Magnetite Thin Films by Solvothermal Synthesis on a Microstructured Si Substrate as a Model to Study Energy Storage Mechanisms of Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Fe3O4 Electrode Synthesis
2.2. Silicon Substrate Treatment
2.3. Characterization
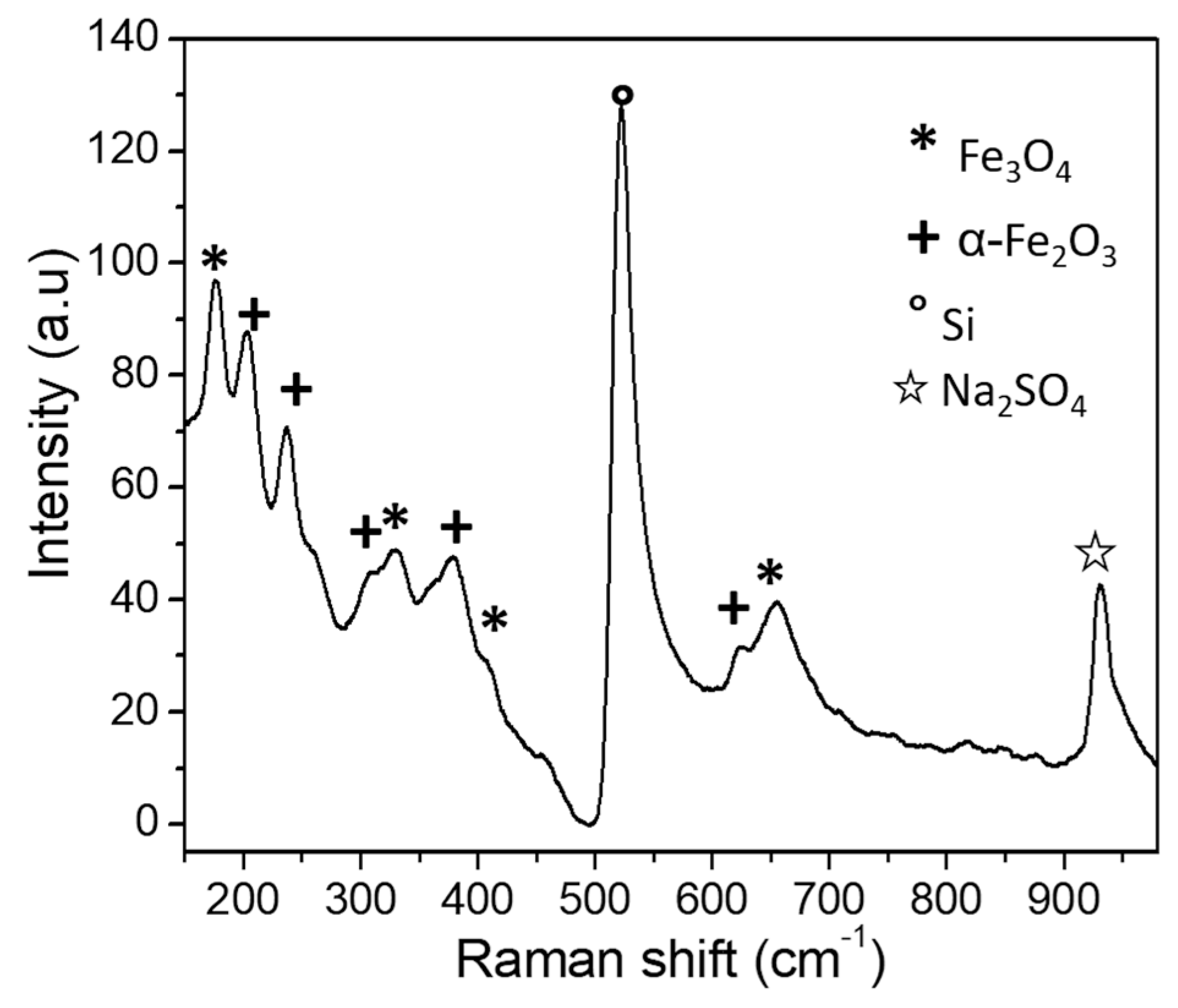
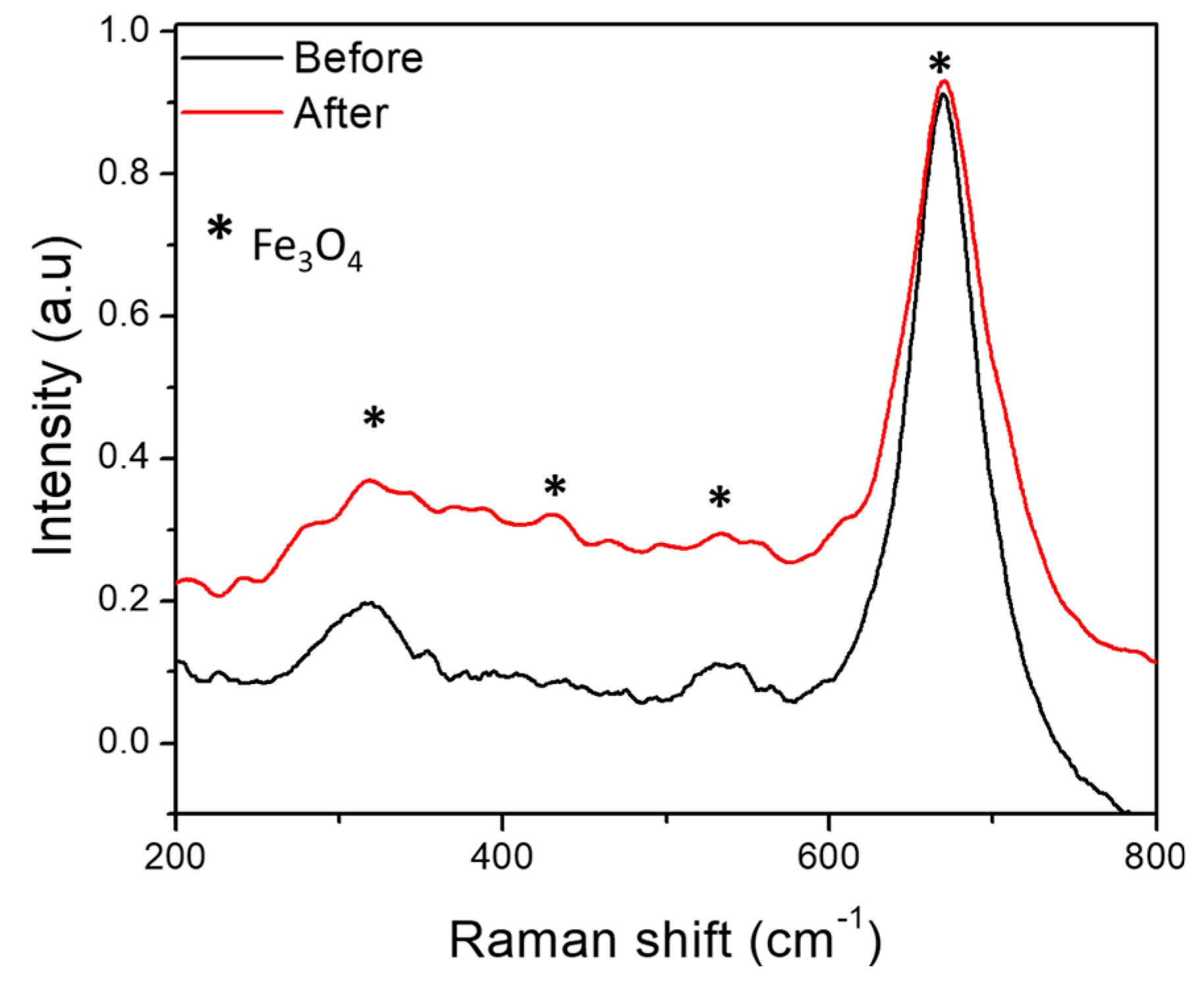
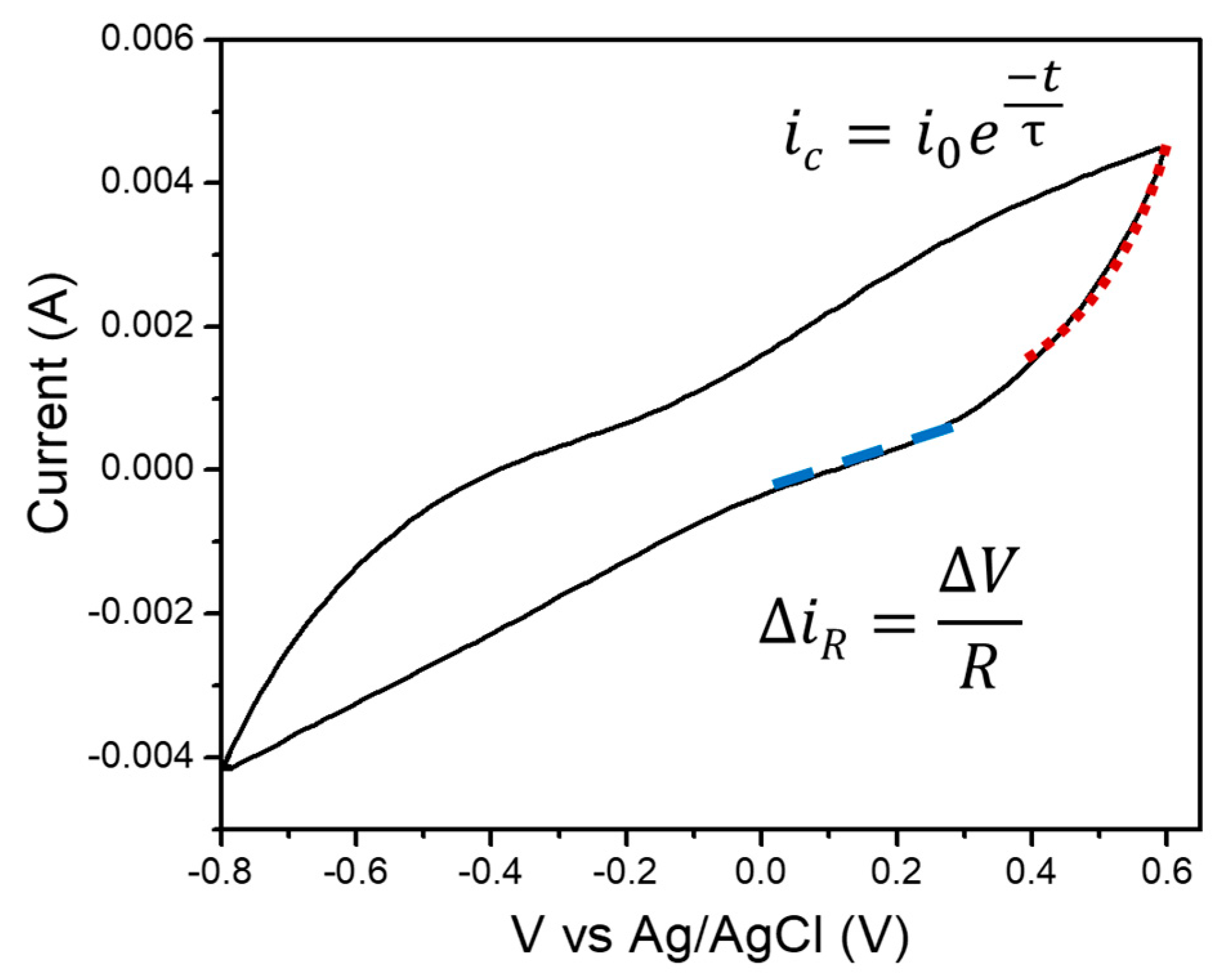
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable energy and energy storage systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Devillers, N.; Jemei, S.; Péra, M.-C.; Bienaimé, D.; Gustin, F. Review of characterization methods for supercapacitor modelling. J. Power Sources 2014, 246, 596–608. [Google Scholar] [CrossRef]
- Attia, S.Y.; Mohamed, S.G.; Barakat, Y.F.; Hassan, H.H.; Zoubi, W.A. Supercapacitor electrode materials: Addressing challenges in mechanism and charge storage. Rev. Inorg. Chem. 2022, 42, 53–88. [Google Scholar] [CrossRef]
- Okwundu, O.S.; Ugwuoke, C.O.; Okaro, A.C. Recent trends in non-faradaic supercapacitor electrode materials. Metall. Mater. Eng. 2019, 25, 105–138. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-L.; Lee, J.-F.; Wu, N.-L. Study on pseudocapacitance mechanism of aqueous MnFe2O4 supercapacitor. J. Electrochem. Soc. 2006, 154, A34. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R. Electrochemical evaluation of the performance of cathodically grown ultra-fine magnetite nanoparticles as electrode material for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 13532–13539. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Ho, K.-C.; Kuo, S.-L.; Wu, N.-L. Investigation on Capacitance Mechanisms of Fe3O4 Electrochemical Capacitors. J. Electrochem. Soc. 2006, 153, A75. [Google Scholar] [CrossRef]
- Wu, N.-L.; Wang, S.-Y.; Han, C.-Y.; Wu, D.-S.; Shiue, L.-R. Electrochemical capacitor of magnetite in aqueous electrolytes. J. Power Sources 2003, 113, 173–178. [Google Scholar] [CrossRef]
