Adhesion and Structural Changes of PEGylated Lipid Nanocarriers on Silica Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Liposomes and Lipodisks
2.3. QCM-D Characterizations
2.4. NPS/QCM-D
2.5. MP-SPR
3. Results and Discussion
3.1. Lipodisks
3.1.1. Adsorption on Silica at 21 °C
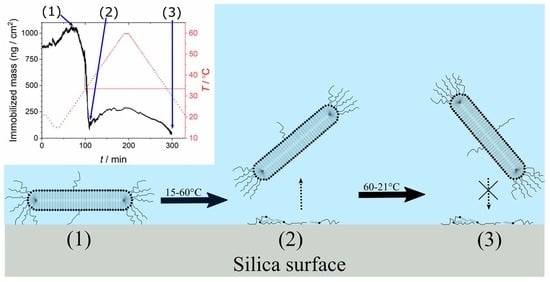
3.1.2. Effect of Temperature
3.1.3. The Effect of the Hydration Layer on the Interaction of Lipodisks with Silica
3.2. Liposomes
3.2.1. Liposomes in the Liquid Disordered Phase State
3.2.2. Liposomes in the Gel Phase State
3.2.3. Liposomes in the Liquid Ordered Phase (DSPC:Cholesterol)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics 2020, 12, 1235. [Google Scholar] [CrossRef]
- Needham, D.; McIntosh, T.J.; Lasic, D.D. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. BBA Biomembr. 1992, 1108, 40–48. [Google Scholar] [CrossRef]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. BBA-Rev. Biomembr. 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Blume, G.; Cevc, G. Liposomes for the sustained drug release in vivo. BBA Biomembr. 1990, 1029, 91–97. [Google Scholar] [CrossRef]
- Ahlgren, S.; Reijmar, K.; Edwards, K. Targeting lipodisks enable selective delivery of anticancer peptides to tumor cells. Nanomedicine 2017, 13, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Kanwaldeep, K.G.; Kaddoumi, A.; Nazzal, S. PEG–lipid micelles as drug carriers: Physiochemical attributes, formulation principles and biological implication. J. Drug Target. 2015, 23, 222–231. [Google Scholar]
- Johan, J.F.V.; Carpenter, J.F.; Anchordoquy, T.J.; Schellekens, H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov. Today 2014, 19, 1945–1952. [Google Scholar]
- Anchordoquy, T.J.; Simberg, D. Watching the gorilla and questioning delivery dogma. J. Control. Release 2017, 262, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.K.; Agmo Hernández, V. Choice of cuvette material can influence spectroscopic leakage and permeability experiments with liposomes. Chem. Phys. Lipids 2018, 215, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Moudgil, B.M. Adsorption Mechanism(s) of Poly(Ethylene Oxide) on Oxide Surfaces. J. Colloid Interface Sci. 1997, 196, 92–98. [Google Scholar] [CrossRef]
- Wind, B.; Killmann, E. Adsorption of polyethylene oxide on surface modified silica—Stability of bare and covered particles in suspension. Colloid Polym. Sci. 1998, 276, 903–912. [Google Scholar] [CrossRef]
- Char, K.; Alice, G.; Curtis, F. Fluorescence studies of polymer adsorption. 1. Rearrangement and displacement of pyrene-terminated polyethylene glycol on colloidal silica particles. Langmuir 1988, 4, 989–998. [Google Scholar] [CrossRef]
- Chang, D.P.; Jankunec, M.; Barauskas, J.; Tiberg, F.; Nylander, T. Adsorption of Lipid Liquid Crystalline Nanoparticles on Cationic, Hydrophilic, and Hydrophobic Surfaces. ACS Appl. Mater. Interface 2012, 4, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.; Kitchener, J.A. The mechanism of adsorption of poly(ethylene oxide) flocculant on silica. J. Colloid Interface Sci. 1976, 57, 132–142. [Google Scholar] [CrossRef]
- Grad, P.; Agmo Hernández, V.; Edwards, K. Avoiding artifacts in liposome leakage measurements via cuvette- and liposome-surface modifications. J. Lipid Res. accepted.
