Geopolymers for Space Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-ray Fluorescence Spectrometry (XRF)
2.2. X-ray Diffraction
2.3. Infrared Spectroscopy
2.4. Thermal Analysis
2.5. Flammability and Toxicity of Smoke
2.6. Thermal Conductivity
2.7. Gamma Radiation
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Synthesis of New High-Temperature Geo-Polymers for Reinforced Plastics/Composites. In Proceedings of the SPE PACTEC ’79, Costa Mesa, CA, USA, 31 January–2 February 1979; pp. 151–154. [Google Scholar]
- Vickers, L.; van Riessen, A.; Rickard, W. Fire-Resistant Geopolymers: Role of Fibers and Fillers to Enhance Thermal Properties; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9789812873101. [Google Scholar]
- Masi, G.; Rickard, W.D.A.; Bignozzi, M.C.; Van Riessen, A. The effect of organic and inorganic fibres on the mechanical and thermal properties of aluminate activated geopolymers. Compos. Part B Eng. 2015, 76, 218–228. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, J.R.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V. The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Lin, T.S.; Jia, D.C.; He, P.G.; Wang, M.R. Thermo-mechanical and microstructural characterization of geopolymers with α-Al2O3 particle filler. Int. J. Thermophys. 2009, 30, 1568–1577. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A.; Segundo, A. Desarrollo de geopolímeros compuestos. Parte II: Efecto de refuerzos de fibra corta y nanopartículas sobre la resistencia a la compresión. Afinidad 2021, 78, 593. Available online: https://raco.cat/index.php/afinidad/article/view/390015 (accessed on 20 March 2024).
- Segundo, A.; Franco-Urquiza, E.A. Desarrollo de geopolímeros compuestos. Parte I: Síntesis y caracterización. Afinidad 2021, 78, 140–147. [Google Scholar]
- Maspoch, M.L.; Franco-Urquiza, E.A.; Gamez-Perez, J.; Santana, O.O.; Sánchez-Soto, M. Fracture behaviour of poly [ethylene–(vinyl alcohol)]/organo-clay composites. Polym. Int. 2009, 58, 648–655. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A. Clay-based polymer nanocomposites: Essential work of fracture. Polymers 2021, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Oshani, F.; Allahverdi, A.; Kargari, A.; Norouzbeigi, R.; Mahmoodi, N.M. Effect of preparation parameters on properties of metakaolin-based geopolymer activated by silica fume-sodium hydroxide alkaline blend. J. Build. Eng. 2022, 60, 104984. [Google Scholar] [CrossRef]
- Abadel, A.A.; Albidah, A.S.; Altheeb, A.H.; Alrshoudi, F.A.; Abbas, H.; Al-Salloum, Y.A. Effect of molar ratios on strength, microstructure & embodied energy of metakaolin geopolymer. Adv. Concr. Constr. 2021, 11, 127–140. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Sata, V.; Chindaprasirt, P. Effect of sodium hydroxide concentration and sodium silicate to sodium hydroxide ratio on properties of calcined kaolin-white portland cement geopolymer. Int. J. Geomate 2018, 14, 121–128. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer-An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Lee, W.K. Solid-Gel Interactions in Geopolymers. Ph.D. Thesis, Department of Chemical Engineering, The University of Melbourne, Parkville, Australia, 2002. [Google Scholar]
- Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.Y.; Javed, S.; Baig, R.U. Optimization of alkaline activator on the strength properties of geopolymer concrete. Polymers 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jiang, Z.; Wang, D.; Lv, Y.; Gao, C.; Xue, G. Effects of alkaline activators on pore structure and mechanical properties of ultrafine metakaolin geopolymers cured at room temperature. Constr. Build. Mater. 2022, 361, 129678. [Google Scholar] [CrossRef]
- Lopes, A.V.; Lopes, S.M.; Pinto, I. Influence of the Composition of the Activator on Mechanical Characteristics of a Geopolymer. Appl. Sci. 2020, 10, 3349. [Google Scholar] [CrossRef]
