Polyvinyl Alcohol Coatings Containing Lamellar Solids with Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
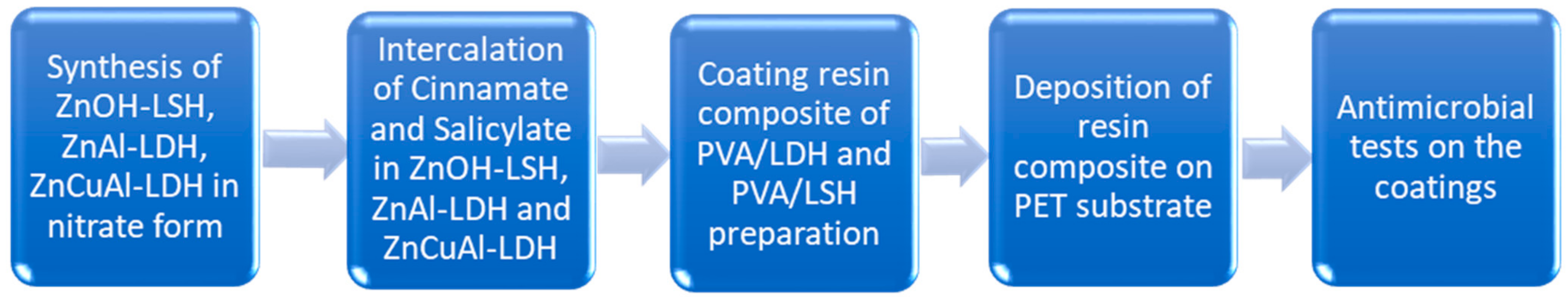
2.2. Synthesis of ZnOH-LSH, ZnAl-LDH, ZnCuAl-LDH in Nitrate Form
2.3. Intercalation of Cinnamate and Salicylate in ZnOH-LSH, ZnAl-LDH, and ZnCuAl-LDH
2.4. Coating Resin Composite of PVA/LDH and PVA/LSH Preparation
2.5. Deposition of Resin Composite on PET Substrate
2.6. Microorganisms
2.7. Antimicrobial Susceptibility Test
2.8. Analytical Procedures and Instrumentation
2.8.1. Inductively Coupled Plasma–Optical Emission Spectrometer
2.8.2. X-ray Diffraction Spectroscopy
2.8.3. FT-IR Analysis
2.8.4. Thermal Analysis
2.8.5. UV-Vis Analysis
2.8.6. SEM Analysis
3. Results and Discussion
3.1. Characterization of the Pristine Powders
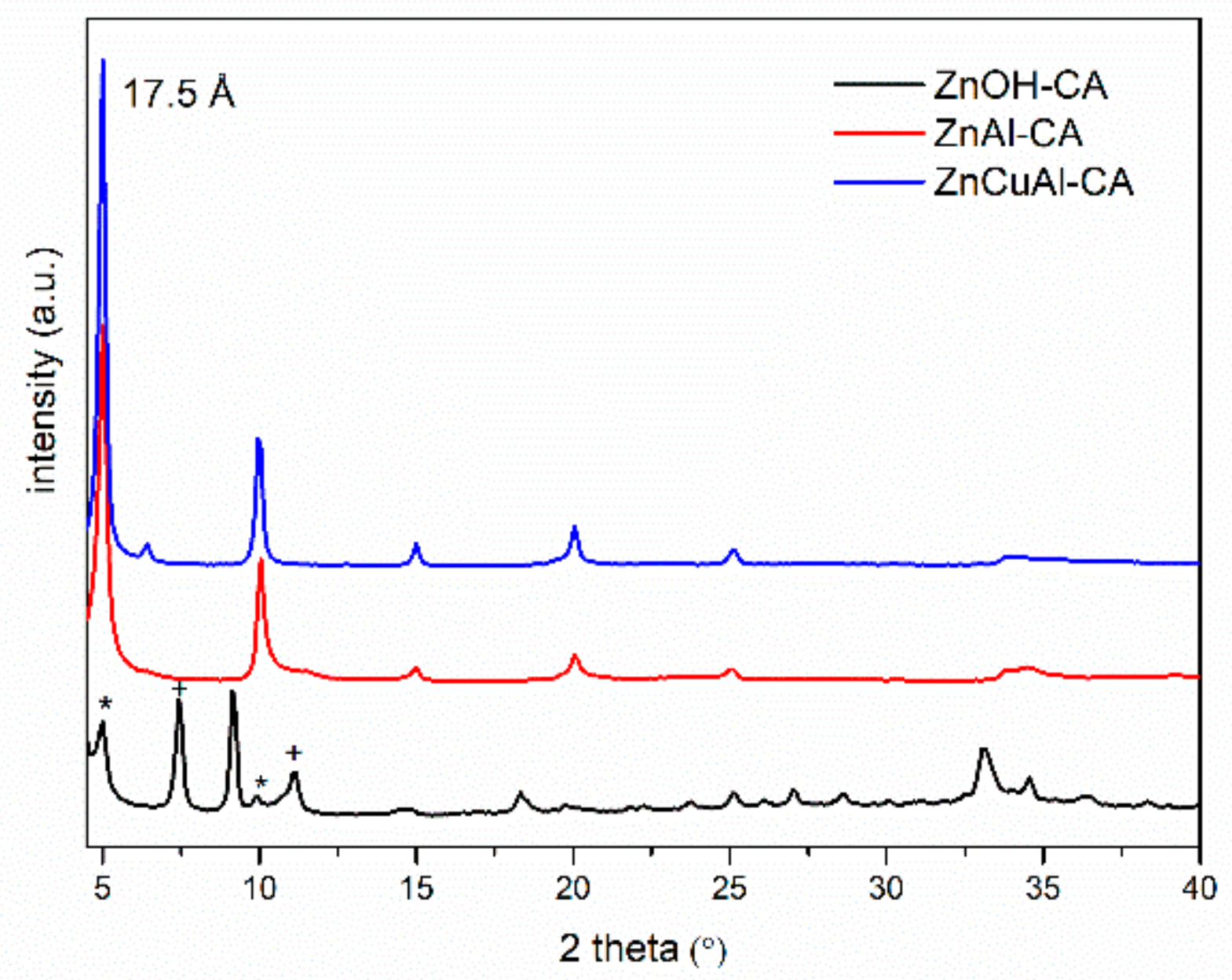
3.2. Characterization of the Intercalated Powders
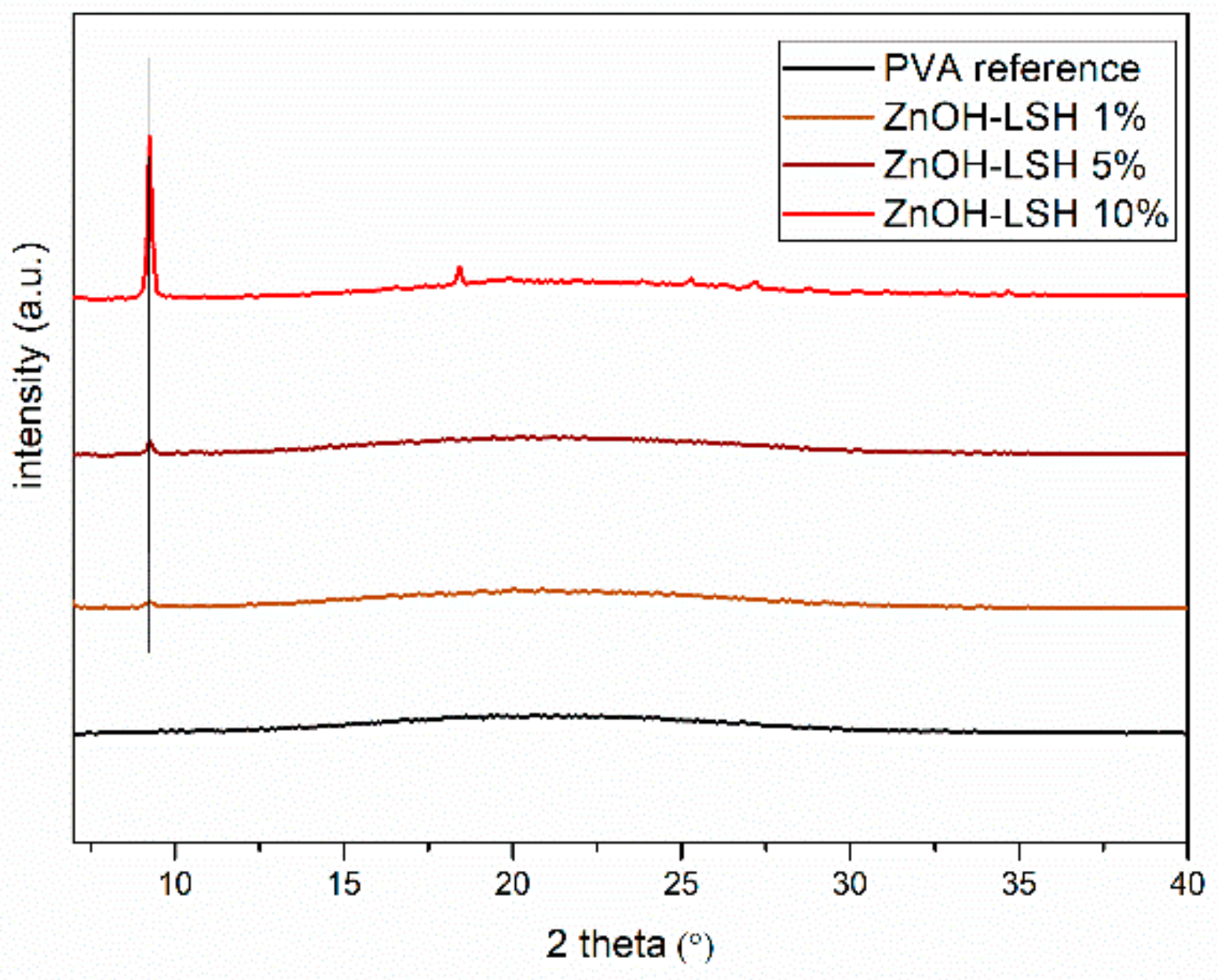
3.3. Chemical–Physical Characterization of the Coatings
3.4. Antimicrobial Tests on the Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Fan, W.; Sun, Y.; Chen, W.; Zhang, Y. Application of antiviral materials in textiles: A review. Nanotechnol. Rev. 2021, 10, 1092–1115. [Google Scholar] [CrossRef]
- Mingoia, M.; Conte, C.; Rienzo, D.A.; Dimmito, M.P.; Marinucci, L.; Magi, G.; Turkez, H.; Cufaro, M.C.; Del Boccio, P.; Di Stefano, A.; et al. Synthesis and Biological Evaluation of Novel Cinnamic Acid-Based Antimicrobials. Pharmaceuticals 2022, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, M.; Scatto, M.; Sisani, M.; Scopece, P.; Patelli, A.; Petracci, A. Innovative Composites Based on Organic Modified Zirconium Phosphate and PEOT/PBT Copolymer. J. Compos. Sci. 2018, 2, 31. [Google Scholar] [CrossRef]