- Tipsawat, P.; Wongpratat, U.; Phumying, S.; Chanlek, N.; Chokprasombat, K.; Maensiri, S. Magnetite (Fe3O4) nanoparticles: Synthesis, characterization and electrochemical properties. Appl. Surf. Sci. 2018, 446, 287–292. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci. Total Environ. 2020, 722, 137972. [Google Scholar] [CrossRef] [PubMed]
- Nkurikiyimfura, I.; Wang, Y.; Safari, B.; Nshingabigwi, E. Temperature-dependent magnetic properties of magnetite nanoparticles synthesized via coprecipitation method. J. Alloys Compd. 2020, 846, 156344. [Google Scholar] [CrossRef]
- Fuentes-García, J.s.A.; Carvalho Alavarse, A.; Moreno Maldonado, A.C.; Toro-Córdova, A.; Ibarra, M.R.; Goya, G.F.n. Simple sonochemical method to optimize the heating efficiency of magnetic nanoparticles for magnetic fluid hyperthermia. ACS Omega 2020, 5, 26357–26364. [Google Scholar] [CrossRef] [PubMed]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Chala, G.H.; Zeleke, T.D. Green synthesis of magnetite nanoparticles using Catha edulis plant leaf extract for removal of hexavalent Chromium from aqueous solution. Int. J. Nano Dimens. 2023, 14, 73–90. [Google Scholar]
- Liang, Y.; Jiang, L.; Xu, S.; Ju, W.; Tao, Z.; Yang, Y.; Peng, X.; Wei, G. Synthesis and Characterization of Fe3O4 Nanoparticles Prepared by Solvothermal Method. J. Mater. Eng. Perform. 2024, 33, 6804–6815. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Delgado, Á.; Iglesias, G.R. Solvothermally synthesized magnetite nanorods for application in magnetic hyperthermia and photothermia. J. Magn. Magn. Mater. 2024, 596, 171990. [Google Scholar] [CrossRef]
- Sahadevan, J.; Sojiya, R.; Padmanathan, N.; Kulathuraan, K.; Shalini, M.; Sivaprakash, P.; Muthu, S.E. Magnetic property of Fe2O3 and Fe3O4 nanoparticle prepared by solvothermal process. Mater. Today Proc. 2022, 58, 895–897. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhao, R.; Meng, F.; Lei, Y.; Zhong, J.; Yang, X.; Liu, X. Oriented growth of magnetite along the carbon nanotubes via covalently bonded method in a simple solvothermal system. Mater. Sci. Eng. B 2011, 176, 779–784. [Google Scholar] [CrossRef]