- Sajan, P.; Jiang, T.; Lau, C.; Tan, G.; Ng, K. Combined effect of curing temperature, curing period and alkaline concentration on the mechanical properties of fly ash-based geopolymer. Cleaner Mater. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Růžek, V.; Dostayeva, A.M.; Walter, J.; Grab, T.; Korniejenko, K. Carbon fiber-reinforced geopolymer composites: A review. Fibers 2023, 11, 17. [Google Scholar] [CrossRef]
- Korniejenko, K.; Łach, M.; Mikuła, J. The influence of short coir, glass and carbon fibers on the properties of composites with geopolymer matrix. Materials 2021, 14, 4599. [Google Scholar] [CrossRef] [PubMed]
- Samal, S. Effect of high temperature on the microstructural evolution of fiber reinforced geopolymer composite. Heliyon 2019, 5, e01779. [Google Scholar] [CrossRef]
- Kozub, B.; Bazan, P.; Mierzwiński, D.; Korniejenko, K. Fly-ash-based geopolymers reinforced by melamine fibers. Materials 2021, 14, 400. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S.; Gomes, J.P.C. Utilization of mining wastes to produce geopolymer binders. In Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing: Sawston, UK, 2009; pp. 267–293. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Introduction to geopolymers. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–11. ISBN 9781845694494. [Google Scholar]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Molin, D.C.C.D.; Vilela, A.C.F.; Da Silva, F.J.; Pavão, B.; Veit, H. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cem. Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Kamseu, E.; Alzari, V.; Nuvoli, D.; Sanna, D.; Lancellotti, I.; Mariani, A.; Leonelli, C. Dependence of the geopolymerization process and end-products to the nature of solid precursors: Challenge of the sustainability. J. Clean. Prod. 2021, 278, 123587. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Characterization of fly-ash-based geopolymeric binders activated withsodium aluminate. Ind. Eng. Chem. Res. 2002, 41, 4242–4251. [Google Scholar] [CrossRef]
- Giancaspro, J.; Balaguru, P.; Lyon, R. Fire protection of flammable materials utilizing geopolymer. Sampe J. 2004, 40, 42–49. [Google Scholar]
- O’Bradaigh, C.; Doyle, A.; Doyle, D.; Feerick, P.J. Electrically-Heated Ceramic Composite Tooling for Out-of-Autoclave Manufacturing of Large Composite Structures. Sampe J. 2011, 47, 6–14. [Google Scholar]
- Lee, S.; Van Riessen, A. A review on geopolymer technology for lunar base construction. Materials 2022, 15, 4516. [Google Scholar] [CrossRef] [PubMed]
- Korniejenko, K.; Pławecka, K.; Kozub, B. An overview for modern energy-efficient solutions for lunar and martian habitats made based on geopolymers composites and 3D printing technology. Energies 2022, 15, 9322. [Google Scholar] [CrossRef]
- Montes, C.; Broussard, K.; Gongre, M.; Simicevic, N.; Mejia, J.; Tham, J.; Allouche, E.; Davis, G. Evaluation of lunar regolith geopolymer binder as a radioactive shielding material for space exploration applications. Adv. Space Res. 2015, 56, 1212–1221. [Google Scholar] [CrossRef]
- Torres, M.; Burdin, L.; Rentería-Rodríguez, A.V.; Franco-Urquiza, E.A. Degradation of Epoxy–Particles Composites Exposed to UV and Gamma Radiation. Chemistry 2023, 5, 559–570. [Google Scholar] [CrossRef]
- Torres, M.; Franco-Urquiza, E.A.; González-García, P.; Bárcena-Balderas, J.; Piedra, S.; Madera-Santana, T.; Melendrez-Amavizca, R.; Quintana-Owen, P. Characterization of epoxy-nanoparticle composites exposed to gamma & UV radiation for aerospace applications. Nano Hybrids Compos. 2019, 27, 53–65. [Google Scholar]
- ANSI/UL94; Tests for Flammability of Plastic Materials for Parts in Devices and Appliances. American National Standard: Washington, DC, USA, 2023.
- FAA. Aircraft Materials Fire Test Handbook, Chapter 6; U.S. Department of Transportation Federal Aviation Administration: Washington, DC, USA, 2000.
- ISO 5659-2; Plastics—Smoke Generation, Part 2: Determination of Optical Density by a Single Chamber Test. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65243.html (accessed on 20 March 2024).
