. Ecol. Appl. 2005, 15, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- Giordani, P.; Brunialti, G.; Benesperi, R.; Rizzi, G.; Frati, L.; Modenesi, P. Rapid biodiversity assessment in lichen diversity surveys: Implications for quality assurance. J. Environ. Monit. 2009, 11, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Jovan, S. Lichen Bioindication of Biodiversity, Air Quality, and Climate: Baseline Results from Monitoring in Washington, Oregon, and California; General Technichal Report, PNW-GTY-737; US Department of Agriculture, Forest Service: Washington, DC, USA, 2008.
- Leavitt, S.D.; St. Clair, L.L. Bio-monitoring in Western North America: What Can Lichens Tell Us about Ecological Disturbances? In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 119–138. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Jovan, S.; Amacher, M.C. Lichen elemental content bioindicators for air quality in upper Midwest, USA: A model for large-scale monitoring. Ecol. Indic. 2017, 78, 253–263. [Google Scholar] [CrossRef]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P. Monitoring with Lichens—Monitoring Lichens; Kluwer Academics: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Seaward, M.R.D. Lichens and sulphur dioxide air pollution: Field studies. Environ. Rev. 1993, 1, 73–91. [Google Scholar] [CrossRef]
- Allen, J.L.; McMullin, R.T.; Tripp, E.A.; Lendemer, J.C. Lichen conservation in North America: A review of current practices and research in Canada and the United States. Biodivers Conserv. 2019, 28, 3103–3138. [Google Scholar] [CrossRef]
- Miller, J.E.D.; Villella, J.; Stone, D.; Hardman, A. Using lichen communities as indicators of forest stand age and conservation value. For. Ecol. Manag. 2020, 475, 118436. [Google Scholar] [CrossRef]
- Hauck, M. Global warming and alternative causes of decline in arctic-alpine and boreal-montane lichens in North-Western Central Europe. Glob. Chang. Biol. 2009, 15, 2653–2661. [Google Scholar] [CrossRef]
- Werth, S.; Wagner, H.H.; Gugerli, F.; Holderegger, R.; Csencsics, D.; Kalwij, J.M.; Scheidegger, C. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 2006, 87, 2037–2046. [Google Scholar] [CrossRef]
- Knick, S.T.; Hanser, S.E.; Preston, K.L. Modeling ecological minimum requirements for distribution of greater sage-grouse leks: Implications for population connectivity across their western range, U.S.A. Ecol. Evol. 2013, 3, 1539–1551. [Google Scholar] [CrossRef]
- Werth, S.; Gugerli, F.; Holderegger, R.; Wagner, H.H.; Csencsics, D.; Scheidegger, C. Landscape-level gene flow in Lobaria pulmonaria, an epiphytic lichen. Mol. Ecol. 2007, 16, 2807–2815. [Google Scholar] [CrossRef] [Green Version]
- Gibson, S.Y.; Van Der Marel, R.C.; Starzomski, B.M. Climate Change and Conservation of Leading-Edge Peripheral Populations. Conserv. Biol. 2009, 23, 1369–1373. [Google Scholar] [CrossRef]