- Ma, J.; Liu, W.; Zhang, S.; Zhao, J.; Li, W. One-step solvothermal approach for preparing soft magnetic hydrophilic PFR coated Fe3O4 nanocrystals. J. Alloys Compd. 2011, 509, 7895–7899. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Zhu, L. Hydrothermal growth and characterization of magnetite (Fe3O4) thin films. Surf. Coat. Technol. 2007, 201, 5870–5874. [Google Scholar] [CrossRef]
- Ooi, F.; DuChene, J.S.; Qiu, J.; Graham, J.O.; Engelhard, M.H.; Cao, G.; Gai, Z.; Wei, W. A Facile Solvothermal Synthesis of Octahedral Fe3O4 Nanoparticles; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2015. [Google Scholar]
- Li, S.; Zhang, T.; Tang, R.; Qiu, H.; Wang, C.; Zhou, Z. Solvothermal synthesis and characterization of monodisperse superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2015, 379, 226–231. [Google Scholar] [CrossRef]
- Aca-López, V.; Quiroga-González, E.; Gómez-Barojas, E.; Światowska, J.; Luna-López, J.A. Effects of the doping level in the production of silicon nanowalls by metal assisted chemical etching. Mater. Sci. Semicond. Process. 2020, 118, 105206. [Google Scholar] [CrossRef]
- Raja, K.; Verma, S.; Karmakar, S.; Kar, S.; Das, S.J.; Bartwal, K. Synthesis and characterization of magnetite nanocrystals. Cryst. Res. Technol. 2011, 46, 497–500. [Google Scholar] [CrossRef]
- Hall, B.; Zanchet, D.; Ugarte, D. Estimating nanoparticle size from diffraction measurements. J. Appl. Crystallogr. 2000, 33, 1335–1341. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, B.; Li, X.; Li, R.; Yu, R. Solvothermal synthesis of hollow Fe3O4 sub-micron spheres and their enhanced electrochemical properties for supercapacitors. Mater. Des. 2016, 101, 35–43. [Google Scholar] [CrossRef]
