Abstract
Rates of fast reactions are inversely proportional to the solvent viscosity (η). However, a quantitative study demonstrates that dynamic viscosity η is often a crude reflection of a viscous drug exerted on a molecule or radical. This paper aims to present an accurate dependence of the rates of fast bi- and monomolecular reactions upon the viscous drug of a media. Different correction coefficients fmicro are discussed, which should lead to a dependence rate ∝ (fmicroη)−1. Microviscosity is viscosity, leading to the expected rate dependence upon shear viscosity. In many cases, experimentally measured diffusion coefficients of molecules of a similar structure to the reactive radicals lead to the correct prediction of radicals’ diffusion coefficients and the rate constants of radicals recombination. Microviscosity of complex non-Newtonian liquids (biological liquids, polymeric solutions) can be measured using low MW molecular probes. Usually, the measured ηmicro is much lower than the shear η of complex biological or polymeric liquids. Cis–trans isomerization of bulky groups in monomolecular reactions is often described with Kramers’ theory. An example of such isomerization of a cyanine dye studied experimentally and theoretically is presented. It is demonstrated in the selected case that Kramers’ theory adequately describes the dependence of cis–trans isomerization of organic compounds upon η.
1. Introduction
The viscosity of a solvent (η) is the dominant factor that affects rates of fast bimolecular and some monomolecular reactions. Bimolecular fast reactions include the termination of free radicals, some swift radical addition reactions, and proton/electron/hydrogen atom transfer. The mini-review will focus on the self-termination of reactive free radicals:
For brevity, Reaction (1) is named recombination. (In general, self-termination of radicals may be disproportionation or competition of both recombination and disproportionation).
Some rearrangements and cis–trans isomerization are examples of fast monomolecular reactions.
The viscous drug of a solvent limits the rates of the listed above chemical transformations. When mutual diffusion of reagents controls the reaction, its rate constant is expected to equal the renowned von Smoluchowski equation [1]:
where ρ is the sum of Van der Waals radii of reagents, and D12 is a mutual diffusion coefficient D1 + D2. The symbol D is used for the diffusion coefficient of an individual molecule or radical. Formula (2) should be modified in the case of diffusion-controlled reactions between free radicals [2]:
where σ = 1/4 is a spin statistic factor. For a diffusion-controlled reaction between identical radicals (1), 2k should equal kdiff. Formula (3) requires further correction for relatively large radicals (molecules) with a localized highly reactive atom. This highly reactive atom is an atom bearing the unpaired electron for radicals. The formula is [2]:
where feff is a steric factor. Such diffusion-controlled reactions with feff < 1 are termed pseudodiffusion. See for details [2,3]. The “usual” diffusion-controlled reactions have feff = 1.0, Equation (3). Bimolecular reactions in the liquid phase are usually divided into two classes- activation-controlled and diffusion-controlled [1]. In this paper, we attract attention to the less common class of biomolecular fast reactions—pseudodiffusion.
k = kdiff = 4πρD12,
k = kdiff = σ4πρD12,
k = kdiff = σ4πρD12feff,
The substitution of D in Equation (2) is expressed by the famous Stokes-Einstein formula
for translational diffusion leads to the well-known Debye formula [1]:
kB is the Boltzmann constant, R—universal gas constant, r—Van der Waals radius of a diffusing molecule (radical), η—solvent viscosity in Equations (5) and (6).
D = kBT/(6πηr),
kdiff = 8RT/(3000η)
Modification of the Equation (4) for the diffusion-controlled reaction between two radicals is:
kdiff = σ8RTfeff/(3000η)
The main focus of this work is the recombination of reactive organic free radicals and their dependence upon solvent viscosity.
Unless stated otherwise, we present below data obtained at 293–298 K and atmospheric pressure.
2. Recombination of Radicals in the Newtonian Liquids
Newtonian liquids are the main subject of this paper, where the dynamic η is the only characteristic of a liquid at the low and high shear rate [4]. (η depends upon temperature and external pressure). The values of η for many Newtonian individual liquids and liquid mixtures can be found in reference books and in the literature on chemistry and physics. Many η values have been measured with high accuracy.
It follows from Equations (6) and (7), that the rate constant of a molecular mobility-controlled reaction is inversely proportional to η at the constant T. (Say, water has η = 1 cP whereas glycerol has η ≈ 1000 cP. The fast bimolecular reaction would proceed slower in glycerol compared to water). There are other additional criteria of a (pseudo)diffusion recombination related to activation energy and activation volume of the reaction and the viscous flow [2,3].
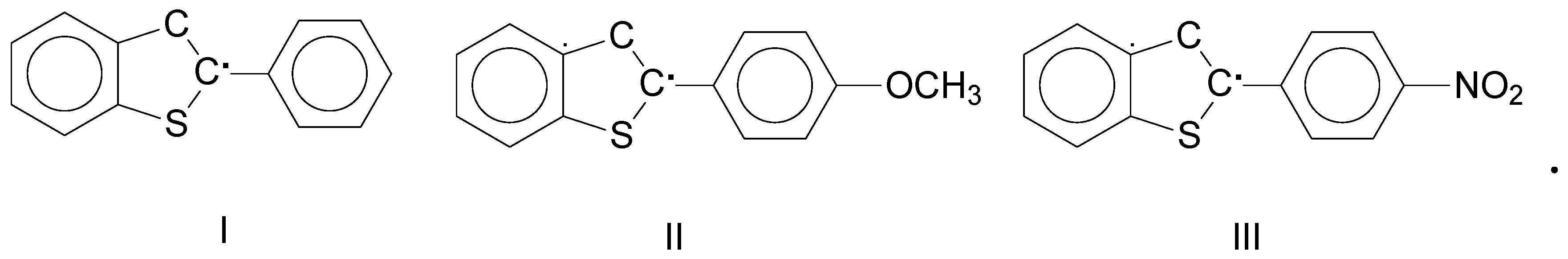
The authors of [3] studied the recombination of highly reactive radicals labeled I–III on Scheme 1 below:
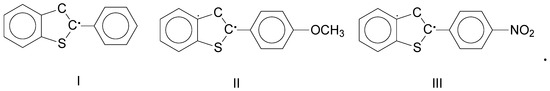
Scheme 1.