- Bergamini, A.; Stofer, S.; Bolliger, J.; Scheidegger, C. Evaluating macrolichens and environmental variables as predictors of the diversity of epiphytic microlichens. Lichenologist 2007, 39, 475–489. [Google Scholar] [CrossRef]
- Rolstad, J.; Ekman, S.; Andersen, H.L.; Rolstad, E. Genetic variation and reproductive mode in two epiphytic lichens of conservation concern: A transatlantic study of Evernia divaricata and Usnea longissima. Botany 2013, 91, 69–81. [Google Scholar] [CrossRef]
- Högberg, N.; Kroken, S.; Thor, G.; Taylor, J.W. Reproductive mode and genetic variation suggest a North American origin of European Letharia vulpina. Mol. Ecol. 2002, 11, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- McCune, B.; Yang, S.; Jovan, S.; Root, H.T. Climate and epiphytic macrolichen communities in the Four Corners region of the U.S.A. Bryologist 2022, 125, 70–90. [Google Scholar] [CrossRef]
- Cometto, A.; Leavitt, S.D.; Millanes, A.M.; Wedin, M.; Grube, M.; Muggia, L. The yeast lichenosphere: High diversity of basidiomycetes from the lichens Tephromela atra and Rhizoplaca melanophthalma. Fungal Biol. 2022, 126, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Banchi, E.; Stankovic, D.; Fernández-Mendoza, F.; Gionechetti, F.; Pallavicini, A.; Muggia, L. ITS2 metabarcoding analysis complements lichen mycobiome diversity data. Mycol. Prog. 2018, 17, 1049–1066. [Google Scholar] [CrossRef]
- Smith, H.B.; Dal Grande, F.; Muggia, L.; Keuler, R.; Divakar, P.K.; Grewe, F.; Schmitt, I.; Lumbsch, H.T.; Leavitt, S.D. Metagenomic data reveal diverse fungal and algal communities associated with the lichen symbiosis. Symbiosis 2020, 82, 133–147. [Google Scholar] [CrossRef]
- Tuovinen, V.; Ekman, S.; Thor, G.; Vanderpool, D.; Spribille, T.; Johannesson, H. Two Basidiomycete Fungi in the Cortex of Wolf Lichens. Curr. Biol. 2019, 29, 476–483. [Google Scholar] [CrossRef]
- Spribille, T. Relative symbiont input and the lichen symbiotic outcome. Curr. Opin. Plant Biol. 2018, 44, 57–63. [Google Scholar] [CrossRef]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488. [Google Scholar] [CrossRef]
- Culberson, C.F. Improved conditions and new data for identification of lichen products by standardized thin-layer chromatographic method. J. Chromatogr. A 1972, 72, 113–125. [Google Scholar] [CrossRef]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2001; p. 101. [Google Scholar]
- Op De Beeck, M.; Lievens, B.; Busschaert, P.; Declerck, S.; Vangronsveld, J.; Colpaert, J.V. Comparison and Validation of Some ITS Primer Pairs Useful for Fungal Metabarcoding Studies. PLoS ONE 2014, 9, e97629. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, M.; Wang, M.; Valencia, B.; Palenik, B. Spatial and temporal variations in Synechococcus microdiversity in the Southern California coastal ecosystem. Environ. Microbiol. 2021, 23, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2017, 34, 1287–1294. [Google Scholar] [CrossRef]
