Structure and Reactivity of CoFe2O4(001) Surfaces in Contact with a Thin Water Film
Abstract
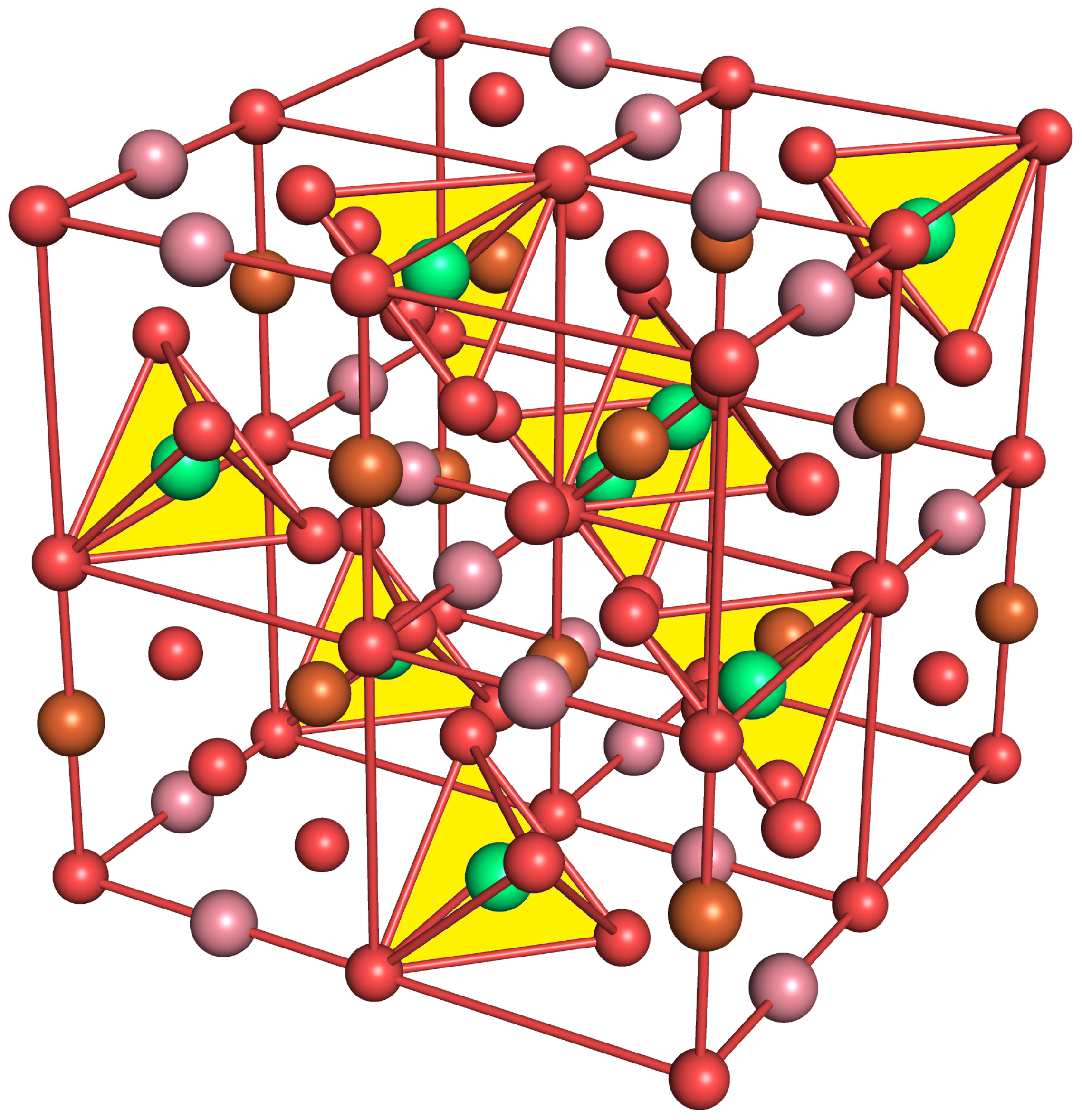
:1. Introduction
2. Computational Details
3. Results and Discussion
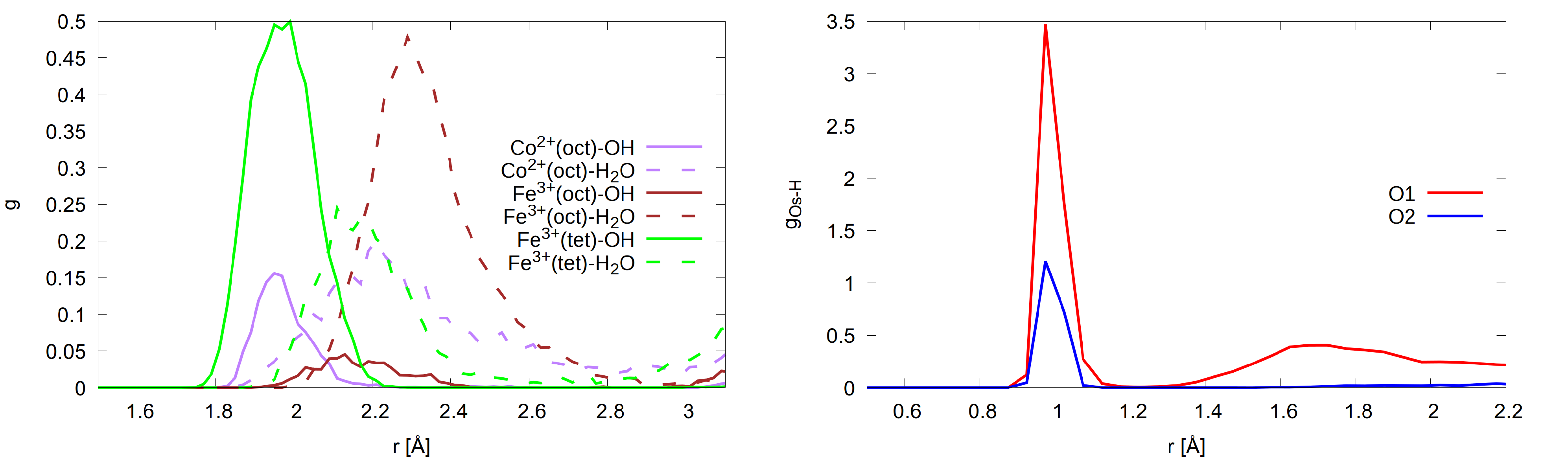
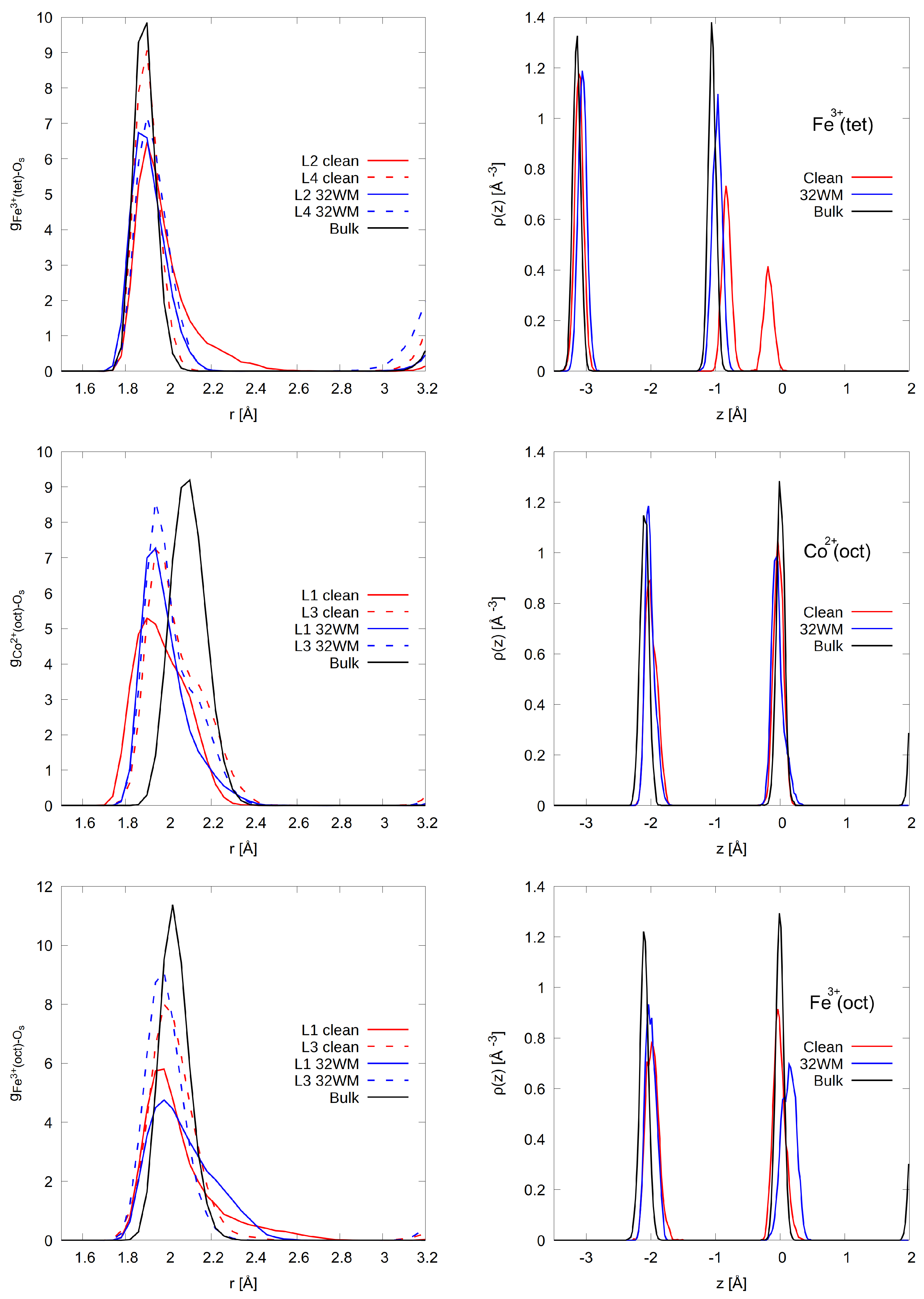
3.1. The Structure of Interfacial Water
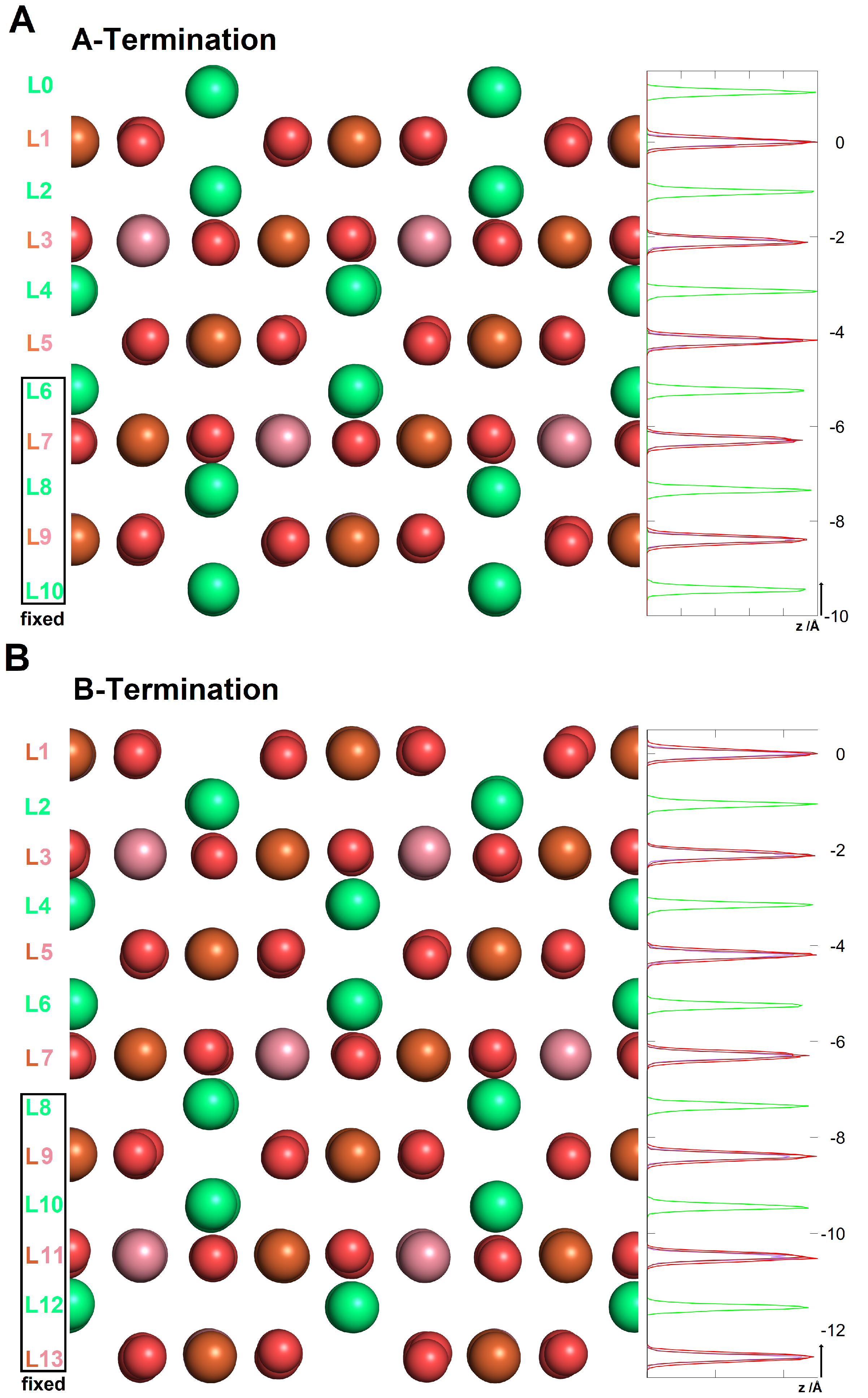
3.1.1. A-Termination
3.1.2. B-Termination
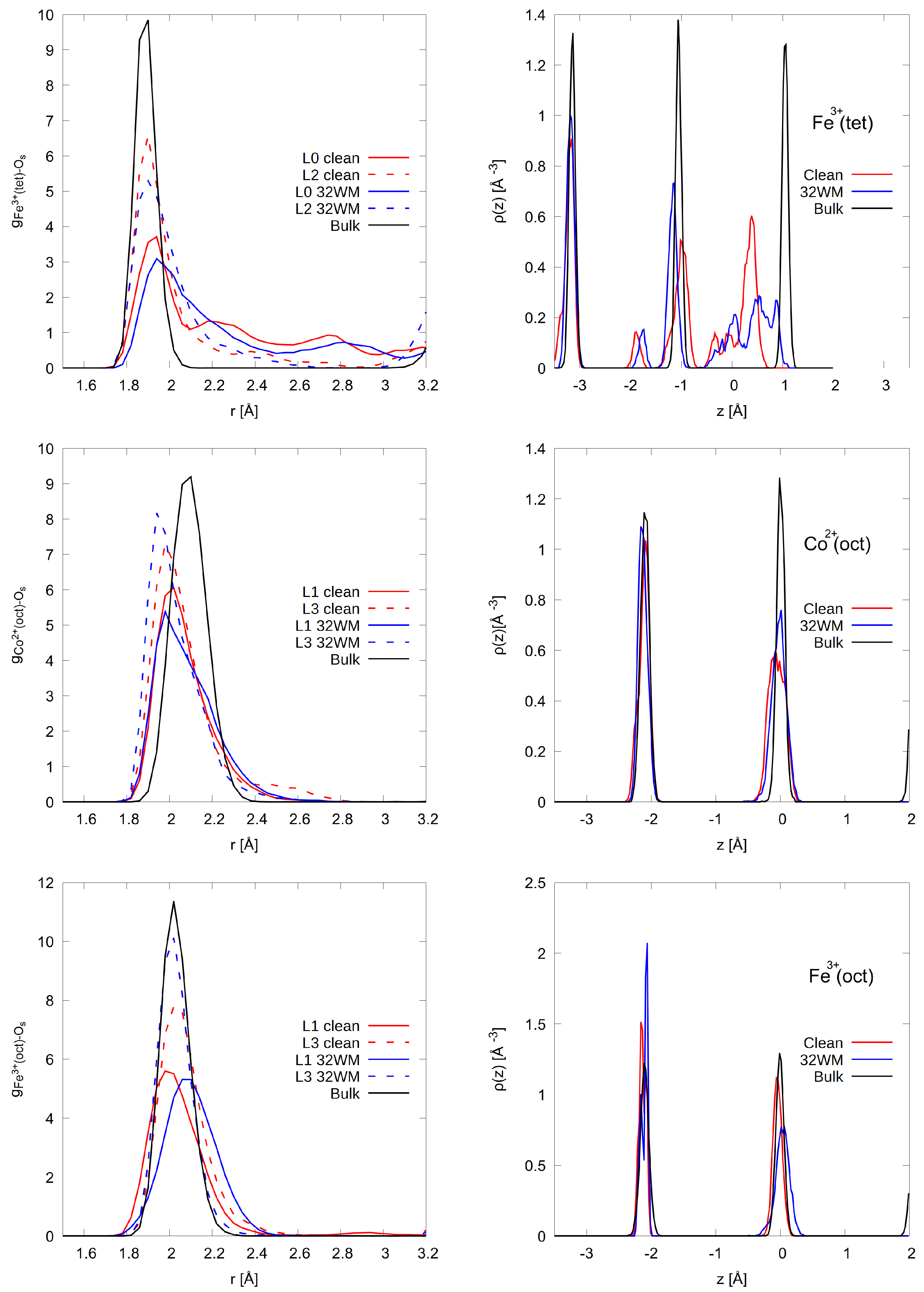
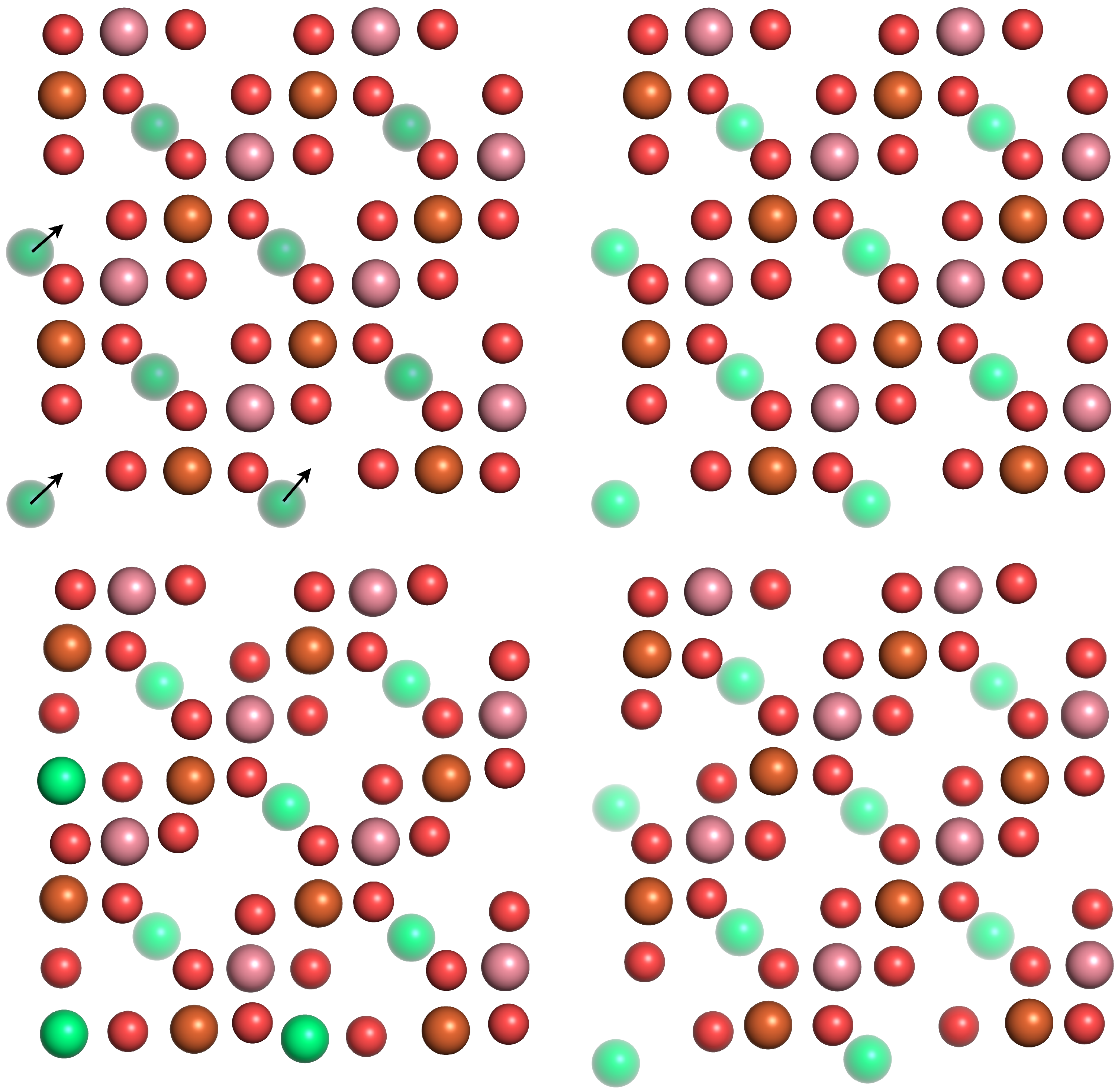
3.2. Surface Relaxations and Reconstructions
3.2.1. A-Termination
3.2.2. B-Termination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E.; Heilmann, E. Physical properties and cation arrangement of oxides with spinel structures I. Cation arrangement in spinels. J. Chem. Phys. 1947, 15, 174–180. [Google Scholar] [CrossRef]
- Guillaud, C. Propriétés magnétiques des ferrites. J. Phys. Radium 1951, 12, 239–248. [Google Scholar] [CrossRef]
- Fritsch, D.; Ederer, C. Epitaxial strain effects in the spinel ferrites CoFe2O4 and NiFe2O4 from first principles. Phys. Rev. B 2010, 82, 104117. [Google Scholar] [CrossRef] [Green Version]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Schwarzrock, I.; Lunkenbein, T.; Salamon, S.; Landers, J.; Wende, H.; Schlögl, R.; Pentcheva, R.; et