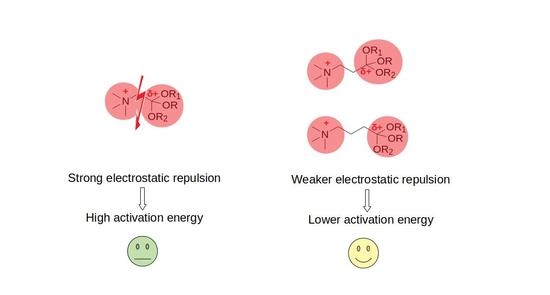
Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism
Abstract
Share and Cite
Moulandou-Koumba, R.D.; Guégan, F.; Ouamba, J.-M.; N’Sikabaka, S.; Frapper, G. Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism. Physchem 2021, 1, 288-296. https://doi.org/10.3390/physchem1030022
Moulandou-Koumba RD, Guégan F, Ouamba J-M, N’Sikabaka S, Frapper G. Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism. Physchem. 2021; 1(3):288-296. https://doi.org/10.3390/physchem1030022
Chicago/Turabian StyleMoulandou-Koumba, Richail Dubien, Frédéric Guégan, Jean-Maurille Ouamba, Samuel N’Sikabaka, and Gilles Frapper. 2021. "Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism" Physchem 1, no. 3: 288-296. https://doi.org/10.3390/physchem1030022
APA StyleMoulandou-Koumba, R. D., Guégan, F., Ouamba, J.-M., N’Sikabaka, S., & Frapper, G. (2021). Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism. Physchem, 1(3), 288-296. https://doi.org/10.3390/physchem1030022