Chemical structures of the studied radicals I–III.
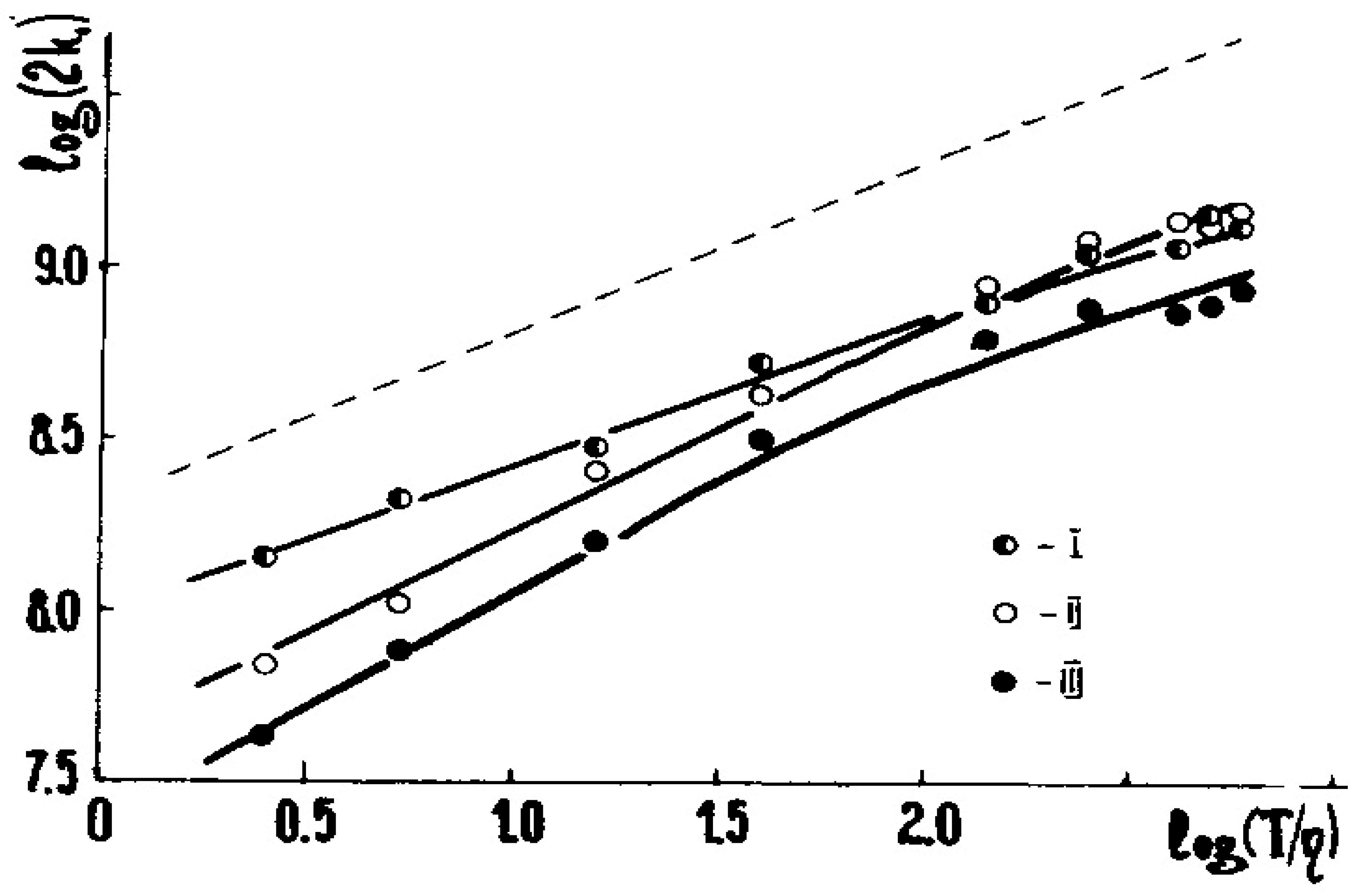
Figure 1 below displays the dependence of 2k1 for these three radicals upon η:
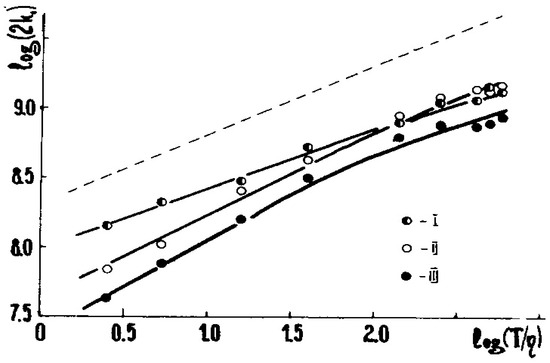
Figure 1.
The log(2k1) vs. log(T/η) relationships for R(I–III) recombination in the toluene-vaseline oil mixture. η is in cP, and 2k1 is in M−1·s−1, see Equation (1). The dotted line is the Formula (7) with the omitted feff; see [3] for details. The reactions are pseudodiffusion ones. (Adapted from [3]).