- Ramos, F.J.T.V.; Vieira, M.D.F.M.; Tienne, L.G.P.; de Oliveira Aguiar, V. Evaluation and characterization of geopolymer foams synthesized from blast furnace with sodium metasilicate. J. Mater. Res. Technol. 2020, 9, 12019–12029. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Zuo, Y.; Chen, W.; Ye, G. Chemical deformation of metakaolin based geopolymer. Cem. Concr. Res. 2019, 120, 108–118. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; An, S.; Yuan, L. Mechanical properties and microstructural characterization of metakaolin geopolymers based on orthogonal tests. Materials 2022, 15, 2957. [Google Scholar] [CrossRef] [PubMed]
- Aouan, B.; Alehyen, S.; Fadil, M.; El Alouani, M.; Saufi, H.; Taibi, M.H. Characteristics, microstructures, and optimization of the geopolymer paste based on three aluminosilicate materials using a mixture design methodology. Constr. Build. Mater. 2023, 384, 131475. [Google Scholar] [CrossRef]
- Moro, D.; Fabbri, R.; Romano, J.; Ulian, G.; Calafato, A.; Solouki, A.; Sangiorgi, C.; Valdrè, G. Thermal, X-ray diffraction and oedometric analyses of silt-waste/NaOH-activated metakaolin geopolymer composite. J. Compos. Sci. 2021, 5, 269. [Google Scholar] [CrossRef]