al. Role of Composition and Size of Cobalt Ferrite Nanocrystals in the Oxygen Evolution Reaction. ChemCatChem 2017, 9, 2988–2995. [Google Scholar] [CrossRef]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Lunkenbein, T.; Greiner, M.T.; Masliuk, L.; Salamon, S.; Landers, J.; Schlögl, R.; Wende, H.; et al. The Role of Composition of Uniform and Highly Dispersed Cobalt Vanadium Iron Spinel Nanocrystals for Oxygen Electrocatalysis. ACS Catal. 2018, 8, 1259–1267. [Google Scholar] [CrossRef]
- Xu, Y.; Özcan, F.; Zielke, P.; Becker, S.; Heimann, M.; Heese, J.; Chakrapani, K.; Behrens, M.; Bredmose Simonsen, S.; Norby, P.; et al. Continuous Hydrothermal Flow Synthesis of Co1−xNixFe2O4 (x = 0–0.8) Nanoparticles and Their Catalytic Properties for CO Oxidation and Oxygen Evolution Reaction. Z. Anorg. Allg. Chem. 2018, 644, 1727–1733. [Google Scholar] [CrossRef]
- El Arrassi, A.; Liu, Z.; Evers, M.V.; Blanc, N.; Bendt, G.; Saddeler, S.; Tetzlaff, D.; Pohl, D.; Damm, C.; Schulz, S.; et al. Intrinsic activity of oxygen evolution catalysts probed at single CoFe2O4 nanoparticles. J. Am. Chem. Soc. 2019, 141, 9197–9201. [Google Scholar] [CrossRef]