Changing the viscosity in an experiment k = F(η) by changing solvents of different chemical nature leads to a large data scatter. (It is a good practice to vary η using alkanes or alcohols of different viscosities. One can start with pentane η = 0.24 cP and end up with heptadecane (C17), the highest MW alkane, a liquid with η = 4.0 cP. The least viscous alcohol is methanol η = 0.55 cP, and undecan-1-ol (C11) is the highest MW linear alcohol, a liquid with η = 17 cP.) Binary mixtures of non-viscous and viscous solvents of similar chemical nature are suitable for measuring the viscosity effect. It was found that the binary mixture toluene—dibutyl phthalate 0.6–18.4 cP or toluene—vaseline oil (η = 120 cP) are quality binary mixtures. Measuring η solvents or solvent mixtures with η ≥ 10 cP in η—dependence experiments is recommended). The solvent’s nature may have a pronounced effect on reaction (1), and solvents (S) can form solvation complexes R.S affecting the rates of recombination of free radicals [5].
However, in many experiments at constant T for (pseudo)diffusion-controlled reactions, Equation (8) holds:
2k1 ~ η−α or log(2k1) = const + α·log(T/η)
The latter is a linear plot convenient for visualization; see, e.g., Figure 1. In this case, α ≈ 1.0 at η > 2 cP. However, in many experiments α = 0.5–1.2; more often, α ≤ 1.0 [3,6].
It would be naïve to expect the precise prediction of the corresponding experimental values by Equation (5) and the consequential Equations (6) and (7).
The derivation of Equation (5) assumes that the molecule/radical is spherical and diffuses in the structureless continuum. A molecule is not necessarily ball-like and diffuses in the structured media among low MW molecules of comparable size. (Probably only fullerene C60 is the ball-like molecule). These facts lead to the deviation of experimental D from its predicted value by Equation (5). Many attempts have been made to introduce corrections into Equation (5), a “microfriction” coefficient fmicro,
which leads to an agreement between the calculated and experimental values [7,8]. There are speculations that a prolate or an oblate form of a molecule, slip or stick boundary condition affects the value of D [7,8]. fmicro accounts for radii or volumes of reactive and solvent molecules [7,8]. A simple Spernol-Wirtz formula for fmicro has often been used in the correction of D since 1953 [7]:
where r is a reagent’s Van der Waals radius (Equation (5)), and rL is such a radius for a solvent molecule. For radicals I–III (Scheme 1) in toluene fmicro ≈ 0.63 per the estimation of [3]. Viscosity, which should be placed in Equation (5) leading to the experimental value of D, is often called microviscosity ηmicro. If a shear viscosity of the media (a Newtonian or non-Newtonian liquid) is η, then:
D = kBT/(6πηfmicro·r),
fmicro = 0.16 + 0.40 r/rL,
ηmicro = η·fmicro
As a reminder, shear viscosity is a rheological property that describes a fluid’s resistance to flow under shear stress [4].
The diffusion of elongated molecules was analyzed in [9].
3. Experimental Measurement of Diffusion Coefficients or Microviscosity
It would be better to measure the D value experimentally instead of estimating D with Equations (5) and (9). It is difficult for transient species and requires much effort, even for stable molecules. Terazima et al. [10,11] developed a transient grating (TG) technique that allows measurements of D of short-live radicals in solution D(R.). In the TG method, two coherent light beams are crossed at a sample to produce the interference pattern of the light intensity. Analysis of the grating pattern eventually allows for D(R.). Several obtained D(R.) were reported [10]. It was interesting to compare D(R.) with D of the parent hydrogenated molecules D(RH). It was observed that in several cases, D(R.) is two to three times smaller than D(RH). The reasons for such differences need to be better understood [9]. Unfortunately, this promising work has not been continued yet.
Reversible recombination of 2,6-diphenyl-4-methoxyphenoxyl radical is controlled by diffusion [12]. It was possible to measure D(R.) in benzene by a capillary method for this system radicals-dimer [12]. The benzene solution of radicals-dimer was put into a thin capillary of the known dimensions, and the opened capillary with reagents was placed into a thermostatted large vessel filled with the same solvent. The concentration of [R.] in the capillary, which decreased with time (t), coincides with periodically measured ESR. A mathematical analysis of [R.] vs. t allowed us to obtain D(R.) [12,13]. The experimentally measured 2k1 = (1.5 ± 0.3) × 109 M−1·s−1 with the experimental error with a value predicted by Equation (3) kdiff = (1.1 ± 0.2) × 109 M−1·s−1. The reaction radius was estimated at ρ = 1.0 ± 0.1 nm [12].
Despite a few observed differences between D(R.) and D(RH) observed in [10], D(RH), or even D of another stable molecule of comparable dimensions, many research publications use the obtained D as a reasonable estimation of D of the reactive radical/molecule or ηmicro. ηmicro can be measured by fluorescence technique with molecular rotors [14], rotational diffusion correlation times by NMR, diffusion-ordered spectroscopy (DOSY) by NMR [15], and by ESR with nitroxyl radicals as spin probes [13]. The reader can find different versions of fluorescence, NMR, and ESR techniques used for the measurements of D in the relevant publications.
Rate constants 2k1 for recombination of 2,6-di-tert-butylphenoxyl and 2,6-di-tert-butyl-4-methylphenoxyl radicals were measured in different solvents by flash photolysis [16]. Kinetics ESR study reproduced these 2k1-values [16]. The abovementioned phenoxyl radicals participate in pseudodifffusion recombination (1). It was concluded that tert-butyl substituted radicals, contrary to phenyl-substituted phenoxyls, do not form solvate complexes [5]. Phenyl-substituted radicals are not sterically hindered and have a large π-electron system. They can form solvation complexes RS. with some solvents. tert-Butyl-substituted radicals are sterically hindered by tert-butyl groups around oxygen atoms and are less prone to form RS [5]. Rotational correlation times τ for the parent two phenols in the same solvents were measured by NMR [16]. It was determined that 2k1 ∝ 1/τ or 2k1·τ = const for these two tert-butyl phenoxyls. Thus, the molecular mobility of the parent phenols nicely reflects the mobility of the corresponding phenoxyls [16].