- Sinan, N.; Unur, E. Fe3O4/carbon nanocomposite: Investigation of capacitive & magnetic properties for supercapacitor applications. Mater. Chem. Phys. 2016, 183, 571–579. [Google Scholar] [CrossRef]
- Dar, M.I.; Shivashankar, S. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Zhao, J.; Brugger, J.; Pring, A. Mechanism and kinetics of hydrothermal replacement of magnetite by hematite. Geosci. Front. 2019, 10, 29–41. [Google Scholar] [CrossRef]
- Patil, S.M.; Shingte, S.R.; Karade, V.C.; Kim, J.H.; Kulkarni, R.M.; Chougale, A.D.; Patil, P.B. Electrochemical performance of magnetic nanoparticle-decorated reduced graphene oxide (MRGO) in various aqueous electrolyte solutions. J. Solid State Electrochem. 2021, 25, 927–938. [Google Scholar] [CrossRef]
- Tang, J.; Myers, M.; Bosnick, K.A.; Brus, L.E. Magnetite Fe3O4 Nanocrystals: Spectroscopic Observation of Aqueous Oxidation Kinetics. J. Phys. Chem. B 2003, 107, 7501–7506. [Google Scholar] [CrossRef]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. Preparation and electrochemical performance of porous hematite (α-Fe2O3) nanostructures as supercapacitor electrode material. J. Solid State Electrochem. 2014, 18, 1057–1066. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Controlled synthesis of mesoporous hematite nanostructures and their application as electrochemical capacitor electrodes. Nanotechnology 2011, 22, 135604. [Google Scholar] [CrossRef] [PubMed]
- Sanei, A.; Irani, M.A.; Kolvari, E.; Koukabi, N.; Dashtian, K. Unveiling magnetite metal-organic frameworks for asymmetric pseudo-supercapacitor with high energy density and magnetocapacitance. J. Energy Storage 2024, 100, 113759. [Google Scholar] [CrossRef]
- Cheng, J.P.; Shou, Q.L.; Wu, J.S.; Liu, F.; Dravid, V.P.; Zhang, X.B. Influence of component content on the capacitance of magnetite/reduced graphene oxide composite. J. Electroanal. Chem. 2013, 698, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Jiang, L.; Wu, H.; Wu, C.; Su, J. Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Sources 2012, 216, 290–296. [Google Scholar] [CrossRef]