- Evans, G.; Kozhevnikov, I.V.; Kozhevnikova, E.F.; Claridge, J.B.; Vaidhyanathan, R.; Dickinson, C.; Wood, C.D.; Cooper, A.I.; Rosseinsky, M.J. Particle size–activity relationship for CoFe2O4 nanoparticleCO oxidation catalysts. J. Mater. Chem. 2008, 18, 5518–5523. [Google Scholar] [CrossRef]
- Mountapmbeme Kouotou, P.; Vieker, H.; Tian, Z.Y.; Tchoua Ngamou, P.H.; El Kasmi, A.; Beyer, A.; Gölzhäuser, A.; Kohse-Höinghaus, K. Structure–activity relation of spinel-type Co–Fe oxides for low-temperature CO oxidation. Catal. Sci. Technol. 2014, 4, 3359–3367. [Google Scholar] [CrossRef]
- Gu, D.; Jia, C.J.; Weidenthaler, C.; Bongard, H.J.; Spliethoff, B.; Schmidt, W.; Schueth, F. Highly ordered mesoporous cobalt-containing oxides: Structure, catalytic properties, and active sites in oxidation of carbon monoxide. J. Am. Chem. Soc. 2015, 137, 11407–11418. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, N.; Girgsdies, F.; Beherns, M.; Huang, X.; Sudheesh, V.; Sebastian, V. Synthesis of cobalt ferrite nanoparticles by constant pH co-precipitation and their high catalytic activity in CO oxidation. New J. Chem. 2017, 41, 7356–7363. [Google Scholar] [CrossRef] [Green Version]
