A Central Role for Troponin C Amino-Terminal α-Helix in Vertebrate Thin Filament Ca2+-Activation
Abstract
1. Introduction
1.1. Troponin C Is Essential for the Ca2+-Regulation of Striated Muscle Contraction
1.2. TnC Function as a Ca2+-Sensor Is Intimately Related to Its Structure
1.3. TnC EF-Hand Affinity and Selectivity Determines Function
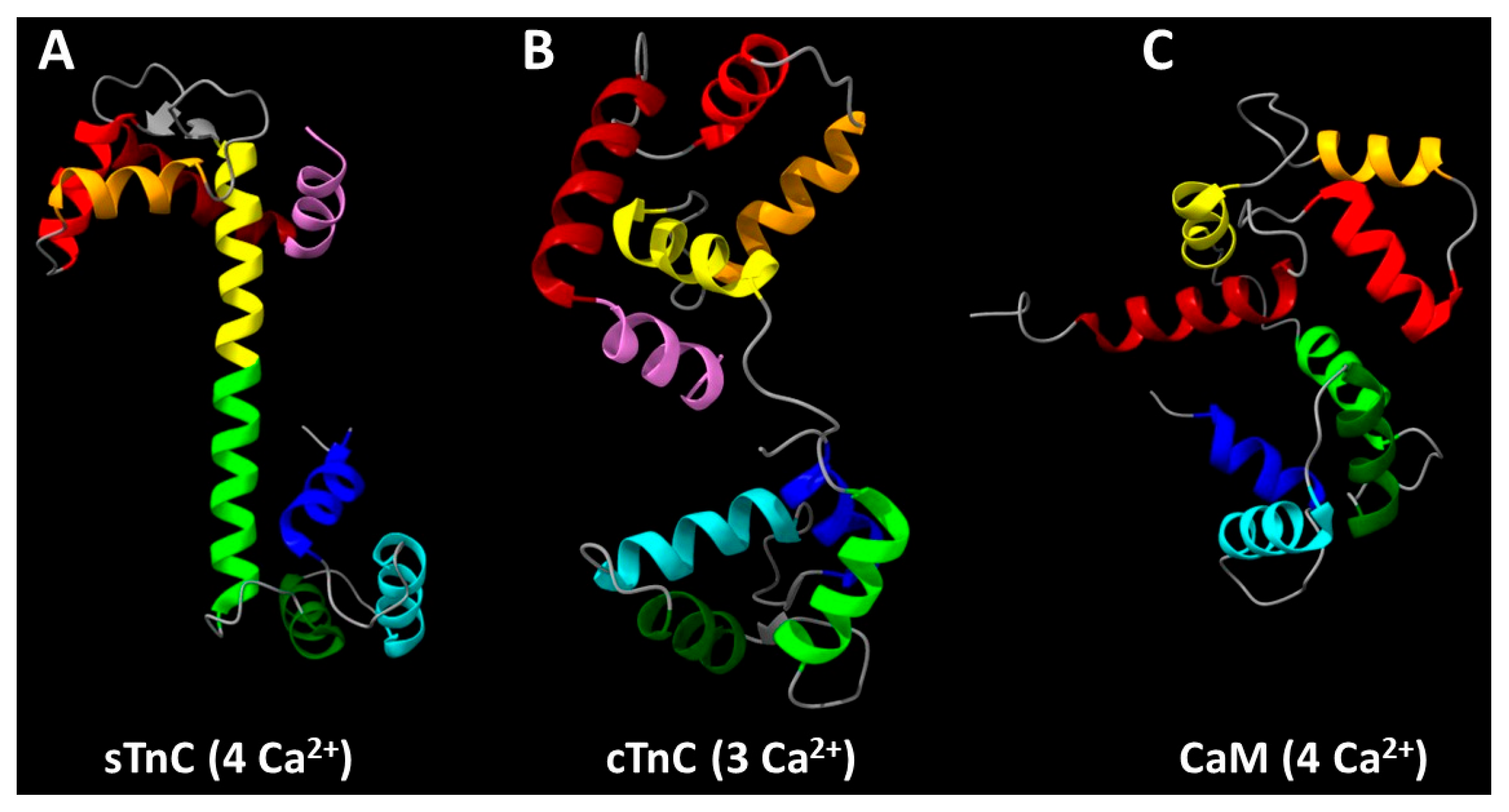
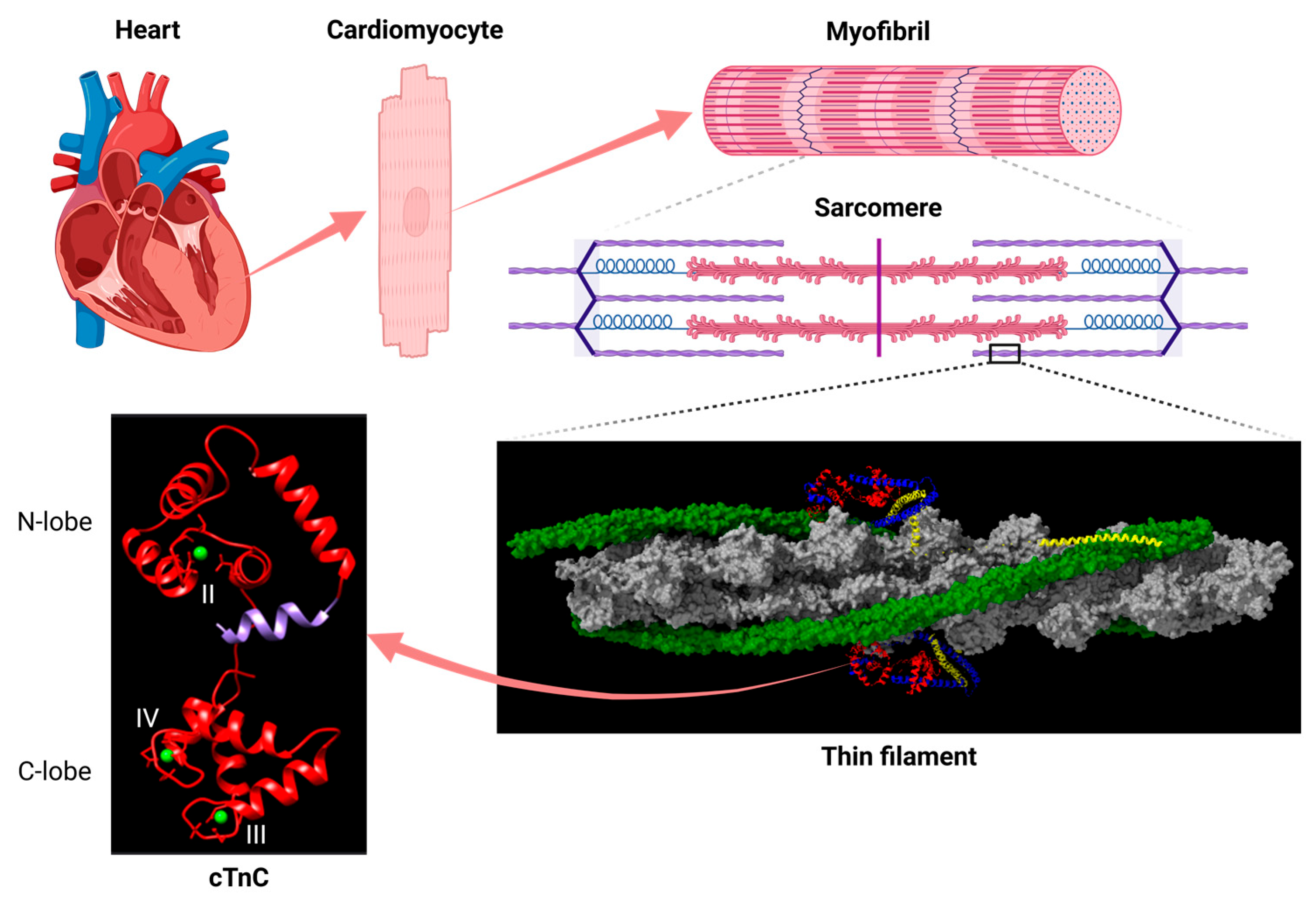
2. Structural and Functional Studies Suggest a Critical Role for the N-Helix of Cardiac Troponin C in Ca2+-Regulation of Cardiac Muscle
2.1. Evolutionary Significance of TnC N-Helix
2.2. Structural Evidence for the Functional Significance of the TnC N-Helix
2.3. Biophysical Studies Demonstrate a Critical Role for the N-Helix of sTnC in Normal Ca2+-Regulation of Skeletal Muscle, but Evidence for That of cTnC Is Lacking
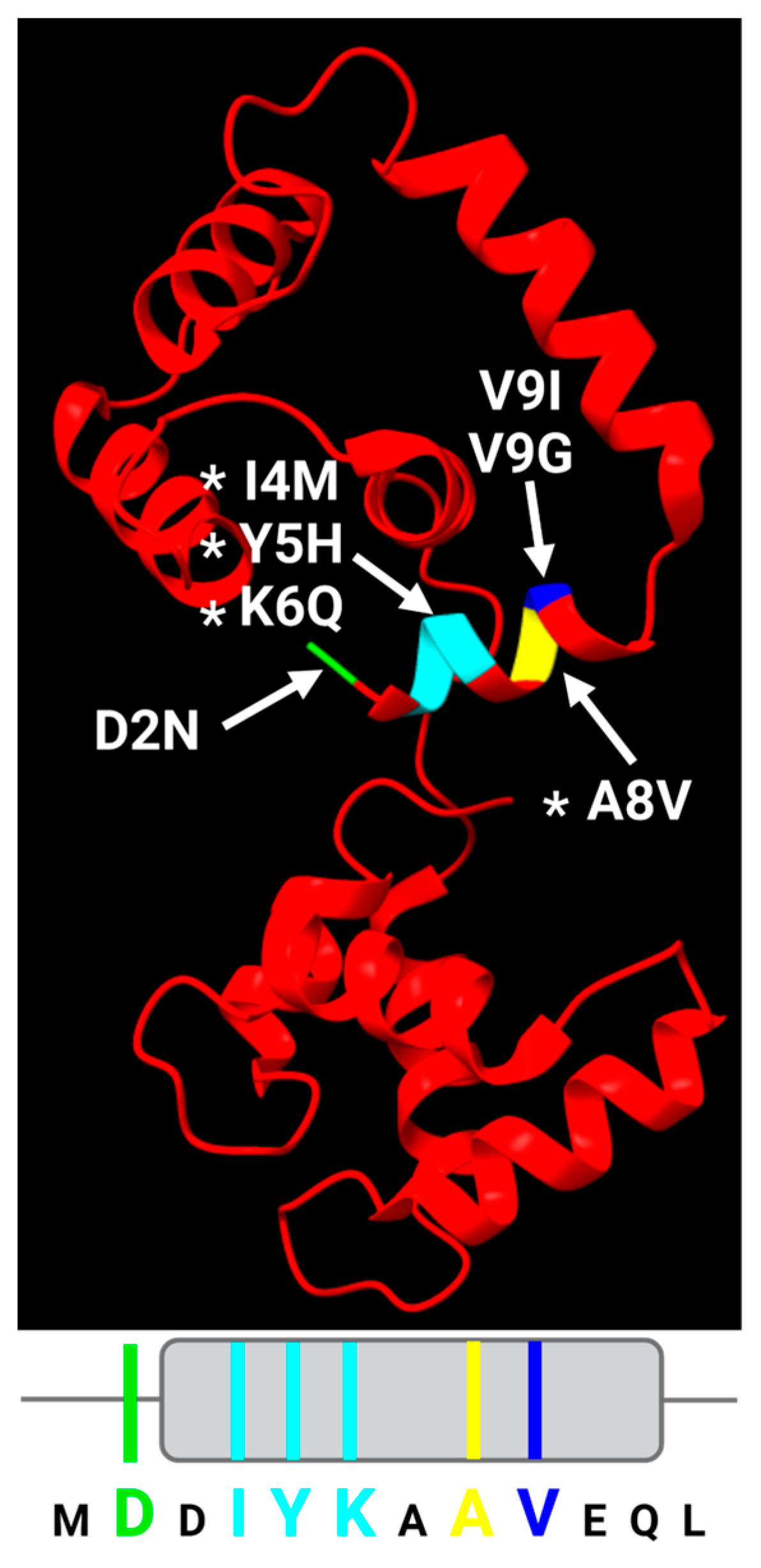
2.4. Pathophysiological Evidence for the Significance of the TnC N-Helix: Variants in the N-Helix of Troponin C Are Associated with Human Cardiomyopathies
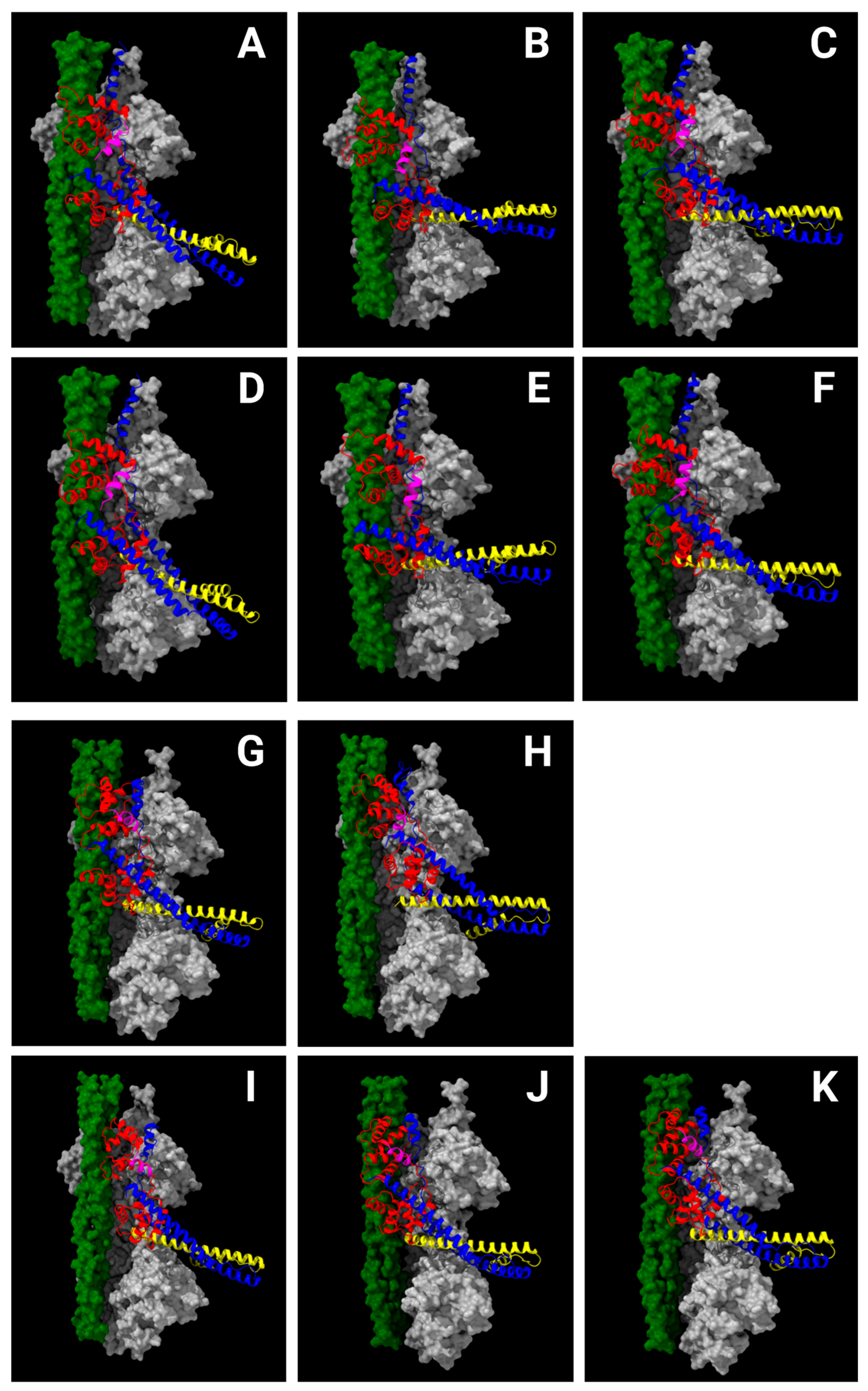
2.5. Cardiomyopathy Variants May Alter Communication Between the cTnC N-Helix and Other Parts of Troponin
3. Conclusions
4. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef] [PubMed]
- Rall, J.A. Discovery of the regulatory role of calcium ion in muscle contraction and relaxation: Setsuro Ebashi and the international emergence of Japanese muscle research. Adv. Physiol. Educ. 2022, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Brunello, E.; Fusi, L. Regulating Striated Muscle Contraction: Through Thick and Thin. Annu. Rev. Physiol. 2024, 86, 255–275. [Google Scholar] [CrossRef]
- Ebashi, S.; Endo, M. Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 1968, 18, 123–183. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.D. The content of troponin, tropomyosin, actin, and myosin in rabbit skeletal muscle myofibrils. Arch. Biochem. Biophys. 1974, 162, 436–441. [Google Scholar] [CrossRef]