- Thakur, A.V.; Lokhande, B.J. Electrolytic anion affected charge storage mechanisms of Fe3O4 flexible thin film electrode in KCl and KOH: A comparative study by cyclic voltammetry and galvanostatic charge–discharge. J. Mater. Sci. Mater. Electron. 2017, 28, 11755–11761. [Google Scholar] [CrossRef]
- Choudhury, B.J.; Moholkar, V.S. Magnetite–Graphene-Based Composites and Their Potential Application as Supercapacitor Electrode Material. In Handbook of Magnetic Hybrid Nanoalloys and their Nanocomposites; Thomas, S., Rezazadeh Nochehdehi, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 879–914. [Google Scholar]
- Chen, J.; Huang, K.; Liu, S. Hydrothermal preparation of octadecahedron Fe3O4 thin film for use in an electrochemical supercapacitor. Electrochim. Acta 2009, 55, 1–5. [Google Scholar] [CrossRef]
- O’Neill, L.; Johnston, C.; Grant, P.S. Enhancing the supercapacitor behaviour of novel Fe3O4/FeOOH nanowire hybrid electrodes in aqueous electrolytes. J. Power Sources 2015, 274, 907–915. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef]
- Eftekhari, A. Surface Diffusion and Adsorption in Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3692–3701. [Google Scholar] [CrossRef]
- Johns, P.A.; Roberts, M.R.; Wakizaka, Y.; Sanders, J.H.; Owen, J.R. How the electrolyte limits fast discharge in nanostructured batteries and supercapacitors. Electrochem. Commun. 2009, 11, 2089–2092. [Google Scholar] [CrossRef]
- Feitknecht, W.; Gallagher, K.J. Mechanisms for the Oxidation of Fe3O4. Nature 1970, 228, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hu, Y.; Qian, H.; Ning, J.; Xu, S. Magnetite (Fe3O4) tetrakaidecahedral microcrystals: Synthesis, characterization, and micro-Raman study. Mater. Charact. 2011, 62, 148–151. [Google Scholar] [CrossRef]