- Anke, S.; Falk, T.; Bendt, G.; Sinev, I.; Haevecker, M.; Antoni, H.; Zegkinoglou, I.; Jeon, H.; Knop-Gericke, A.; Schlögl, R.; et al. On the reversible deactivation of cobalt ferrite spinel nanoparticles applied in selective 2-propanol oxidation. J. Catal. 2020, 382, 57–68. [Google Scholar] [CrossRef]
- Falk, T.; Budiyanto, E.; Dreyer, M.; Pflieger, C.; Waffel, D.; Büker, J.; Weidenthaler, C.; Ortega, K.F.; Behrens, M.; Tüysüz, H.; et al. Identification of Active Sites in the Catalytic Oxidation of 2-Propanol over Co1+xFe2-xO4 Spinel Oxides at Solid/Liquid and Solid/Gas Interfaces. ChemCatChem 2021, 13, 2942–2951. [Google Scholar] [CrossRef]
- Kooti, M.; Afshari, M. Magnetic cobalt ferrite nanoparticles as an efficient catalyst for oxidation of alkenes. Sci. Iran. 2012, 19, 1991–1995. [Google Scholar] [CrossRef] [Green Version]
- Dumitru, R.; Papa, F.; Balint, I.; Culita, D.C.; Munteanu, C.; Stanica, N.; Ianculescu, A.; Diamandescu, L.; Carp, O. Mesoporous cobalt ferrite: A rival of platinum catalyst in methane combustion reaction. Appl. Catal. A Gen. 2013, 467, 178–186. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Tătăruş, A.; CuliŢă, D.C.; Stănică, N.; Ionescu, I.A.; Butoi, B.; Banici, A.M. Comparative Study of CoFe2O4 Nanoparticles and CoFe2O4-Chitosan Composite for Congo Red and Methyl Orange Removal by Adsorption. Nanomaterials 2021, 11, 711. [Google Scholar] [CrossRef]