- Coiai, S.; Cicogna, F.; Pinna, S.; Spiniello, R.; Onor, M.; Oberhauser, W.; Coltelli, M.-B.; Passaglia, E. Antibacterial LDPE-based nanocomposites with salicylic and rosmarinic acid-modified layered double hydroxides. Appl. Clay Sci. 2021, 214, 106276. [Google Scholar] [CrossRef]
- Puccetti, M.; Donnadio, A.; Ricci, M.; Latterini, L.; Quaglia, G.; Pietrella, D.; Di Michele, A.; Ambrogi, V. Alginate Ag/AgCl Nanoparticles Composite Films for Wound Dressings with Antibiofilm and Antimicrobial Activities. J. Funct. Biomater. 2023, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.M.E.; White, D.; McLaughlin, S.; Kumar, A. Salicylic acids and pathogenic bacteria: New perspectives on an old compound. Can. J. Microbiol. 2023, 14, 1–14. [Google Scholar] [CrossRef]
- Moulay, S. Review: Poly(vinyl alcohol) Functionalizations and Applications. Polym. Plast. Technol. Eng. 2015, 54, 1289–1319. [Google Scholar] [CrossRef]
- Yeun, J.H.; Bang, G.S.; Park, B.J.; Ham, S.K.; Chang, J.H. Poly(vinyl alcohol) nanocomposite films: Thermooptical properties, morphology, and gas permeability. J. Appl. Polym. Sci. 2006, 101, 591–596. [Google Scholar] [CrossRef]
- Bastianini, M.; Sisani, M.; Naryyev, E.; Petracci, A.; Di Guida, I.; Narducci, R. Composite membranes based on polyvinyl alcohol and lamellar solids for water decontamination. New J. Chem. 2024, 48, 2128. [Google Scholar] [CrossRef]
- Ruiz, C.V.; López-González, M.; Giraldo, O. Composite films based on salicylic acid-layered zinc hydroxide and polyvinyl alcohol: Preparation, characterization, properties and potential applications. Polym. Test. 2021, 94, 107057. [Google Scholar] [CrossRef]
- Li, P.; Xu, Z.P.; Hampton, M.A.; Vu, D.T.; Huang, L.; Rudolph, V.; Nguyen, A.V. Control preparation of zinc hydroxide nitrate nanocrystals and examination of the chemical and structural stability. J. Phys. Chem. C 2012, 116, 10325–10332. [Google Scholar] [CrossRef]