- Parmacek, M.S.; Leiden, J.M. Structure, function, and regulation of troponin C. Circulation 1991, 84, 991–1003. [Google Scholar] [CrossRef]
- Solaro, R.J.; Rarick, H.M. Troponin and tropomyosin: Proteins that switch on and tune in the activity of cardiac myofilaments. Circ. Res. 1998, 83, 471–480. [Google Scholar] [CrossRef]
- Gomes, A.V.; Potter, J.D.; Szczesna-Cordary, D. The role of troponins in muscle contraction. IUBMB Life 2002, 54, 323–333. [Google Scholar] [CrossRef]
- Li, M.X.; Hwang, P.M. Structure and function of cardiac troponin C (TNNC1): Implications for heart failure, cardiomyopathies, and troponin modulating drugs. Gene 2015, 571, 153–166. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Cao, T.; Thongam, U.; Jin, J.-P. Invertebrate troponin: Insights into the evolution and regulation of striated muscle contraction. Arch. Biochem. Biophys. 2019, 666, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Joyce, W.; Ripley, D.M.; Gillis, T.; Black, A.C.; Shiels, H.A.; Hoffmann, F.G. A revised perspective on the evolution of troponin I and troponin T gene families in vertebrates. Genome Biol. Evol. 2023, 15, evac173. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; Solís, C. Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage. J. Muscle Res. Cell Motil. 2021, 42, 367–380. [Google Scholar] [CrossRef] [PubMed]
- McKillop, D.F.A.; Geeves, M.A. Regulation of the interaction between actin and myosin subfragment 1: Evidence for three states of the thin filament. Biophys. J. 1993, 65, 693–701. [Google Scholar] [CrossRef]
- Araujo, A.; Walker, J.W. Phosphate release and force generation in cardiac myocytes investigated with caged phosphate and caged calcium. Biophys. J. 1996, 70, 2316–2326. [Google Scholar] [CrossRef]
- Sun, Y.-B.; Brandmeier, B.; Irving, M. Structural changes in troponin in response to Ca2+ and myosin binding to thin filaments during activation of skeletal muscle. Proc. Natl. Acad. Sci. USA 2006, 103, 17771–17776. [Google Scholar] [CrossRef]
- Sun, Y.-B.; Lou, F.; Irving, M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J. Physiol. 2009, 587, 155–163. [Google Scholar] [CrossRef]