The kinetic ESR study in different solvents studied recombination (1) of benzyl radicals and substituted benzyls [17]. The reactions are diffusion-controlled [17]. D of toluene and substituted toluene were measured in the same solvents. It was found that 2k1 coincides with the experimental error with corresponding values calculated by Equation (3) [17]. Thus, D(RH) is usually a good estimation of D(R.).
4. Cage Effect in Different Solvents
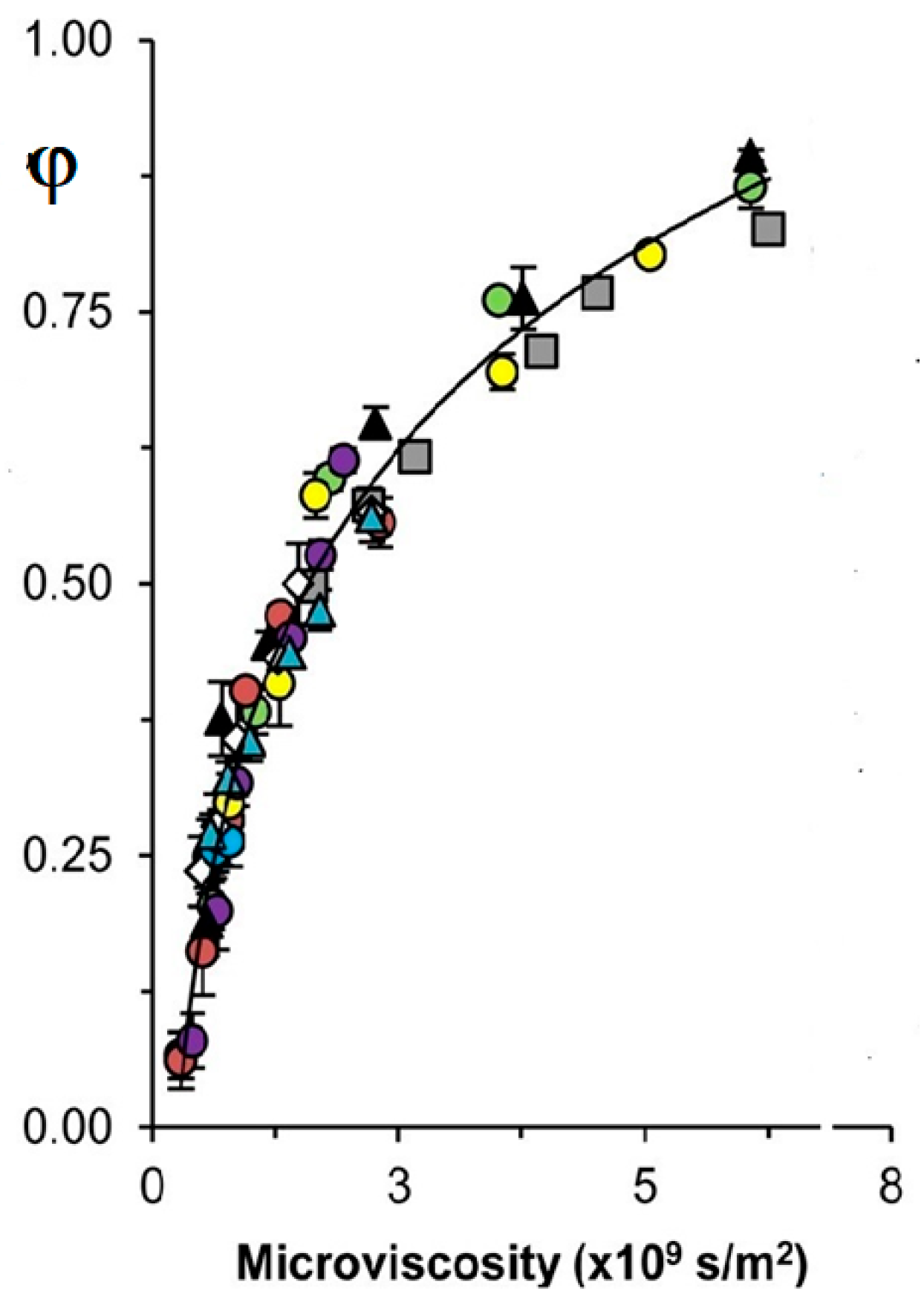
The cage effect (φ) plays a vital role in the photoinitiation of chemical reactions and free-radical chemistry [18,19,20,21]. φ is the fraction of radicals that decayed near each other after photolysis or thermolysis of molecules in solution; 0 ≤ φ ≤ 1. It is known that φ increases with the solvent viscosity η increase [18,19,20,21]. Tyler and coworkers [18,19] studied the dependence of experimentally measured φ vs. η under photolysis of an organometallic dimer. Solvents of different chemical nature, including a solution of a polymer, were used. Dependences φ vs. η demonstrated much scatter. However, the authors measured the microviscosity ηmicro of a stable molecule of similar structure to the studied organometallic radical by NMR [18,19]. Interestingly, all dependencies of φ vs. ηmicro lay on the one smooth and increased with viscosity as expected [19]:
Figure 2 is an awe-inspiring result confirming the expectation related to the φ and its microviscosity dependence [15,18,19]. The work [19] aimed to find a method to quantitatively correlate φ values to the properties of the solvent system. Although bulk viscosity qualitatively correlates with φ cage, the shortcomings of using solvent η for quantitative work are well-known. The new method developed in [19] employs ηmicro, a parameter straightforwardly measured using an NMR probe. As demonstrated herein, microviscosity provides a reliable correlation between the cage recombination efficiency in all categories of solvents, thus lending itself to quantitative predictions. The correlation between φ and microviscosity holds for a wide range of solvent systems, spanning nonpolar, polar, aromatic, and hydrogen-bonding solvents [19]. In addition, selective solvation from mixed-type solvent systems was shown to insignificantly affect the predictive power of the method [19]. It is important to emphasize the straightforwardness of the method: to predict φ of a particular couple of radicals in a particular solvent, all that is required is to measure the diffusion coefficient of an appropriate NMR probe [19].