- Di Michele, A.; Boccalon, E.; Costantino, F.; Bastianini, M.; Vivani, R.; Nocchetti, M. Insight into the synthesis of LDH using the urea method: Morphology and intercalated anion. Dalton Trans. 2024, accepted. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Standard, Approval CDM-A. In M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018; Volume 91. [Google Scholar]
- Costantino, U.; Bugatti, V.; Gorrasi, G.; Montanari, F.; Nocchetti, M.; Tammaro, L.; Vittoria, V. New polymeric composites based on poly(ε-caprolactone) and layered double hydroxides containing antimicrobial species. ACS Appl. Mater. Interfaces 2009, 1, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Bovio, B.; Locchi, S. Crystal structure of the orthorhombic basic copper nitrate, Cu2(OH)3NO3. J. Crystallogr. Spectrosc. Res. 1982, 12, 6. [Google Scholar] [CrossRef]
- Ruiz, C.V.; Rodríguez-Castellón, E.; Giraldo, O. Hybrid materials based on a layered zinc hydroxide solid and gallic acid: Structural characterization and evaluation of the controlled release behavior as a function of the gallic acid content. Appl. Clay Sci. 2019, 181, 105228. [Google Scholar] [CrossRef]
- Nabipour, H.; Sadr, M.H.; Thomas, N. Synthesis, controlled release and antibacterial studies of nalidixic acid-zinc hydroxide nitrate nanocomposites. New J. Chem. 2016, 40, 238–244. [Google Scholar] [CrossRef]
- Adam, N.; Mohd Ghazali, S.A.I.S.; Dzulkifli, N.N.; Hak, C.R.C.; Sarijo, S.H. Intercalations and characterization of zinc/aluminium layered double hydroxide-cinnamic acid. Bull. Chem. React. Eng. Catal. 2019, 14, 165–172. [Google Scholar] [CrossRef]
- Pérez, M.R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.C.; Ulibarri, M.A. Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]