- Zheng, H.; Zhan, Q.; Zavaliche, F.; Sherburne, M.; Straub, F.; Cruz, M.P.; Chen, L.Q.; Dahmen, U.; Ramesh, R. Controlling Self-Assembled Perovskite—Spinel Nanostructures. Nano Lett. 2006, 6, 1401–1407. [Google Scholar] [CrossRef]
- Fan, X.; Guan, J.; Cao, X.; Wang, W.; Mou, F. Low-Temperature Synthesis, Magnetic and Microwave Electromagnetic Properties of Substoichiometric Spinel Cobalt Ferrite Octahedra. Eur. J. Inorg. Chem. 2010, 2010, 419–426. [Google Scholar] [CrossRef]
- Kenmoe, S.; Biedermann, P.U. Water adsorbate phases on ZnO and impact of vapor pressure on the equilibrium shape of nanoparticles. J. Chem. Phys. 2018, 148, 054701. [Google Scholar] [CrossRef]
- Hajiyani, H.; Pentcheva, R. Surface Termination and Composition Control of Activity of the CoxNi1−xFe2O4(001) Surface for Water Oxidation: Insights from DFT+U Calculations. ACS Catal. 2018, 8, 11773–11782. [Google Scholar] [CrossRef]
- Lu, L.T.; Dung, N.T.; Tung, L.D.; Thanh, C.T.; Quy, O.K.; Chuc, N.V.; Maenosono, S.; Thanh, N.T.K. Synthesis of magnetic cobalt ferrite nanoparticles with controlled morphology, monodispersity and composition: The influence of solvent, surfactant, reductant and synthetic