- Kouamo, H.T.; Elimbi, A.; Mbey, J.A.; Sabouang, C.N.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019, 14, 4212901. [Google Scholar] [CrossRef]
- Mustakim, S.M.; Das, S.K.; Mishra, J.; Aftab, A.; Alomayri, T.S.; Assaedi, H.S.; Kaze, C.R.; Fresh, I.I. Mechanical and Microstructural Properties of Fly Ash- Blast Furnace Slag Based Geopolymer Concrete By Addition of Nano and Micro Silica. Silicon 2021, 13, 2415–2428. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Kaya, K.; Soyer-Uzun, S. Evolution of structural characteristics and compressive strength in red mud-metakaolin based geopolymer systems. Ceram. Int. 2016, 42, 7406–7413. [Google Scholar] [CrossRef]
- Poggetto, G.D.; Kittisayarm, P.; Pintasiri, S.; Chiyasak, P.; Leonelli, C.; Chaysuwan, D. Chemical and Mechanical Properties of Metakaolin-Based Geopolymers with Waste Corundum Powder Resulting from Erosion Testing. Polymers 2022, 14, 5091. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zheng, Y.; Tang, Y.; Yu, J.; Yu, X.; Liu, B. Effect of phosphoric acid content on the microstructure and compressive strength of phosphoric acid-based metakaolin geopolymers. Heliyon 2020, 6, e03853. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Nenadović, S.; Pavlović, V.P.; Radovic, I.; Kijevcanin, M.; Pavlovic, V.; Kljajević, L. The influence of thermodynamic parameters on alkaline activator of geopolymers and structure of geopolymers. Maced. J. Chem. Chem. Eng. 2021, 40, 107–117. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Adediran, A.; Yliniemi, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Thermal stability of one-part metakaolin geopolymer composites containing high volume of spodumene tailings and glass wool. Cem. Concr. Comp. 2020, 114, 103792. [Google Scholar] [CrossRef]
- Sivasakthi, M.; Jeyalakshmi, R.; Rajamane, N.P.; Jose, R. Thermal and structural micro analysis of micro silica blended fly ash based geopolymer composites. J. Non-Cryst. Solids. 2018, 499, 117–130. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Sawan, S.A.; Khattab, R.M.; Abdel-Shafi, A.A. Effect of nano sand on the properties of metakaolin-based geopolymer: Study on its low rate sintering. Constr. Build. Mater. 2020, 246, 118486. [Google Scholar] [CrossRef]
- He, R.; Dai, N.; Wang, Z. Thermal and Mechanical Properties of Geopolymers Exposed to High Temperature: A Literature Review. Adv. Civ. Eng. 2020, 1, 7532703. [Google Scholar] [CrossRef]