- Fusi, L.; Brunello, E.; Sevrieva, I.R.; Sun, Y.-B.; Irving, M. Structural dynamics of troponin during activation of skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 4626–4631. [Google Scholar] [CrossRef]
- Yamada, Y.; Namba, K.; Fujii, T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 2020, 11, 153. [Google Scholar] [CrossRef]
- Risi, C.M.; Pepper, I.; Belknap, B.; Landim-Vieira, M.; White, H.D.; Dryden, K.; Pinto, J.R.; Chase, P.B.; Galkin, V.E. The Structure of the Native Cardiac Thin Filament at Systolic Ca2+ Levels. Proc. Natl. Acad. Sci. USA 2021, 118, e2024288118. [Google Scholar] [CrossRef]
- Brunello, E.; Marcucci, L.; Irving, M.; Fusi, L. Activation of skeletal muscle is controlled by a dual-filament mechano-sensing mechanism. Proc. Natl. Acad. Sci. USA 2023, 120, e2302837120. [Google Scholar] [CrossRef] [PubMed]
- Risi, C.M.; Belknap, B.; Atherton, J.; Coscarella, I.L.; White, H.D.; Chase, P.B.; Pinto, J.R.; Galkin, V.E. Troponin structural dynamics in the native cardiac thin filament revealed by cryo electron microscopy. J. Mol. Biol. 2024, 436, 168498. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.; Tikunova, S.B.; Janssen, P.M.L. Mechanisms of Muscle Contraction and Relaxation. In Muscle and Exercise Physiology; Zoladz, J.A., Ed.; Academic Press: London, UK, 2019; pp. 39–50. [Google Scholar] [CrossRef]
- Kreutziger, K.L.; Gillis, T.E.; Davis, J.P.; Tikunova, S.B.; Regnier, M. Influence of enhanced troponin C Ca2+-binding affinity on cooperative thin filament activation in rabbit skeletal muscle. J. Physiol. 2007, 583, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Tobacman, L.S. Troponin Revealed: Uncovering the Structure of the Thin Filament On-Off Switch in Striated Muscle. Biophys. J. 2021, 120, 1–9. [Google Scholar] [CrossRef]
- Davis, J.P.; Tikunova, S.B. Ca2+ exchange with troponin C and cardiac muscle dynamics. Cardiovasc. Res. 2008, 77, 619–626. [Google Scholar] [CrossRef]