conditions. Nanoscale 2015, 7, 19596–19610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eom, Y.; Abbas, M.; Noh, H.; Kim, C. Morphology-controlled synthesis of highly crystalline Fe3O4 and CoFe2O4 nanoparticles using a facile thermal decomposition method. RSC Adv. 2016, 6, 15861–15867. [Google Scholar] [CrossRef]
- Rushiti, A.; Hättig, C.; Wen, B.; Selloni, A. Structure and Reactivity of Pristine and Reduced Spinel CoFe2O4 (001)/(100) Surfaces. J. Phys. Chem. C 2021, 125, 9774–9781. [Google Scholar] [CrossRef]
- Kenmoe, S.; Biedermann, P.U. Water aggregation and dissociation on the ZnO(100) surface. Phys. Chem. Chem. Phys. 2017, 19, 1466–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kox, T.; Spohr, E.; Kenmoe, S. Impact of Solvation on the Structure and Reactivity of the Co3O4 (001)/H2O Interface: Insights From Molecular Dynamics Simulations. Front. Energy Res. 2020, 8, 312. [Google Scholar] [CrossRef]
- Kenmoe, S.; Lisovski, O.; Piskunov, S.; Bocharov, D.; Zhukovskii, Y.F.; Spohr, E. Water Adsorption on Clean and Defective Anatase TiO2 (001) Nanotube Surfaces: A Surface Science Approach. J. Phys. Chem. B 2018, 122, 5432–5440. [Google Scholar] [CrossRef] [PubMed]