- Bernard, M.; Rué, O.; Mariadassou, M.; Pascal, G. FROGS: A powerful tool to analyse the diversity of fungi with special management of internal transcribed spacers. Brief. Bioinform. 2021, 22, bbab318. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2013, 42, D633–D642. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Lücking, R.; Leavitt, S.D.; Hawksworth, D.L. Species in lichen-forming fungi: Balancing between conceptual and practical considerations, and between phenotype and phylogenomics. Fungal Divers. 2021, 109, 99–154. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef]
- Moncada, B.; Sipman, H.; Lücking, R. Testing DNA barcoding in Usnea (Parmeliaceae) in Colombia using the internal transcribed spacer (ITS). Plant Fungal Syst. 2020, 65, 358–385. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Bungartz, F.; Nash III, T.H.; Ryan, B.D. Morphology and anatomy of chasmolithic versus epilithic growth: A taxonomic revision of inconspicuous saxicolous Buellia species from the Sonoran Desert Region generally ascribed to the "Buellia punctata" group. Can. J. Bot. 2004, 82, 540–562. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.L.; Zhao, Z.T.; Wang, W.C.; Leavitt, S.D.; Lumbsch, H.T. A molecular phylogeny of the lichen genus Lecidella focusing on species from mainland China. PLoS ONE 2015, 10, e0139405. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Esslinger, T.L.; Spribille, T.; Divakar, P.K.; Lumbsch, H.T. Multilocus phylogeny of the lichen-forming fungal genus Melanohalea (Parmeliaceae, Ascomycota): Insights on diversity, distributions, and a comparison of species tree and concatenated topologies. Mol. Phylogenetics Evol. 2013, 66, 138–152. [Google Scholar] [CrossRef]
- Muggia, L.; Baloch, E.; Stabentheiner, E.; Grube, M.; Wedin, M. Photobiont association and genetic diversity of the optionally lichenized fungus Schizoxylon albescens. FEMS Microbiol. Ecol. 2011, 75, 255–272. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Lumbsch, H.T.; St. Clair, L.L. Contrasting demographic histories of two species in the lichen-forming fungal genus Xanthomendoza (Teloschistaceae, Ascomycota). Bryol. 2013, 116, 337–349. [Google Scholar] [CrossRef]
- Copeland, S.M.; Bradford, J.B.; Duniway, M.C.; Schuster, R.M. Potential impacts of overlapping land-use and climate in a sensitive dryland: A case study of the Colorado Plateau, USA. Ecosphere 2017, 8, e01823. [Google Scholar] [CrossRef]
- Munson, S.M.; Belnap, J.; Schelz, C.D.; Moran, M.; Carolin, T.W. On the brink of change: Plant responses to climate on the Colorado Plateau. Ecosphere 2011, 2, art68. [Google Scholar] [CrossRef]