- Eisner, D.; Neher, E.; Taschenberger, H.; Smith, G. Physiology of intracellular calcium buffering. Physiol. Rev. 2023, 103, 2767–2845. [Google Scholar] [CrossRef]
- Bers, D.M. Excitation-Contraction Coupling and Cardiac Contractile Force, 2nd ed.; Kluwer Academic Press: Dordrecht, The Netherlands, 2001; 427p. [Google Scholar]
- Smith, G.L.; Eisner, D.A. Calcium Buffering in the Heart in Health and Disease. Circulation 2019, 139, 2358–2371. [Google Scholar] [CrossRef]
- Fakuade, F.E.; Hubricht, D.; Möller, V.; Sobitov, I.; Liutkute, A.; Döring, Y.; Seibertz, F.; Gerloff, M.; Pronto, J.R.D.; Haghighi, F.; et al. Impaired Intracellular Calcium Buffering Contributes to the Arrhythmogenic Substrate in Atrial Myocytes From Patients With Atrial Fibrillation. Circulation 2024, 150, 544–559. [Google Scholar] [CrossRef]
- Fine, R.; Lehman, W.; Head, J.; Blitz, A. Troponin C in brain. Nature 1975, 258, 260–267. [Google Scholar] [CrossRef]
- Reddy, K.K.; Oitomen, F.M.; Patel, G.P.; Bag, J. Perinuclear localization of slow troponin C m RNA in muscle cells is controlled by a cis-element located at its 3′ untranslated region. RNA 2005, 11, 294–307. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Alkass, K.; Druid, H.; Bernard, S.; Frisén, J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp. Cell Res. 2011, 317, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Asumda, F.Z.; Chase, P.B. Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation 2012, 83, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chase, P.B.; Szczypinski, M.P.; Soto, E.P. Nuclear tropomyosin and troponin in striated muscle: New roles in a new locale? J. Muscle Res. Cell Motil. 2013, 34, 275–284. [Google Scholar] [CrossRef]
- Zhang, T.; Taylor, J.; Jiang, Y.; Pereyra, A.S.; Messi, M.L.; Wang, Z.-M.; Hereñú, C.; Delbono, O. Troponin T3 regulates nuclear localization of the calcium channel Cavb1a subunit in skeletal muscle. Exp. Cell Res. 2015, 336, 276–286. [Google Scholar] [CrossRef][Green Version]
- Nunez Lopez, Y.O.; Messi, M.L.; Pratley, R.E.; Zhang, T.; Delbono, O. Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs. Exp. Gerontol. 2018, 108, 35–40. [Google Scholar] [CrossRef]