- Palacio, L.A.; Velásquez, J.; Echavarría, A.; Faro, A.; Ribeiro, F.R.; Ribeiro, M.F. Total oxidation of toluene over calcined trimetallic hydrotalcites type catalysts. J. Hazard. Mater. 2010, 177, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.M.N.; Hussein, M.Z.; Sarijo, S.H.; Fakurazi, S.; Arulselven, P.; Yun Hin, T.-Y. Synthesis of (cinnamate-zinc layered hydroxide) intercalation compound for sunscreen application. Chem. Cent. J. 2013, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Arizaga, G.G.C.; Satyanarayana, K.G.; Wypych, F. Layered hydroxide salts: Synthesis, properties and potential applications. Solid State Ionics 2007, 178, 1143–1162. [Google Scholar] [CrossRef]
- Silion, M.; Hritcu, D.; Popa, M.I. Intercalation of salicylic acid into ZnAl layered double hydroxides by ionic-exchange method. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 817–820. [Google Scholar]
- Shamraiz, U.; Badshah, A.; Hussain, R.A.; Nadeem, M.A.; Saba, S. Surfactant-Free Fabrication of Copper Sulfides (CuS, Cu2S) via Hydrothermal Method. J. Clust. Sci. 2013, 24, 1181–1191. [Google Scholar] [CrossRef]
- Cheng, H.M.; Gao, X.W.; Zhang, K.; Wang, X.-R.; Zhou, W.; Li, S.-H.; Cao, X.-L.; Yan, D.-P. A novel antimicrobial composite: ZnAl-hydrotalcite with: P-hydroxybenzoic acid intercalation and its possible application as a food packaging material. New J. Chem. 2019, 43, 19408–19414. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Congradyova, A.; Jomová, K.; Kucková, L.; Kozísek, J.; Moncol, J.; Valko, M. Antimicrobial activity of copper (II) complexes. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 67. [Google Scholar]



| Film | PVA Amount (g) | Water (mL) | Filler (g) |
|---|---|---|---|
| 1% | 9.9 | 100 | 0.1 |
| 5% | 9.5 | 100 | 0.5 |