- Baker, W.L.; Veblen, T.T. Spruce beetles and fires in the nineteenth-century subalpine forests of western Colorado, USA. Arct. Alp. Res. 1990, 22, 65–80. [Google Scholar] [CrossRef]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe—A review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Garrido-Benavent, I.; Pérez-Ortega, S. Past, present, and future research in bipolar lichen-forming fungi and their photobionts. Am. J. Bot. 2017, 104, 1660–1674. [Google Scholar] [CrossRef]
- Printzen, C.; Domaschke, S.; Fernández-Mendoza, F.; Pérez-Ortega, S. Biogeography and ecology of Cetraria aculeata, a widely distributed lichen with a bipolar distribution. MycoKeys 2013, 6, 33–53. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Arnold, A.E. Community Analysis Reveals Close Affinities Between Endophytic and Endolichenic Fungi in Mosses and Lichens. Microb. Ecol. 2010, 60, 340–353. [Google Scholar] [CrossRef]
- Muggia, L.; Grube, M. Fungal Diversity in Lichens: From Extremotolerance to Interactions with Algae. Life 2018, 8, 15. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Thirunavukkarasu, N. Endolichenic fungi: The lesser known fungal associates of lichens. Mycology 2017, 8, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Tagirdzhanova, G.; Saary, P.; Tingley, J.P.; Díaz-Escandón, D.; Abbott, D.W.; Finn, R.D.; Spribille, T. Predicted Input of Uncultured Fungal Symbionts to a Lichen Symbiosis from Metagenome-Assembled Genomes. Genome Biol. Evol. 2021, 13, evab047. [Google Scholar] [CrossRef]
- Millanes, A.M.; Diederich, P.; Wedin, M. Cyphobasidium gen. nov., a new lichen-inhabiting lineage in the Cystobasidiomycetes (Pucciniomycotina, Basidiomycota, Fungi). Fungal Biol. 2016, 120, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Beck, J.L.; Bradford, J.B.; Bybee, J.; Campbell, S.; Carlson, J.; Christiansen, T.J.; Clause, K.J.; Collins, G.; Crist, M.R. Science Framework for Conservation and Restoration of the Sagebrush Biome: Linking the Department of the Interior’s Integrated Rangeland Fire Management Strategy to Long-Term strategic Conservation Actions; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2017; p. 213.
- Farkas, E.; Varga, N.; Veres, K.; Matus, G.; Sinigla, M.; Lőkös, L. Distribution Types of Lichens in Hungary That Indicate Changing Environmental Conditions. J. Fungi 2022, 8, 600. [Google Scholar] [CrossRef] [PubMed]
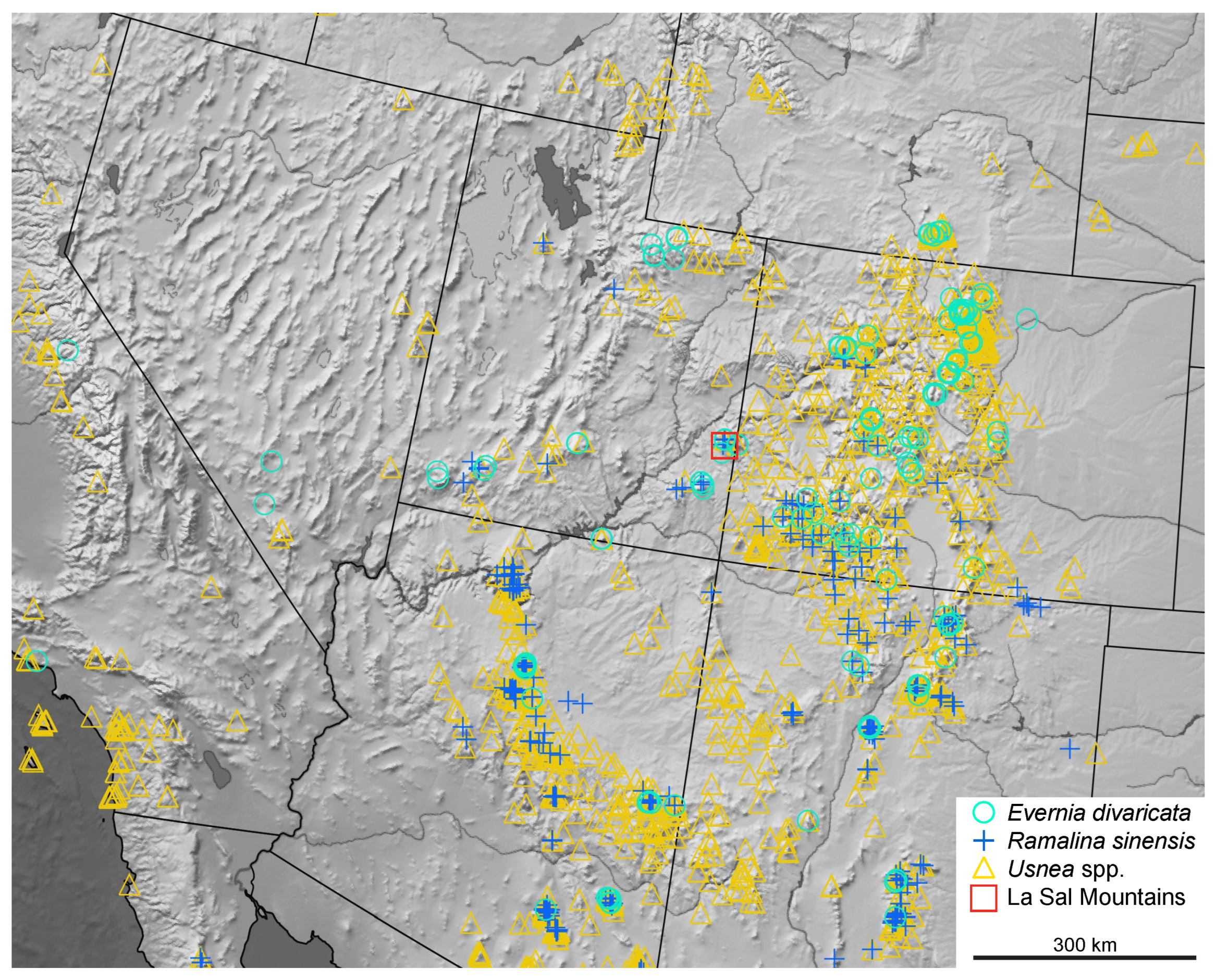


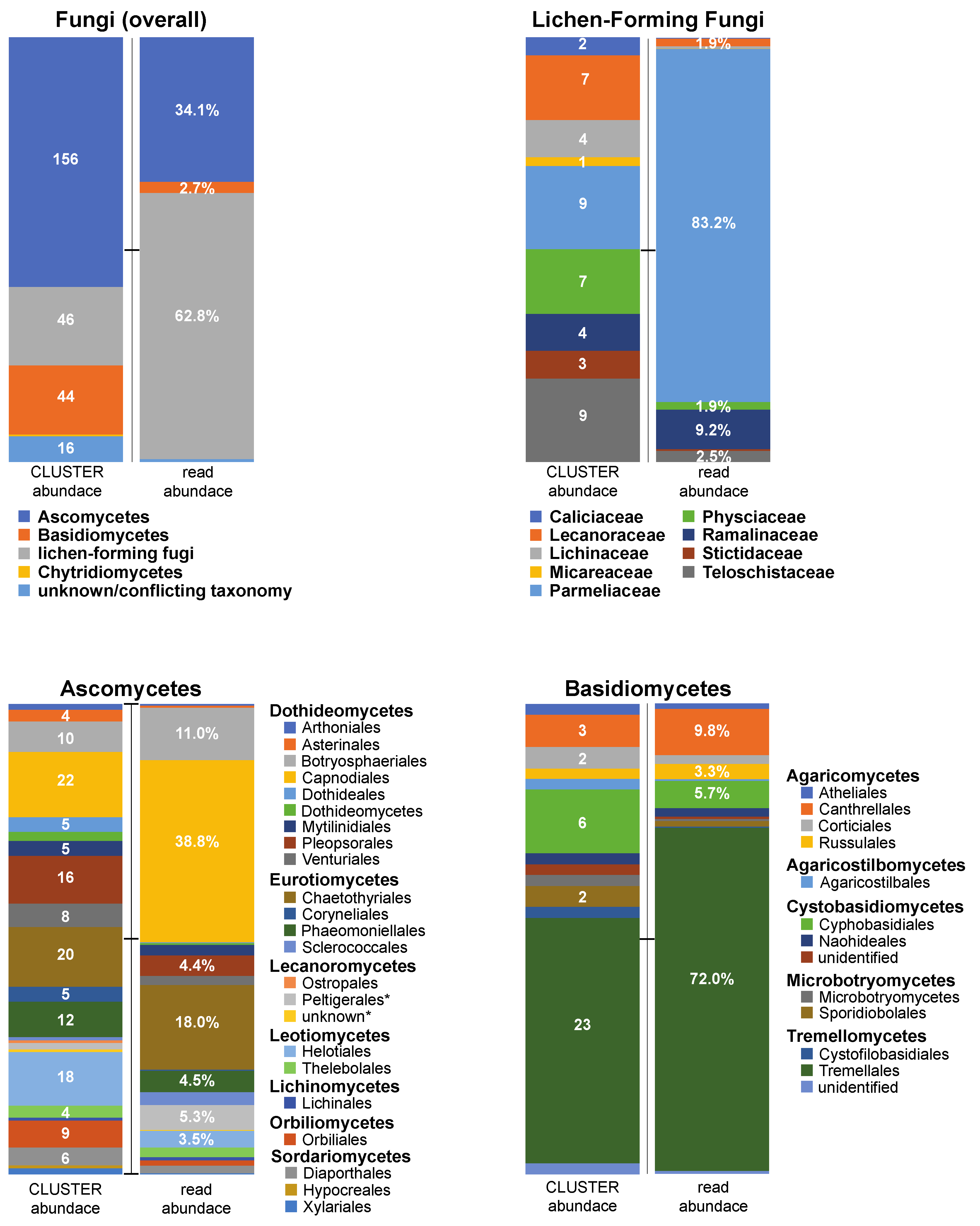

| Name | Estimated Area | Coordinates | Altitude (m a.s.l.) |
|---|---|---|---|
| Geyser Pass 1 | 10,000 m2 | 38.4864, −109.2491 | 3050 |
| Geyser Pass 2 | 19,000 m2 | 38.4821, −109.2368 | 3200 |
| Medicine Lake | 65,000 m2 | 38.4118, −109.2458 | 3040 |
| Taxon | #Genetic Clusters | Notes |
|---|---|---|
| Amandinea aff. punctata (Hoffm.) Coppins & Scheid. | 2 | cosmopolitan lichen likely comprising multiple, distinct species-level mycobiont lineages [48]; common on Colorado Plateau |
| Bibbya vermifera (Nyl.) Kistenich et al. | 1 | uncommon in North America, and this is likely the first report from the Colorado Plateau |