- Kenmoe, S.; Lisovski, O.; Piskunov, S.; Zhukovskii, Y.F.; Spohr, E. Electronic and optical properties of pristine, N- and S-doped water-covered TiO2 nanotube surfaces. J. Chem. Phys. 2019, 150, 041714. [Google Scholar] [CrossRef]
- CP2K Is Freely. T.C.d.g. 2016. Available online: http://www.cp2k.org/.
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Hubbard, J.; Flowers, B.H. Electron correlations in narrow energy bands. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1963, 276, 238–257. [Google Scholar]
- Zigla, A.A.; Kox, T.; Mevoa, D.; Assaouka, H.T.; Nsangou, I.N.; Daawe, D.M.; Kenmoe, S.; Kouotou, P.M. Magnesium-Modified Co3O4 Catalyst with Remarkable Performance for Toluene Low Temperature Deep Oxidation. Catalysts 2022, 12, 411. [Google Scholar] [CrossRef]
- Zerebecki, S.; Salamon, S.; Landers, J.; Yang, Y.; Tong, Y.; Budiyanto, E.; Waffel, D.; Dreyer, M.; Saddeler, S.; Kox, T.; et al. Engineering of Cation Occupancy of CoFe2O4 Oxidation Catalysts by Nanosecond, Single-Pulse Laser Excitation in Water. ChemCatChem 2022, 14, e202101785. [Google Scholar]
- Budiyanto, E.; Zerebecki, S.; Weidenthaler, C.; Kox, T.; Kenmoe, S.; Spohr, E.; DeBeer, S.; Rüdiger, O.; Reichenberger, S.; Barcikowski, S.; et al. Impact of Single-Pulse, Low-Intensity Laser Post-Processing on Structure and Activity of Mesostructured Cobalt Oxide for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 51962–51973. [Google Scholar] [CrossRef]