- Iruthayaraj, J.; Poptoshev, E.; Vareikis, A.; Makuška, R.; van der Wal, A.; Claesson, P.M. Adsorption of Low Charge Density Polyelectrolyte Containing Poly(ethylene oxide) Side Chains on Silica: Effects of Ionic Strength and pH. Macromolecules 2005, 38, 6152–6160. [Google Scholar] [CrossRef]
- Liese, S.; Gensler, M.; Krysiak, S.; Schwarzl, R.; Achazi, A.; Paulus, B.; Hugel, T.; Rabe, J.P.; Netz, R.R. Hydration Effects Turn a Highly Stretched Polymer from an Entropic into an Energetic Spring. ACS Nano 2017, 11, 702–712. [Google Scholar] [CrossRef] [Green Version]
- Kolberg, A.; Wenzel, C.; Hackenstrass, K.; Schwarzl, R.; Rüttiger, C.; Hugel, T.; Gallei, M.; Netz, R.R.; Balzer, B.N. Opposing Temperature Dependence of the Stretching Response of Single PEG and PNiPAM Polymers. J. Am. Chem. Soc. 2019, 141, 11603–11613. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Ortega, F.; Rubio, R.G. Adsorption of Mixtures of a Pegylated Lipid with Anionic and Zwitterionic Surfactants at Solid/Liquid. Colloids Interfaces 2020, 4, 47. [Google Scholar] [CrossRef]
- Zetterberg, M.M.; Ahlgren, S.; Hernández, V.A.; Parveen, N.; Edwards, K. Optimization of lipodisk properties by modification of the extent and density of the PEG corona. J. Colloid Interface Sci. 2016, 484, 86–96. [Google Scholar] [CrossRef]
- Agmo Hernández, V.; Reijmar, K.; Edwards, K. Label-Free Characterization of Peptide–Lipid Interactions Using Immobilized Lipodisks. Anal. Chem. 2013, 85, 7377–7384. [Google Scholar] [CrossRef] [Green Version]
- Ferhan, A.R.; Jackman, J.A.; Cho, N.-J. Integration of Quartz Crystal Microbalance-Dissipation and Reflection-Mode Localized Surface Plasmon Resonance Sensors for Biomacromolecular Interaction Analysis. Anal. Chem. 2016, 88, 12524–12531. [Google Scholar] [CrossRef]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Rupert, D.L.M.; Shelke, G.V.; Emilsson, G.; Claudio, V.; Block, S.; Lässer, C.; Dahlin, A.; Lötvall, J.O.; Bally, M.; Zhdanov, V.P.; et al. Dual-Wavelength Surface Plasmon Resonance for Determining the Size and Concentration of Sub-Populations of Extracellular Vesicles. Anal. Chem. 2016, 88, 9980–9988. [Google Scholar] [CrossRef] [PubMed]
- Saigal, T.; Riley, J.K.; Lynn Golas, P.; Bodvik, R.; Claesson, P.M.; Matyjaszewski, K.; Tilton, R.D. Poly(Ethylene Oxide) Star Polymer Adsorption at the Silica/Aqueous Interface and Displacement by Linear Poly(Ethylene Oxide). Langmuir 2013, 29, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Nawal, D.; Sylvère, S.; Yves, G.; René, O.; Mireille, P. Polyethylene Glycol Adsorption on Silica: From Bulk Phase Behavior to Surface Phase Diagram. Langmuir 2007, 23, 6631–6637. [Google Scholar]
- Pashley, R.M.; Israelachvili, J.N. Dlvo and hydration forces between mica surfaces in Mg2+, Ca2+, Sr2+, and Ba2+ chloride solutions. J. Colloid Interface Sci. 1984, 97, 446–455. [Google Scholar] [CrossRef]
- Pashley, R.M.; Quirk, J.P. The effect of cation valency on DLVO and hydration forces between macroscopic sheets of muscovite mica in relation to clay swelling. Colloid Surf. 1984, 9, 1–17. [Google Scholar] [CrossRef]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.A.; Glasmastar, K.; Zhdanov, V.P.; Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett. 2000, 84, 5443–5446. [Google Scholar] [CrossRef]
- Reijmar, K.; Edwards, K.; Andersson, K.; Agmo Hernandez, V. Characterizing and controlling the loading and release of cationic amphiphilic peptides onto and from PEG-stabilized lipodisks. Langmuir ACS J. Surf. Colloids 2016, 32, 12091–12099. [Google Scholar] [CrossRef]
- Lundsten, S.; Agmo Hernandez, V.; Gedda, L.; Saren, T.; Brown, C.J.; Lane, D.P.; Edwards, K.; Nestor, M. Tumor-Targeted Delivery of the p53-Activating Peptide VIP116 with PEG-Stabilized Lipodisks. Nanomaterials 2020, 10, 783. [Google Scholar] [CrossRef] [Green Version]
- Seifert, U.; Lipowsky, R. Adhesion of Vesicles. Phys. Rev. A 1990, 42, 4768–4771. [Google Scholar] [CrossRef]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Szewczuk-Karpisz, K.; Ostolska, I. Temperature effect on the adsorption equilibrium at the silica–polyethylene glycol solution interface. Fluid Phase Equilibr. 2013, 360, 10–15. [Google Scholar] [CrossRef]


















| Temperature | Time |
|---|---|
| 21 °C | 10 min |
| From 21 to 15 °C | 20 min |
| 15 °C | 10 min |
| From 15 to 60 °C | 150 min |
| 60 °C | 10 min |
| From 60 to 21 °C | 130 min |
| 21 °C | 10 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grad, P.; Edwards, K.; Agmo Hernández, V. Adhesion and Structural Changes of PEGylated Lipid Nanocarriers on Silica Surfaces. Physchem 2021, 1, 133-151. https://doi.org/10.3390/physchem1020009
Grad P, Edwards K, Agmo Hernández V. Adhesion and Structural Changes of PEGylated Lipid Nanocarriers on Silica Surfaces. Physchem. 2021; 1(2):133-151. https://doi.org/10.3390/physchem1020009
Chicago/Turabian StyleGrad, Philipp, Katarina Edwards, and Víctor Agmo Hernández. 2021. "Adhesion and Structural Changes of PEGylated Lipid Nanocarriers on Silica Surfaces" Physchem 1, no. 2: 133-151. https://doi.org/10.3390/physchem1020009
APA StyleGrad, P., Edwards, K., & Agmo Hernández, V. (2021). Adhesion and Structural Changes of PEGylated Lipid Nanocarriers on Silica Surfaces. Physchem, 1(2), 133-151. https://doi.org/10.3390/physchem1020009