| Caloplaca sp. | 1 | ITS2 sequence from La Sals was 99.4% similar to unidentified Caloplaca from central Europe |
| Micarea sp. | 1 | ITS2 sequence from La Sals was 91.4% similar to Micarea sequences on GenBank |
| Evernia divaricata (L.) Ach. | 2 | occurs worldwide on conifers in montane to subalpine forests; red-listed in all European countries where it occurs [25] |
| Lecidella euphorea (Flörke) Hertel | 3 | occurs worldwide and likely comprises multiple, distinct species-level mycobiont lineages [49]; common on Colorado Plateau |
| Lecidella sp. | 1 | ITS2 sequence from the La Sals was recovered in the “Lecidella elaeochroma clade” [49] |
| Melanohalea exasperatula (De Not.) O. Blanco et al. | 2 | widespread across Europe and western North America [50]; common on Colorado Plateau |
| Melanohalea subolivacea (Nyl.) O. Blanco et al. | 2 | widespread across western North America [50]; common on Colorado Plateau |
| Myriolecis juniperina (Śliwa) Śliwa, Zhao Xin & Lumbsch | 1 | occurs on the Colorado Plateau at mid elevations—this is the first known report from subalpine forests |
| Myriolecis sp. | 1 | ITS2 sequence from the La Sals was recovered with a provisionally named species M. “altaterrae” nom. prov. |
| Myriolecis wetmorei (Śliwa) Śliwa, Zhao Xin & Lumbsch | 1 | occurs at higher elevations throughout western North America (and Armenia); common on Colorado Plateau |
| Parvoplaca sp. | 1 | ITS2 sequence was 97.4% similar to P. nigroblastidiata from Turkey |
| Phaeophyscia sp. | 1 | Phaeophyscia species commonly occur in montane habitats throughout western North America |
| Phylliscum aff. demangeonii (Moug. & Mont.) Nyl. | 4 | ID uncertain: ITS2 sequence was 97.8% similar to ITS sequence from type (NR_120130); however, BLAST searches also shown high similarity to uncultured Rhinocladiella (Eurotiomycetes) |
| Physcia adscendens (Fr.) H. Olivier | 1 | widely distributed in temperate and boreal areas in all continents; common on Colorado Plateau |
| Polycauliona sp. | 1 | NA. P. candelaria occurs scattered throughout the Intermountain West, but the ITS2 sequence from the La Sals was highly dissimilar to P. candelaria sequences on GenBank (ca. 92% similarity) |
| Ramalina sinensis Jatta | 3 | cosmopolitan in temperate regions, and common in montane habitats on the Colorado Plateau |
| Rinodina sp. 1 | 1 | NA—voucher specimen required for identification |
| Rinodina sp. 2 | 2 | NA—voucher specimen required for identification |
| Rinodina sp. 3 | 1 | NA–voucher specimen required for identification |
| Schizoxylon albescens Gilenstam, Döring & Wedin | 1 | occurs both as lichen and as saprobe [51]; not previously reported in North America |
| Stictidaceae sp. | 2 | ITS2 sequence from La Sals was 93.6% similar to Stictis brunnescens. If these clusters truly represent a species in Stictis, they are one of the first members of the genus reported for western North America |