| 10% | 9.0 | 100 | 1.0 |
| Sample | Water Loss% | Total Loss% | Active Weight Content% |
|---|---|---|---|
| ZnOH-CA | 4.0 | 49.5 | 37 |
| ZnAl-CA | 18.0 | 54.4 | 27 |
| ZnCuAl-CA | 12.0 | 51.0 | 30 |
| ZnOH-SAL | 5.0 | 53.0 | 35 |
| ZnAl-SAL | 21.0 | 56.8 | 25 |
| ZnCuAl-SAL | 5.0 | 53.1 | 30 |
| S. aureus | E. faecalis | A. baumannii | K. pneumoniae | C. albicans | |
|---|---|---|---|---|---|
| ZnAl-LDH 1% | 6.33 ± 1.53 | 8.00 ± 3.61 | - | Nt | 7.00 ± 0.00 |
| ZnAl-LDH 5% | 7.33 ± 2.08 | 7.33 ± 2.52 | - | Nt | 7.00 ± 0.00 |
| ZnAl-LDH 10% | 7.33 ± 2.08 | 8.67 ± 3.21 | - | Nt | 8.33 ± 1.53 |
| ZnCuAl-LDH 1% | - | - | - | - | 6.33 ± 0.58 |
| ZnCuAl-LDH 5% | - | 5.08 ± 3.04 | - | - | 6.33 ± 1.15 |
| ZnCuAl-LDH 10% | - | - | - | - | 8.00 ± 1.00 |
| ZnOH-LSH 1% | - | 8.33 ± 1.53 | - | Nt | 7.67 ± 3.79 |
| ZnOH-LSH 5% | - | 10.33 ± 0.58 | - | Nt | 9.33 ± 4.93 |
| ZnOH-LSH 10% | 8.33 ± 3.06 | 11.33 ± 2.08 | 7.33 ± 2.08 | Nt | 11.00 ± 3.61 |
| Erytromycin (15 µg) | 16.33 ± 1.15 | 20.00 ± 2.65 | 14.00 ± 2.65 | - | - |
| Fluconazole (25 µg) | - | - | - | - | 5.67 ± 1.15 |
| S. aureus | E. faecalis | A. baumannii | K. pneumoniae | C. albicans | |
|---|---|---|---|---|---|
| ZnAl-CA 1% | - | 7.00 ± 0.00 | - | Nt | 5.33 ± 0.58 |
| ZnAl-CA 5% | - | 8.00 ± 1.41 | - | Nt | 8.67 ± 3.79 |
| ZnAl-CA 10% | - | 10.00 ± 1.73 | - | Nt | 12.33 ± 0.58 |
| ZnCuAl-CA 1% | - | - | - | 5.33 ± 0.58 | 5.33 ± 0.58 |
| ZnCuAl-CA 5% | - | 5.67 ± 4.17 | - | 5.33 ± 0.58 | 7.00 ± 1.00 |
| ZnCuAl-CA 10% | - | 5.50 ± 3.73 | - | 6.00 ± 1.00 | 11.33 ± 1.15 |
| ZnOH-CA 1% | - | 10.00 ± 5.00 | - | Nt | - |
| ZnOH-CA 5% | - | 10.67 ± 5.13 | - | Nt | 5.67 ± 1.15 |
| ZnOH-CA 10% | - | 10.00 ± 6.24 | - | Nt | 8.00 ± 1.00 |
| Erytromycin (15 µg) | 16.33 ± 1.15 | 20.00 ± 2.65 | 14.00 ± 2.65 | - | - |