- Chen, D.; Ding, D.; Li, X.; Waller, G.H.; Xiong, X.; El-Sayed, M.A.; Liu, M. Probing the Charge Storage Mechanism of a Pseudocapacitive MnO2 Electrode Using in Operando Raman Spectroscopy. Chem. Mater. 2015, 27, 6608–6619. [Google Scholar] [CrossRef]
- Meng, C.; Das, P.; Shi, X.; Fu, Q.; Müllen, K.; Wu, Z.-S. In Situ and Operando Characterizations of 2D Materials in Electrochemical Energy Storage Devices. Small Sci. 2021, 1, 2000076. [Google Scholar] [CrossRef]
- Gupta, S.; Carrizosa, S.B.; Jasinski, J.; Dimakis, N. Charge transfer dynamical processes at graphene-transition metal oxides/electrolyte interface for energy storage: Insights from in-situ Raman spectroelectrochemistry. AIP Adv. 2018, 8, 065225. [Google Scholar] [CrossRef]
- Flores, E.; Novák, P.; Berg, E.J. In situ and Operando Raman Spectroscopy of Layered Transition Metal Oxides for Li-ion Battery Cathodes. Front. Energy Res. 2018, 6, 82. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Hu, A.; Zhou, M.; Chen, W.; Lei, T.; Yan, Y.; Huang, J.; Yang, C.; Wang, X. In Situ/operando Raman techniques in lithium–sulfur batteries. Small Struct. 2022, 3, 2100170. [Google Scholar] [CrossRef]
- Li, G.; Lan, J.; Li, G. Chrysanthemum-like 3D hierarchical magnetic γ-Fe2O3 and Fe3O4 superstructures: Facile synthesis and application in adsorption of organic pollutants from water. RSC Adv. 2015, 5, 1705–1711. [Google Scholar] [CrossRef]
- Naser, A.M.; Higgins, E.M.; Arman, S.; Ercumen, A.; Ashraf, S.; Das, K.K.; Rahman, M.; Luby, S.P.; Unicomb, L. Effect of groundwater iron on residual chlorine in water treated with sodium dichloroisocyanurate tablets in rural Bangladesh. Am. J. Trop. Med. Hyg. 2018, 98, 977. [Google Scholar] [CrossRef] [PubMed]
- Tuschel, D. Stress, strain, and Raman spectroscopy. Spectroscopy 2019, 34, 10–21. [Google Scholar]
- Kumar, P.; No-Lee, H.; Kumar, R. Synthesis of phase pure iron oxide polymorphs thin films and their enhanced magnetic properties. J. Mater. Sci. Mater. Electron. 2014, 25, 4553–4561. [Google Scholar] [CrossRef]






| Scan Rate [V/s] | R [Ω] | τ [s] | C [F] | C [F/g] |