| Tetramelas pulverulentus (Anzi) A. Nordin & Tibell | 1 | endoparasite within members of the Physciaceae; likely first report from the Colorado Plateau |
| Usnea aff. barbata (L.) F.H. Wigg. | 2 (haplotypes) | widespread across western North America; rarely collected on Colorado Plateau. |
| Usnea cavernosa Tuck. | 98 (haplotypes) | Eurasian and North American distribution; occurring sporadically on the Colorado Plateau |
| Usnea lapponica | 1 (haplotypes) | likely cosmopolitan, occurring throughout the Intermountain West |
| Usnea perplexans Stirt. | 2 (haplotypes) | likely cosmopolitan, occurring throughout the Intermountain West |
| Usnea sp. | 1 (haplotypes) | NA |
| Xanthomendoza montana (L. Lindblom) Søchting et al. | 6 | widespread across western North America [52]; common on Colorado Plateau |
| Taxon | # of Hapoltypes | Notes |
|---|---|---|
| Evernia divaricata | 8 | Haplotypes from the La Sals were distributed across multiple, weakly supported clades in the ITS topology. |
| Ramalina sinensis | 13 | Haplotypes from the La Sals were recovered within a single clade comprised exclusively of closely related haplotypes from the La Sals and sister to a clade comprised of two specimens from Arizona and New Mexico. |
| Usnea spp. | 104 total U. aff. barbata (2) U. cavernosa (98) U. lapponica (1) U. perplexans (2) U. sp. (1) | The U. cavernosa haplotypes were recovered within a clade comprised of closely related sequences from Idaho (USA) and Switzerland. The phylogenetic position of specimens identified as U. barbata, U. perplexans, and U. sp. were unresolved; the U. lapponica haplotype was recovered within a clade of other U. lapponica sequences from Austria, Estonia, Canada, India, Spain, Switzerland, and the USA. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robison, A.; Baugh, M.; Muggia, L.; Leavitt, S.D. Fruticose Lichen Communities at the Edge: Distribution and Diversity in a Desert Sky Island on the Colorado Plateau. Conservation 2022, 2, 550-565. https://doi.org/10.3390/conservation2040037
Robison A, Baugh M, Muggia L, Leavitt SD. Fruticose Lichen Communities at the Edge: Distribution and Diversity in a Desert Sky Island on the Colorado Plateau. Conservation. 2022; 2(4):550-565. https://doi.org/10.3390/conservation2040037
Chicago/Turabian StyleRobison, Abigail, Mikele Baugh, Lucia Muggia, and Steven D. Leavitt. 2022. "Fruticose Lichen Communities at the Edge: Distribution and Diversity in a Desert Sky Island on the Colorado Plateau" Conservation 2, no. 4: 550-565. https://doi.org/10.3390/conservation2040037
APA StyleRobison, A., Baugh, M., Muggia, L., & Leavitt, S. D. (2022). Fruticose Lichen Communities at the Edge: Distribution and Diversity in a Desert Sky Island on the Colorado Plateau. Conservation, 2(4), 550-565. https://doi.org/10.3390/conservation2040037