- Karunadasa, K.S.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- da Silva Rocha, T.; Dias, D.P.; França, F.C.C.; de Salles Guerra, R.R.; de Oliveira, L.R.D.C. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Chaipanich, A.; Wianglor, K.; Piyaworapaiboon, M.; Sinthupinyo, S. Thermogravimetric analysis and microstructure of alkali-activated metakaolin cement pastes. J. Therm. Anal. Calorim. 2019, 138, 1965–1970. [Google Scholar] [CrossRef]
- Caballero, L.R.; Paiva, M.D.D.M.; Fairbairn, E.D.M.R.; Toledo Filho, R.D. Thermal, mechanical and microstructural analysis of metakaolin based geopolymers. Mater. Res. 2019, 22, e20180716. [Google Scholar] [CrossRef]
- Abramowitz, M. Handbook of Mathematical Functions, with Formulas, Graphs, and Mathematical Tables; Dover Publications, Inc.: Mineola, NY, USA, 1974; ISBN 0486612724. [Google Scholar]
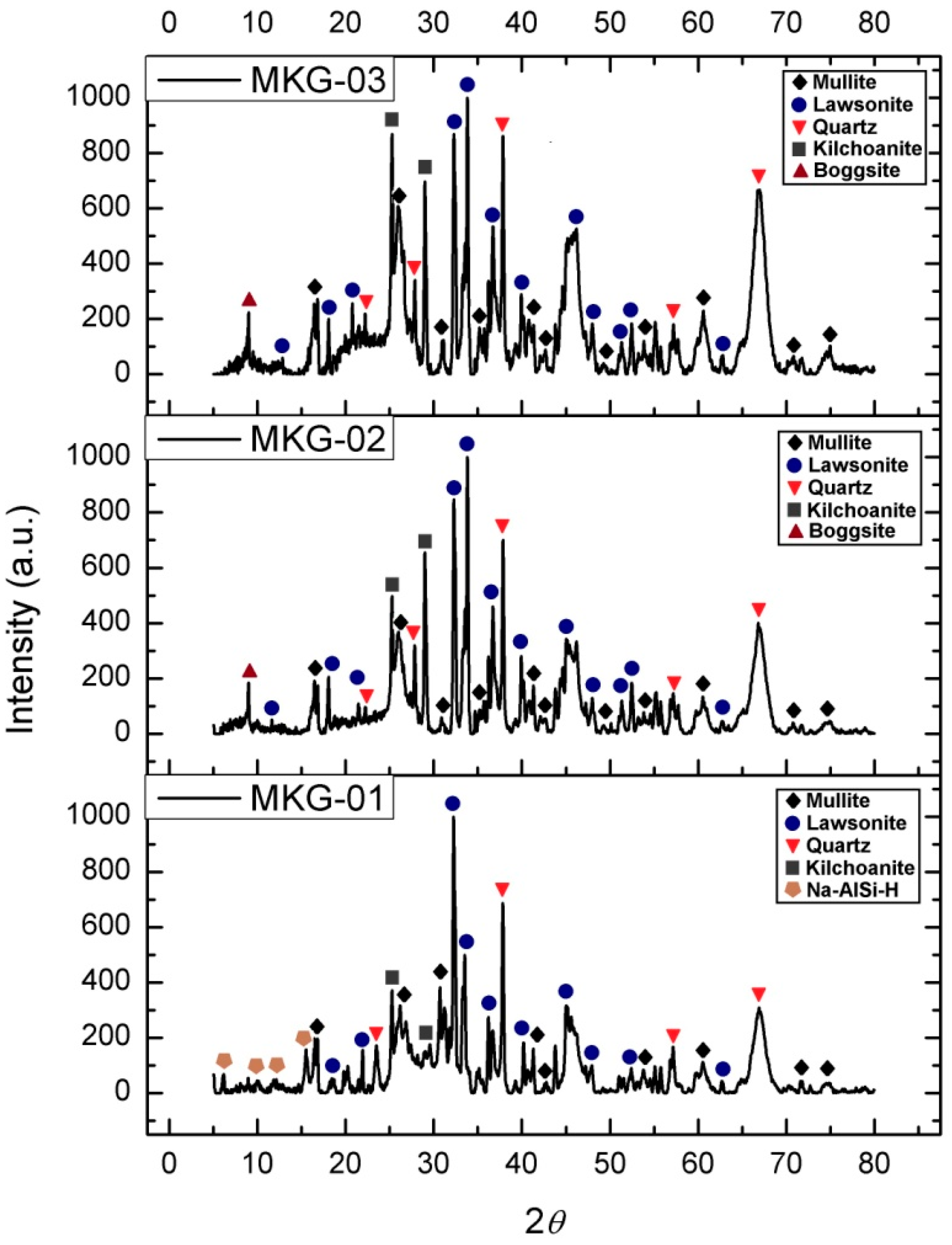

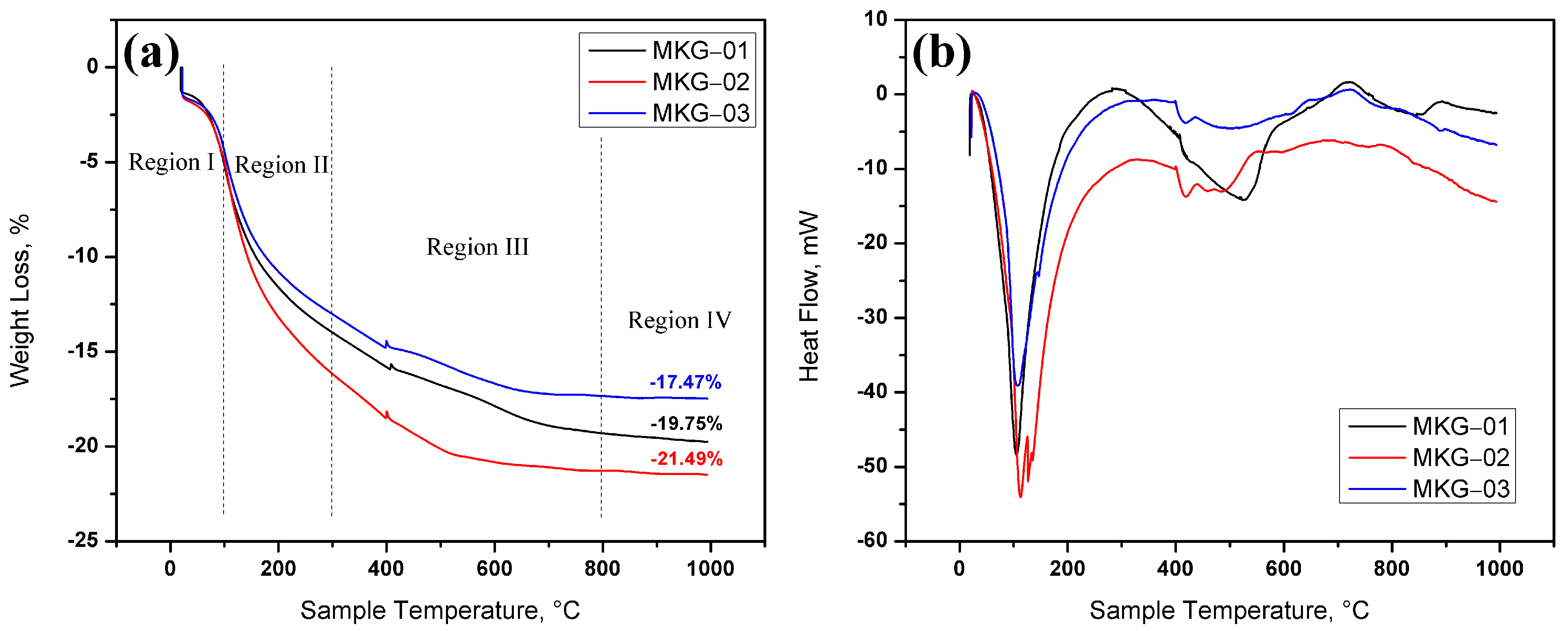
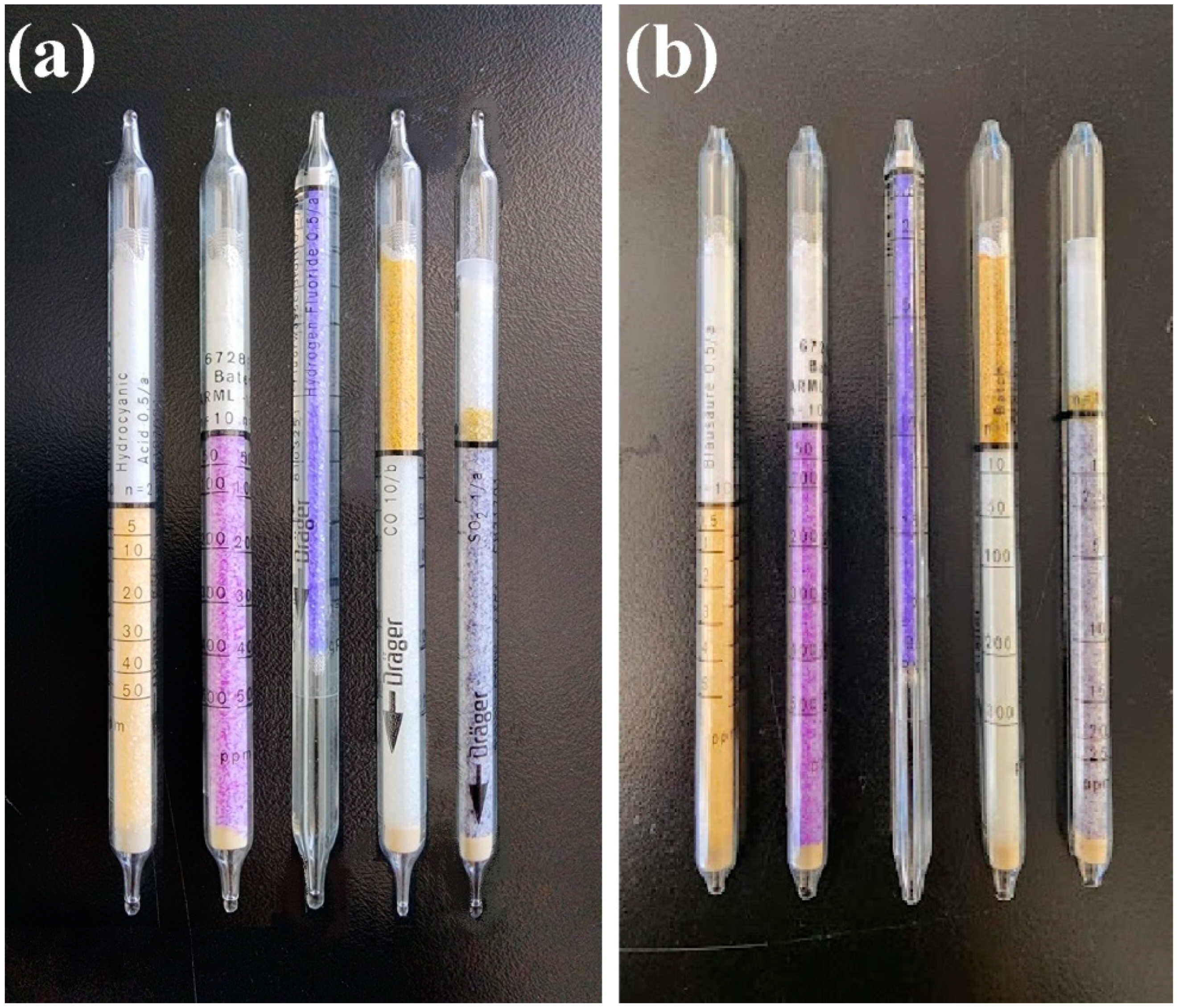
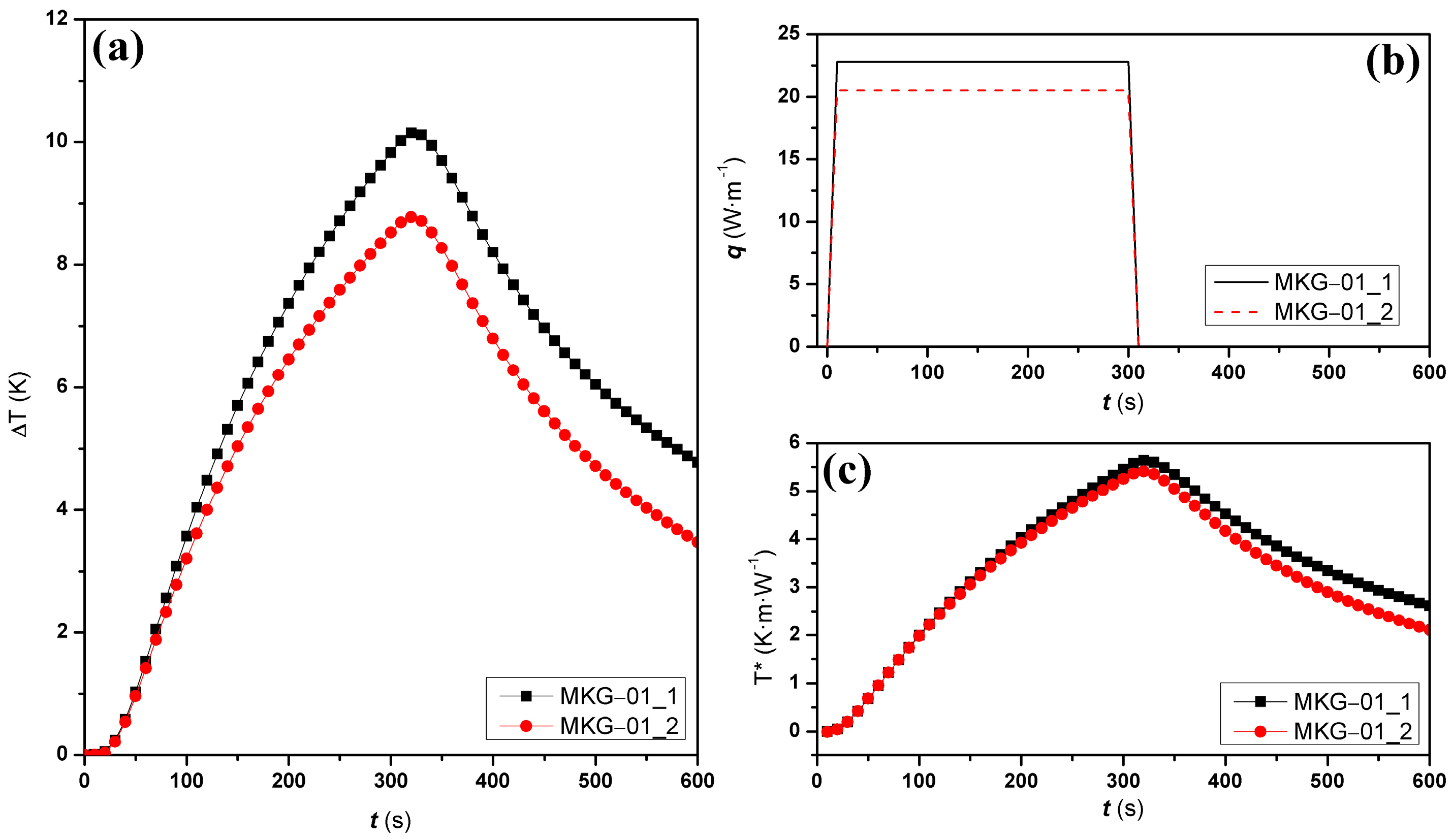


| Chemical Composition | Metakaolin |
|---|---|
| Al2O3 | 51.035 |
| SiO2 | 44.495 |
| P2O5 | 1.4850 |
| TiO2 | 1.4230 |
| Fe2O3 | 0.9201 |
| K2O | 0.3005 |
| CaO | 0.1466 |
| V2O5 | 0.0605 |
| NiO | 0.0259 |
| Cr2O3 | 0.0234 |
| Chemical Composition | MKG-01 | MKG-02 | MKG-03 |
|---|---|---|---|
| SiO2 | 20.060 | 35.310 | 36.130 |