|---|---|---|---|---|
| 0.002 | 881.05727 | 45.79298 | 0.05198 | 16.65866 |
| 0.005 | 505.61798 | 22.94127 | 0.04537 | 14.54254 |
| 0.01 | 321.94598 | 14.39368 | 0.04471 | 14.32961 |
| 0.02 | 242.71845 | 9.96214 | 0.04104 | 13.15513 |
| 0.05 | 271.96653 | 6.22587 | 0.02289 | 7.33719 |
| 0.1 | 240.32043 | 4.2858 | 0.01783 | 5.71593 |
| 0.2 | 283.28612 | 2.6158 | 0.00923 | 2.95954 |
| Scan Rate [V/s] | C = τ/R [F] | icap | idif | icap/idif |
|---|---|---|---|---|
| 0.002 | 0.05198 | 1.04 × 10−4 | 7.42 × 10−4 | 0.1401 |
| 0.005 | 0.04537 | 2.27 × 10−4 | 0.00109 | 0.20898 |
| 0.01 | 0.04471 | 4.47 × 10−4 | 0.00134 | 0.33273 |
| 0.02 | 0.04104 | 8.21 × 10−4 | 0.00125 | 0.65907 |
| 0.05 | 0.02289 | 0.00114 | 0.00156 | 0.73582 |
| 0.1 | 0.01783 | 0.00178 | 7.93 × 10−4 | 2.24978 |
| 0.2 | 0.00923 | 0.00185 | 9.69 × 10−4 | 1.90594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez, K.; Quiroga-González, E. Magnetite Thin Films by Solvothermal Synthesis on a Microstructured Si Substrate as a Model to Study Energy Storage Mechanisms of Supercapacitors. Physchem 2024, 4, 536-547. https://doi.org/10.3390/physchem4040037
Chavez K, Quiroga-González E. Magnetite Thin Films by Solvothermal Synthesis on a Microstructured Si Substrate as a Model to Study Energy Storage Mechanisms of Supercapacitors. Physchem. 2024; 4(4):536-547. https://doi.org/10.3390/physchem4040037
Chicago/Turabian StyleChavez, Karina, and Enrique Quiroga-González. 2024. "Magnetite Thin Films by Solvothermal Synthesis on a Microstructured Si Substrate as a Model to Study Energy Storage Mechanisms of Supercapacitors" Physchem 4, no. 4: 536-547. https://doi.org/10.3390/physchem4040037
APA StyleChavez, K., & Quiroga-González, E. (2024). Magnetite Thin Films by Solvothermal Synthesis on a Microstructured Si Substrate as a Model to Study Energy Storage Mechanisms of Supercapacitors. Physchem, 4(4), 536-547. https://doi.org/10.3390/physchem4040037