- Ulman, K.; Poli, E.; Seriani, N.; Piccinin, S.; Gebauer, R. Understanding the electrochemical double layer at the hematite/water interface: A first principles molecular dynamics study. J. Chem. Phys. 2019, 150, 041707. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kox, T.; Omranpoor, A.H.; Kenmoe, S. Structure and Reactivity of CoFe2O4(001) Surfaces in Contact with a Thin Water Film. Physchem 2022, 2, 321-333. https://doi.org/10.3390/physchem2040023
Kox T, Omranpoor AH, Kenmoe S. Structure and Reactivity of CoFe2O4(001) Surfaces in Contact with a Thin Water Film. Physchem. 2022; 2(4):321-333. https://doi.org/10.3390/physchem2040023
Chicago/Turabian StyleKox, Tim, Amir Hossein Omranpoor, and Stephane Kenmoe. 2022. "Structure and Reactivity of CoFe2O4(001) Surfaces in Contact with a Thin Water Film" Physchem 2, no. 4: 321-333. https://doi.org/10.3390/physchem2040023
APA StyleKox, T., Omranpoor, A. H., & Kenmoe, S. (2022). Structure and Reactivity of CoFe2O4(001) Surfaces in Contact with a Thin Water Film. Physchem, 2(4), 321-333. https://doi.org/10.3390/physchem2040023