- Johnston, J.R.; Chase, P.B.; Pinto, J.R. Troponin through the looking-glass: Emerging roles beyond regulation of striated muscle contraction. Oncotarget 2018, 9, 1461–1482. [Google Scholar] [CrossRef]
- Kharitonov, A.V.; Shubina, M.Y.; Nosov, G.A.; Mamontova, A.V.; Arifulin, E.A.; Lisitsyna, O.M.; Nalobin, D.S.; Musinova, Y.R.; Sheval, E.V. Switching of cardiac troponin I between nuclear and cytoplasmic localization during muscle differentiation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118601. [Google Scholar] [CrossRef]
- Solís, C.; Solaro, R.J. Novel insights into sarcomere regulatory systems control of cardiac thin filament activation. J. Gen. Physiol. 2021, 153. [Google Scholar] [CrossRef]
- Zhang, T.; Birbrair, A.; Delbono, O. Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis. Cytoskeleton 2013, 70, 134–147. [Google Scholar] [CrossRef]
- Elezaby, A.; Lin, A.J.; Vijayan, V.; Pokhrel, S.; Kraemer, B.R.; Bechara, L.R.G.; Larus, I.; Sun, J.; Baena, V.; Syed, Z.A.; et al. Cardiac troponin I directly binds and inhibits mitochondrial ATP synthase with a noncanonical role in the post-ischemic heart. Nat. Cardiovasc. Res. 2024, 3, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lee, J.; Vincent, L.G.; Wang, Q.; Gu, M.; Lan, F.; Churko, J.M.; Sallam, K.I.; Matsa, E.; Sharma, A.; et al. Epigenetic Regulation of Phosphodiesterases 2A and 3A Underlies Compromised β-Adrenergic Signaling in an iPSC Model of Dilated Cardiomyopathy. Cell Stem Cell 2015, 17, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Schreier, T.; Kedes, L.; Gahlmann, R. Cloning, structural analysis, and expression of the human slow twitch skeletal muscle/cardiac troponin C gene. J. Biol. Chem. 1990, 265, 21247–21253. [Google Scholar] [CrossRef]
- Gahlmann, R.; Kedes, L. Cloning, structural analysis, and expression of the human fast twitch skeletal muscle troponin C gene. J. Biol. Chem. 1990, 265, 12520–12528. [Google Scholar] [CrossRef] [PubMed]