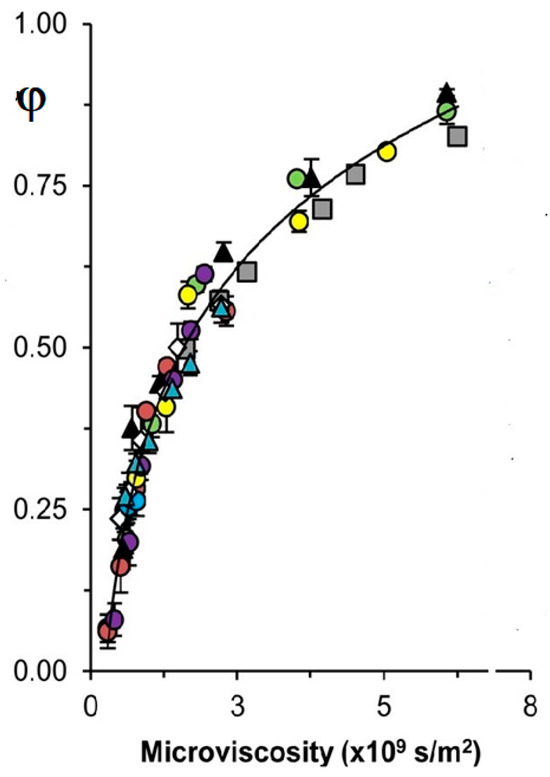
Figure 2.
Dependence of cage effect (φ) upon microviscosity of solvents of different natures at [19]. See for details [19]. (Adapted from [19]).
It is expected that 1/φ = 1 + const/microviscosity is to be a linear plot [20,21].
The kinetics of free radicals recombination in the solvent cage and recombination in the solvent bulk are analyzed in [22].
5. Microviscosity of Complex Liquids
A solvent or a reaction media is not necessarily a Newtonian liquid. Much effort was devoted to studying diffusion in micelles, biological objects (vesicles, membranes), and liquid polymers or polymer solutions. These liquids are usually viscous, their shear η does not reflect a viscous drag imposed on low MW molecules or radicals.
Such media viscosity is measured with one or another viscometer. In particular, the polymer and coatings industry measures η with Brookfield viscometer under specified conditions. That measurement can be replicated at another laboratory with a standard Brookfield.
In non-Newtonian complex liquids, a notion of microviscosity (ηmicro) is often used. ηmicro is measured with a molecular or a radical probe; see above.
The local viscosity or ηmicro supposedly plays an important role in biology across several length scales [23]. There is growing evidence that the fluidity of the cell’s lipid plasma membrane is regulated to enable optimal cellular function, and ηmicro within the cell’s nucleus may influence the formation of non-canonical DNA assemblies [23]. However, unlike in the cases of bulk industrial applications (see above), including additive manufacturing, biological viscosity is challenging to measure and requires the development of methodologies that can probe viscoelastic properties of microscopic volumes of individual biological cells and organelles [23]. While a myriad of different dyes have been developed to study the microenvironment of lipid membranes, Molecular fluorescent rotors give valuable ηmicro of their surroundings in a biological object [23]. At the same time, many developed dyes-probes of ηmicro often respond to multiple biophysical features and/or are not fully calibrated against the various membrane parameters [23]. This is a problem for biologists and biophysicists. Microviscosity can indicate the condition of living cells [24]. It is closely linked to numerous diseases [23,24]. It is necessary to design tools to effectively monitor ηmicro changes, which could provide promising avenues for treating diseases. A novel mitochondria-targeting fluorescent probe P was suggested in [25] for the detection of ηmicro changes in vivo and in vitro. P has advantages such as long emission wavelength (650 nm), large Stokes shift (105 nm), significant fluorescence enhancement (59-fold), high sensitivity, good biocompatibility, and so on. Biological experiments showed that P could target mitochondria and detect viscosity alterations in HeLa cells. (HeLa cells are the first immortal human cell line [24]). Moreover, P allows the exploration of fluctuations in ηmicro within living organisms [24].
The authors [25] aimed to measure microviscosity in dimyristoyllecithin vesicles with a fluorescent probe. The system turns out to be rather complex [25]. At the same time, nanosecond time-dependent depolarization measurements of fluorescence are capable of reporting in detail the complex rotational motion of fluorescent probes in biological membranes thereby providing information on both their ηmicro and structure [25].