| Fluconazole (25 µg) | - | - | - | - | 5.67 ± 1.15 |
| S. aureus | E. faecalis | A. baumannii | K. pneumoniae | C. albicans | |
|---|---|---|---|---|---|
| ZnAl-SAL 1% | 6.33 ± 1.15 | 9.67 ± 5.03 | - | Nt | 6.67 ± 2.89 |
| ZnAl-SAL 5% | 7.00 ± 2.00 | 10.00 ± 4.36 | 7.00 ± 2.00 | Nt | 7.33 ± 4.04 |
| ZnAl-SAL 10% | 8.00 ± 2.65 | 12.33 ± 2.31 | 5.67 ± 1.15 | Nt | 7.00 ± 3.46 |
| ZnCuAl-SAL 1% | - | 5.42 ± 3.30 | 7.33 ± 4.04 | 10.00 ± 5.20 | - |
| ZnCuAl-SAL 5% | 8.33 ± 1.53 | 6.57 ± 3.49 | 9.00 ± 6.93 | 10.00 ± 2.65 | 6.50 ± 2.12 |
| ZnCuAl-SAL 10% | 9.33 ± 1.53 | 6.49 ± 3.46 | 10.67 ± 7.37 | 11.33 ± 2.31 | - |
| ZnOH-SAL 1% | - | 7.33 ± 2.52 | 9.67 ± 3.79 | Nt | 5.33 ± 0.58 |
| ZnOH-SAL 5% | - | 6.67 ± 2.89 | 10.00 ± 4.36 | Nt | 6.00 ± 1.73 |
| ZnOH-SAL 10% | - | 9.00 ± 5.29 | 10.33 ± 4.16 | Nt | 10.33 ± 4.16 |
| Erytromycin (15 µg) | 16.33 ± 1.15 | 20.00 ± 2.65 | 14.00 ± 2.65 | - | - |
| Fluconazole (25 µg) | - | - | - | - | 5.67 ± 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastianini, M.; Sisani, M.; Escudero García, R.; Di Guida, I.; Russo, C.; Pietrella, D.; Narducci, R. Polyvinyl Alcohol Coatings Containing Lamellar Solids with Antimicrobial Activity. Physchem 2024, 4, 272-284. https://doi.org/10.3390/physchem4030019
Bastianini M, Sisani M, Escudero García R, Di Guida I, Russo C, Pietrella D, Narducci R. Polyvinyl Alcohol Coatings Containing Lamellar Solids with Antimicrobial Activity. Physchem. 2024; 4(3):272-284. https://doi.org/10.3390/physchem4030019
Chicago/Turabian StyleBastianini, Maria, Michele Sisani, Raúl Escudero García, Irene Di Guida, Carla Russo, Donatella Pietrella, and Riccardo Narducci. 2024. "Polyvinyl Alcohol Coatings Containing Lamellar Solids with Antimicrobial Activity" Physchem 4, no. 3: 272-284. https://doi.org/10.3390/physchem4030019