- Gahlmann, R.; Wade, R.; Gunning, P.; Kedes, L. Differential expression of slow and fast skeletal muscle troponin C. J. Mol. Biol. 1988, 201, 379–391. [Google Scholar] [CrossRef]
- Collins, J.H.; Greaser, M.L.; Potter, J.D.; Horn, M.J. Determination of the amino acid sequence of troponin C from rabbit skeletal muscle. J. Biol. Chem. 1977, 252, 6356–6362. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takagi, T.; Konishi, K.; Morimoto, S.; Ohtsuki, I. Amino acid sequence of porcine cardiac muscle troponin C. J. Biochem. 1989, 106, 55–59. [Google Scholar] [CrossRef]
- Parmacek, M.S.; Leiden, J.M. Structure and expression of the murine slow/cardiac troponin C gene. J. Biol. Chem. 1989, 264, 13217–13225. [Google Scholar] [CrossRef]
- Ding, X.-L.; Akella, Á.B.; Su, H.; Gulati, J. The role of glycine (residue 89) in the central helix of EF-hand protein troponin-C exposed following amino-terminal α-helix deletion. Protein Sci. 1994, 3, 2089–2096. [Google Scholar] [CrossRef]
- Smith, L.; Greenfield, N.J.; Hitchcock-DeGregori, S.E. Mutations in the N- and D-helices of the N-domain of troponin C affect the C-domain and regulatory function. Biophys. J. 1999, 76, 400–408. [Google Scholar] [CrossRef]
- Chandra, M.; da Silva, E.F.; Sorenson, M.M.; Ferro, J.A.; Pearlstone, J.R.; Nash, B.E.; Borgford, T.; Kay, C.M.; Smillie, L.B. The effects of N helix deletion and mutant F29W on the Ca2+ binding and functional properties of chicken skeletal muscle troponin C. J. Biol. Chem. 1994, 269, 14988–14994. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Greenfield, N.J.; Hitchcock-DeGregori, S.E. The effects of deletion of the amino-terminal helix on troponin C function and stability. J. Biol. Chem. 1994, 269, 9857–9863. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, O.; James, M.N.G. Structure of the calcium regulatory muscle protein troponin-C at 2.8 Å resolution. Nature 1985, 313, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, O.; James, M.N.G. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 Å resolution. J. Mol. Biol. 1988, 203, 761–779. [Google Scholar] [CrossRef]