The viscosity of cell membranes is a crucial parameter that affects the diffusion of small molecules both across and within the lipidic membrane [26]. It was mentioned above that such diffusion can be related to several diseases [26]. The possibility to measure quantitatively membrane viscosity ηmicro on the nanoscale is of great interest [26]. The paper [26] reports a complete investigation of the photophysics of an amphiphilic membrane-targeted azobenzene, and the results validate its use as a viscosity probe for some other cell membranes. Trans-cis photoisomerization azobenzene was a method to develop a molecular viscometer and to assess the viscosity of E. coli bacteria membranes employing time-resolved fluorescence spectroscopy. Lifetime measurements of the probe in E. coli bacteria suspensions correctly indicate membrane ηmicro value in live E. coli cells [26]. ηmicro changes from 10 to 5 cP when increasing the temperature from 295 K up to 313 K [26].
Microviscositry of the studied mitochondria measured with a fluorescent probe varies from 63 to 130 cP [27]. Supposedly, it gives valuable information on the subcellular microenvironment [27].
According to [28], “how intracellular contents affect the mobility of single molecules is a key aspect of cell biology”. A molecular probe gives such info for solutions of calmodulin labeled with green fluorescent protein as a diffusing probe and containing polymer dextran with MW 10 to 500 kg/mol [28]. Not surprisingly, microviscosity essentially differs from the shear η and depends upon the concentration and MW of a polymer [28].
Near-infrared emissive indolizine squaraine fluorophores turn out to be efficient molecular viscosity sensors of intracellular microenvironments [29]. Moreover, the quantum yield of fluorescence of these fluorophores strongly depends upon viscosity [29].
Below, we list a few examples not related to biology.
Typical micelles sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), and some others) have ηmicro in the range of 12–45 cP measured with an ESR probe [30]. Microviscosities of the several studied micelles obtained by the ESR method are in concordance with those obtained by other techniques [30].
The processing of initially liquid materials often involves a drying step, during which a solvent evaporates from a multicomponent mixture. Such materials are usually confined in a thin film or a droplet [31]. Examples of such materials may be paints or coatings. Evaporation leads to the concentration of non-volatile material in the product. The observed η of the material measured one or another way cannot reflect the occurring changes in different parts of the drying material. The authors of [31] found a way to measure microviscosity for local and time-dependent mapping of concentration fields in such confined systems. The use of fluorescence lifetime imaging microscopy and fluorescent molecular rotors is suggested as a versatile quantitative method to map ηmicro. Measured microviscosity and concentration fields allow the characterization of drying time dependence in agreement with simple modeling of the evaporation [31].
High MW commercial poly(dimethylsiloxane) (PDMS) with added reagents has η ~ 103 P [32]. However, reactive radicals recombine in such media very fast like it had η ~ 1 cP. Thus, microviscosity is ~105 times lower than the shear viscosity [32].
Knowledge of microviscosity is important in polymer-based lubrication systems [33]. A relationship between the change in microviscosity and the friction coefficient is established in [33]. ηmicro was measured with molecular rotors [33]. In contrast to viscosity η, ηmicro enables a precise characterization of each component’s diffusion and adsorption rates within the contact region at micro and nano scales. Therefore, ηmicro exhibits a more rapid response, which is very important for studying polymer aqueous lubrication friction regulation systems and for revealing the friction coefficient’s response to viscosity changes within the contact region and the underlying mutation mechanism. However, the existing mechanical viscosity measurement methods pose challenges when it comes to detecting ηmicro at polymer-modified interfaces [33].
Thus, microviscosity indicates the diffusivity of low MW molecules/radicals in a complex media. At the same time, ηmicro is a characteristic of a specific media and cannot be considered a parameter that could be used for another similar media.
6. Fast Monomolecular Reactions
Almost any rearrangement in the condensed phase experiences the viscous drag of the media to one or another extent. Much effort was devoted to cis–trans (photo)isomerization in the liquid phase and solvent viscosity effect on this process. The movement of relatively large groups (phenyl and bulkier) behaving like blades is retarded by η [34,35,36,37,38,39]. At the same time, an excellent approximation to cis–trans isomerization is a movement along one reaction coordinate, usually the angle between the moving groups. Such a reaction is expected to be described by Kramers’ theory [38]. There are many problems related to applying Kramers’ theory to isomerization and other monomolecular reactions [39,40,41]. They are beyond the scope of this manuscript. Some authors consider volumes of the rotating group and a solvent and fmicro [36,37]. One of the appealing to the experimenter applications of Kramers’ theory is presented by Formula (12) [36]:
where kiso/s−1 is a (photo)isomerization rate constant, 0 < α < 1.0 is a constant parameter for an experiment, and Eo (kJ/mol) is the height of the isoviscosity barrier. Eo is measured at different temperatures, preserving a certain viscosity. It is an intrinsic activation energy for overcoming a barrier separating the reagent and its isomer.
kiso = const × η−α × exp(−Eo/RT),
It is improbable to observe in the experiment α = 1.0, Equation (12), especially in a system with a high and narrow barrier, the solvent molecules will not have enough time to slow the reaction [30].
One can suggest that in the following Equation (12), the experimental activation energy of isomerization Eobs should be equal to a sum:
where B (kJ/mol) is an activation energy of viscous flow obtained from Andrade Equation (14):
Eobs = αB + Eo,
η = ηo exp(B/RT)
We will use experimental values obtained for the isomerization of the cyanine dye DODCI (3,3′-diethyloxadicarbocynanine iodide) in the ground (gr) and the excited (ex) singlet state in two solvents—ethanol (EtOH) and decan-1-ol (DecOH) [36], see Table 1.

Table 1.
Activation parameters of DODCI isomerization. Experimental data of [36].
There is good agreement between the observed calculated values of Eobs generally; see the two last columns in Table 1. In the case of EtOH, “ex” demonstrates a worse agreement.
Equation (12) may be applied for the analysis of different cis–trans isomerizations.
Photochemical and thermal spiropyran–merocyanine interconversion in photoswitches depends upon viscosity. However, the process is too complex to be analyzed by Kramers’ theory [42].