| Al2O3 | 14.110 | 27.960 | 31.820 |
| TiO2 | 0.6464 | 0.7912 | 0.7831 |
| Fe2O3 | 0.6097 | 0.6175 | 0.6148 |
| P2O5 | 0.5110 | 0.8915 | 0.8201 |
| K2O | 0.1275 | 0.1672 | 0.1638 |
| CaO | 0.0918 | 0.1076 | 0.0872 |
| NiO | 0.0309 | 0.0336 | 0.0354 |
| V2O5 | 0.0178 | 0.0218 | 0.0188 |
| Cr2O3 | 0.0117 | 0.0139 | 0.0134 |
| Weight Loss, % | |||
|---|---|---|---|
| Region | MKG-01 | MKG-02 | MKG-03 |
| I | 5.186 | 4.994 | 4.476 |
| II | 13.994 | 16.169 | 13.026 |
| III | 19.305 | 21.279 | 17.341 |
| IV | 19.750 | 21.490 | 17.470 |
| Sample ID | MKG-01 | MKG-02 | MKG-03 |
|---|---|---|---|
| Optical Density | 0.0 | 0.0 | 0.0 |
| Transmittance, % | 99.1 | 99.1 | 99.2 |
| Sample | (Wm−1K−1) | (×10−6m2s−1) | (×106Jm−3K−1) | R2 | RMSE (KmW−1) |
|---|---|---|---|---|---|
| MKG-01_1 | 0.215 ± 0.002 | 0.144 ± 0.001 | 1.492 ± 0.021 | 0.9995 | 0.035 |
| MKG-01_2 | 0.221 ± 0.001 | 0.151 ± 0.001 | 1.461 ± 0.018 | 0.9996 | 0.032 |
| Sample | (Wm−1K−1) | (×10−6m2s−1) | (×106Jm−3K−1) | R2 | RMSE (KmW−1) |
|---|---|---|---|---|---|
| MKG-02_1 | 0.228 ± 0.002 | 0.143 ± 0.001 | 1.592 ± 0.024 | 0.9994 | 0.036 |
| MKG-02_2 | 0.230 ± 0.002 | 0.143 ± 0.001 | 1.608 ± 0.022 | 0.9995 | 0.033 |
| Sample ID | L | a | b | Hue | Croma |
|---|---|---|---|---|---|