- Satyshur, K.A.; Rao, S.T.; Pyzalska, D.; Drendel, W.; Greaser, M.; Sundaralingam, M. Refined structure of chicken muscle troponin C in the two-calcium state at 2-Å resolution. J. Biol. Chem. 1988, 263, 1628–1647. [Google Scholar] [CrossRef]
- Putkey, J.A.; Liu, W.; Sweeney, H.L. Function of the N-terminal calcium-binding sites in cardiac/slow troponin C assessed in fast skeletal muscle fibers. J. Biol. Chem. 1991, 266, 14881–14884. [Google Scholar] [CrossRef]
- Slupsky, C.M.; Sykes, B.D. NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry 1995, 34, 15953–15964. [Google Scholar] [CrossRef]
- Houdusse, A.; Love, M.L.; Dominguez, R.; Grabarek, Z.; Cohen, C. Structures of four Ca2+-bound troponin C at 2.0 Å resolution: Further insights into the Ca2+-switch in the calmodulin superfamily. Structure 1997, 5, 1695–1711. [Google Scholar] [CrossRef]
- Sia, S.K.; Li, M.X.; Spyracopoulos, L.; Gagné, S.M.; Liu, W.; Putkey, J.A.; Sykes, B.D. Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. J. Biol. Chem. 1997, 272, 18216–18221. [Google Scholar] [CrossRef]
- Takeda, S.; Yamashita, A.; Maeda, K.; Maéda, Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature 2003, 424, 35–41. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G. Relaxation, [Ca2+]i and [Mg2+]i during prolonged tetanic stimulation of intact, single fibres from mouse skeletal muscle. J. Physiol. 1994, 480, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, M.W.; Brinkmeier, H.; Müntener, M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef] [PubMed]
- Kushmerick, M.J.; Dillon, P.F.; Meyer, R.A.; Brown, T.R.; Krisanda, J.M.; Sweeney, H.L. 31P NMR spectroscopy, chemical analysis, and free Mg2+ of rabbit bladder and uterine smooth muscle. J. Biol. Chem. 1986, 261, 14420–14429. [Google Scholar] [CrossRef]
- Godt, R.E.; Maughan, D.W. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am. J. Physiol. 1988, 254, C591–C604. [Google Scholar] [CrossRef]
- Homsher, E.; Kean, C.J. Skeletal muscle energetics and metabolism. Annu. Rev. Physiol. 1978, 40, 93–131. [Google Scholar] [CrossRef]