It was demonstrated that in some enzyme catalysis (allosteric regulation), free energy surfaces along the reaction coordinate and the diffusion coefficient of the reaction dynamics can be analyzed within the framework of Kramers’ theory [43]. That approach allows the authors [43] to explore various factors underlying the reaction kinetics and their allosteric regulation. The results provide new insights into the detailed mechanism of the allosteric fine-tuning of enzyme catalysis, and the tools developed can be valuable for studying other allosteric systems [43].
7. Conclusions
The detailed analysis of highly reactive free radical self-termination demonstrated that, in several cases, there is a quantitative agreement between the rate constant of self-termination of free radicals and the well-known von Smoluchowski or Debye formula. The widely used Stokes-Einstein equation assumes that (a highly reactive) molecule or a free radical is a sphere in a solvent–a structureless continuum. That known assumption deviates from the expected rate ∝ η−1 dependence. A viscosity placed in the Stokes-Einstein equation that leads to the expected value of a diffusion coefficient is often called microviscosity. Molecular/radical probes of comparable size as that of a reagent molecule give much more accurate information on the viscous drag of molecules in each solvent.
Microviscosity of different solvents measured by NMR led to one smooth dependence of cage effect under photolysis or organometallic dimer vs. microviscosity.
Measured in one- or another-way shear η of non-Newtonian polymeric liquids, biological liquids usually do not reflect a viscous drag on the molecular movement of low MW species, and the same term, microviscosity, is widely used. Microviscosity of complex liquids has a limited value: it applies to a specific liquid media.
Much progress has been achieved by studying fast cis–trans isomerization with laser flash photolysis and corresponding analysis by Kramers’ theory.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Connors, K.A. Chemical Kinetics. In The Study of Reaction Rates in Solution; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Burshtein, A.I.; Khudyakov, I.V.; Yakobson, B.I. Fast reactions between radicals. Pseudodiffusion control. Prog. React. Kinet. 1984, 13, 221–306. [Google Scholar]
- Margulis, L.A.; Khudyakov, I.V.; Kuzmin, V.A.; Prokof’ev, A.I.; Yasmenko, A.I.; Smets, G. Kinetics of reversible recombination of aromatic C-centered radicals. Int. J. Chem. Kinet. 1985, 17, 735–747. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. An Introduction to Polymer Rheology and Processing; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Khudyakov, I.V.; Kuzmin, V.A.; Yasmenko, A.I.; Smit, W.; Salve, J.; de Jonge, C.R.H.I. Solvent effect on reversible self-termination of aromatic free radicals. Int. J. Chem. Kinet. 1984, 16, 1481–1494. [Google Scholar] [CrossRef]
- Pisarenko, L.M.; Nikulin, V.I.; Khudyakov, I.V. Kinetics of the reversible recombination of substituted 2-(p-dimethylaminophenyl)indane-1,3-dione-2-yl-radicals. Bull. Acad. Sci. USSR Div. Chem. Sci. 1988, 37, 1544–1548. [Google Scholar] [CrossRef]
- Spernol, A.; Wirtz, K. Zur Mikroreibung in Flüssigkeiten. Z. Naturforschung 1953, 89, 522–553. [Google Scholar] [CrossRef]
- Edward, J.T. Molecular volumes and Stokes-Einstein equation. J. Chem. Educ. 1970, 47, 261–266. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Luo, H.; Jing, G. Anisotropic diffusion of elongated particles in active coherent flows. Micromashines 2024, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Terazima, M. Is the translational diffusion of organic radicals different from that of closed-shell molecules? Acc. Chem. Res. 2000, 33, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Terazima, M. Diffusion coefficients as a monitor of reaction kinetics of biological molecules. Phys. Chem. Chem. Phys. 2006, 8, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Nikitaev, V.T.; Nikitaeva, G.A.; Khudyakov, I.V.; Prokof’ev, A.I.; Levin, P.P.; Burshtein, A.I. ESR study of spin exchange and diffusion rates in the solutions of reversibly recombining radicals. Doklady AN SSSR 1979, 247, 391–393. [Google Scholar]
- Wasserman, A.M.; Kovarskii, A.L. Spin Labels and Probes in Physical Chemistry of Polymers; Nauka Publishers: Moscow, Russia, 1986. [Google Scholar]
- Lakowicz, J.R. Principle of Fluorescence Spectroscopy; Kluwer: New York, NY, USA, 1999. [Google Scholar]
- Claridge, T.D.W. High-Resolution NMR Technology in Organic Chemistry; Pergamon: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Daragan, V.A.; Il’ina, E.E.; Khudyakov, I.V. Recombination constants of aroxyl radicals and correlation rotation times of the corresponding phenols in different solvents. Russ. J. Phys. Chem. 1986, 60, 572–575. [Google Scholar]
- Claridge, R.F.C.; Fischer, H. Self-termination and electronic spectra of substituted benzyl radicals in solution. J. Phys. Chem. 1983, 87, 1960–1967. [Google Scholar] [CrossRef]