| MKG-01 | 79.78 | 1.34 | 8.72 | 81.23 | 8.93 |
| MKG-02 | 89.37 | 0.22 | 6.29 | 88.25 | 6.26 |
| MKG-03 | 84.18 | 0.47 | 6.89 | 85.92 | 6.44 |
| Sample ID | L | a | b | Hue | Croma |
|---|---|---|---|---|---|
| MKG-01 | 80.86 | 1.66 | 9.46 | 80.05 | 9.60 |
| MKG-02 | 87.32 | 0.48 | 5.83 | 85.31 | 5.85 |
| MKG-03 | 87.24 | 0.67 | 5.95 | 83.55 | 5.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Cachú, D.; Rojas-Trigos, J.B.; Hernández-Wong, J.; Madera-Santana, T.J.; Franco-Urquiza, E.A. Geopolymers for Space Applications. Physchem 2024, 4, 197-213. https://doi.org/10.3390/physchem4030015
Mendoza-Cachú D, Rojas-Trigos JB, Hernández-Wong J, Madera-Santana TJ, Franco-Urquiza EA. Geopolymers for Space Applications. Physchem. 2024; 4(3):197-213. https://doi.org/10.3390/physchem4030015
Chicago/Turabian StyleMendoza-Cachú, D., J. B. Rojas-Trigos, J. Hernández-Wong, T. J. Madera-Santana, and E. A. Franco-Urquiza. 2024. "Geopolymers for Space Applications" Physchem 4, no. 3: 197-213. https://doi.org/10.3390/physchem4030015
APA StyleMendoza-Cachú, D., Rojas-Trigos, J. B., Hernández-Wong, J., Madera-Santana, T. J., & Franco-Urquiza, E. A. (2024). Geopolymers for Space Applications. Physchem, 4(3), 197-213. https://doi.org/10.3390/physchem4030015