- Barry, J.T.; Berg, D.J.; Tyler, D.R. Radical cage effects: Comparison of solvent bulk viscosity and microviscosity in predicting the recombination efficiencies of radical cage pairs. J. Am. Chem. Soc. 2016, 138, 9389–9392. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.T.; Berg, D.J.; Tyler, D.R. Radical cage effects: The prediction of radical cage pair recombination efficiencies using microviscosity across a range of solvent types. J. Am. Chem. Soc. 2017, 139, 14399–14406. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.V.; Levin, P.P.; Efremkin, A.F. Cage effect under photolysis in polymer matrices. Coatings 2019, 9, 111. [Google Scholar] [CrossRef]
- Khudyakov, I.V.; Yakobson, B.I. Influence of the solvent viscosity on cage effect. Russ. J. Gen. Chem. 1984, 54, 3–23. [Google Scholar]
- Doktorov, A.B.; Lukzen, N.N. General relationships between the kinetic characteristics of bulk and geminate recombination of radicals in solutions. J. Math. Chem. 2024, 62, 502–521. [Google Scholar] [CrossRef]
- Paez-Perez, M.; Kuimova, M.K. Molecular rotors: Fluorescent sensors for microviscosity and conformation of biomolecules. Angew. Chem. Int. Ed. 2024, 136, e202311233. [Google Scholar] [CrossRef]
- Gong, X.; Guo, R.; Li, X.; Yang, Y.; Lin, W. A red-emitting mitochondria targetable fluorescent probe for detecting viscosity in HeLa, zebrafish, and mice. Anal. Methods 2024, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.A.; Dale, R.E.; Roth, S.; Brand, L. Nanosecond time-dependent fluorescence depolarization of diphenylhexatriene in dimyristoyllecithin vesicles and the determination of “microviscosity”. J. Biol. Chem. 1977, 252, 2163–2178. [Google Scholar] [CrossRef] [PubMed]
- Magni, A.; Bondelli, G.; Paternò, G.M.; Sardar, S.; Sesti, V.; D’Andrea, C.; Bertarelli, C.; Lanzani, G. Azobenzene photoisomerization probes cell membrane nanoviscosity. Phys. Chem. Chem. Phys. 2022, 24, 8716–8722. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, A.; Lei, E.K.; Kelley, S.O. A multifunctional chemical probe for the measurement of local micropolarity and microviscosity in mitochondria. Angew. Chem. Int. Ed. 2018, 57, 8891–8899. [Google Scholar] [CrossRef]
- Goins, A.B.; Sanabria, H.; Waxham, M.N. Macromolecular crowding and size effects on probe microviscosity. Biophys. J. 2008, 95, 5362–5373. [Google Scholar] [CrossRef] [PubMed]
- Ndaleh, D.; Meador, W.E.; Smith, C.; Friedman, H.C.; McGuire, M.; Caram, J.R.; Hammer, N.I.; Delcamp, J.H. Near-infrared emissive indolizine squaraine fluorophores as strong molecular viscosity sensors. Chem. Eur. 2024, 8, e202300212. [Google Scholar] [CrossRef]
- Bahri, M.A.; Hoebeke, M.; Grammenos, A.; Delaney, L.; Vandewalle, N.; Seret, A. Investigation of SDS, DTAB and CTAB micelle microviscosities by electron spin resonance. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 206–230. [Google Scholar] [CrossRef]
- Gibouin, F.; Nalatamby, D.; Lidon, P.; Medina-Gonzalez, Y. Molecular rotors for in situ viscosity mapping during evaporation of confined fluid mixtures. Am. Chem. Soc. Appl. Mater. Interfaces 2024, 16, 8066–8076. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, V.A.; Levin, P.P.; Khudyakov, I.V. Kinetics of the geminate recombination of aromatic radicals in viscous polymers. Bull. Acad. Sci. USSR. Ser. Khim. 1987, 74, 395–396. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Liang, L.; Li, Y.; Cheng, Y.; Liu, Z.; Liu, M. Fast-response mechanism for regulating friction coefficients induced by microviscosity in polymer-based aqueous lubrication systems. Appl. Surf. Sci. 2024, 654, 159410–159424. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1834. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Saltiel, J. Application of the Kramers equation to stilbene photoisomerization in n-alkanes using translational diffusion coefficients to describe microviscosity. J. Phys. Chem. 1989, 93, 8310–8316. [Google Scholar] [CrossRef]
- Velsko, S.P.; Waldeck, D.H.; Fleming, G.R. Breakdown of Kramers theory description of photochemical isomerization and the possible involvement of frequency dependent friction. J. Chem. Phys. 1983, 78, 249–258. [Google Scholar] [CrossRef]
- Åkesson, E.; Hakkarainen, A.; Laitinen, E.; Helenius, V.; Gillbro, T.; Korppi-Tommola, J.; Sundström, V.J. Analysis of microviscosity and reaction coordinate concepts in isomerization dynamics described by Kramers’ theory. J. Chem. Phys. 1991, 95, 6508–6522. [Google Scholar] [CrossRef]
- Kramers, H.A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 1940, 7, 284–304. [Google Scholar] [CrossRef]
- Bagchi, B. Isomerization dynamics in solution. Int. Rev. Phys. Chem. 1987, 6, 1–38. [Google Scholar] [CrossRef]
- Donatia, L.; Schütte, C.; Weber, M. The Kramers turnover in terms of a macro-state projection on phase space. arXiv 2024, arXiv:2402.00211. [Google Scholar]
- Lyons, A.; Devi, A.; Hoffer, N.Q.; Woodside, M.T. Quantifying the properties of nonproductive attempts at thermally activated energy-barrier crossing through direct observation. Phys. Rev. X 2024, 14, 011017. [Google Scholar] [CrossRef]
- Whelan, J.; Abdallah, D.; Piskorz, K.; Wojtyk, J.T.C.; Dust, J.M.; Nunzi, J.-M.; Hoz, S.; Buncel, E. Photochemical and thermal spiropyran (SP) merocyanine (MC) interconversion: A dichotomy in dependence on viscosity. Phys. Chem. Chem. Physic 2012, 14, 13684–13694. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hamelberg, D. Dissecting the allosteric fine-tuning of enzyme catalysis. J. Am. Chem. Soc. Au 2024, 4, 837–846. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).