- Hou, T.-T.; Johnson, J.D.; Rall, J.A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J. Physiol. 1991, 441, 285–304. [Google Scholar] [CrossRef]
- Vinogradova, M.V.; Stone, D.B.; Malanina, G.G.; Karatzaferi, C.; Cooke, R.; Mendelson, R.A.; Fletterick, R.J. Ca2+-regulated structural changes in troponin. Proc. Natl. Acad. Sci. USA 2005, 102, 5038–5043. [Google Scholar] [CrossRef]
- Ikura, M.; Clore, G.M.; Gronenborn, A.M.; Zhu, G.; Klee, C.B.; Bax, A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science 1992, 256, 632–638. [Google Scholar] [CrossRef]
- Potter, J.D.; Gergely, J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J. Biol. Chem. 1975, 250, 4628–4633. [Google Scholar] [CrossRef]
- Negele, J.C.; Dotson, D.G.; Liu, W.; Sweeney, H.L.; Putkey, J.A. Mutation of the high affinity calcium binding sites in cardiac troponin C. J. Biol. Chem. 1992, 267, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.A.; Pinto, J.R.; Davidson, M.W.; Chase, P.B. Fluorescent protein-based Ca2+ sensor reveals global, divalent cation-dependent conformational changes in cardiac troponin C. PLoS ONE 2016, 11, e0164222. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.; Rall, J.A.; Reiser, P.J.; Smillie, L.B.; Tikunova, S.B. Engineering competitive magnesium binding into the first EF-hand of skeletal troponin C. J. Biol. Chem. 2002, 277, 49716–49726. [Google Scholar] [CrossRef]
- Skowronsky, R.A.; Schroeter, M.; Baxley, T.; Li, Y.; Chalovich, J.M.; Spuches, A.M. Thermodynamics and molecular dynamics simulations of calcium binding to the regulatory site of human cardiac troponin C: Evidence for communication with the structural calcium binding sites. J. Biol. Inorg. Chem. 2013, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rayani, K.; Seffernick, J.; Li, A.Y.; Davis, J.P.; Spuches, A.M.; Van Petegem, F.; Solaro, R.J.; Lindert, S.; Tibbits, G.F. Binding of calcium and magnesium to human cardiac troponin C. J. Biol. Chem. 2021, 296, 100350. [Google Scholar] [CrossRef]
- van Eerd, J.-P.; Takahashi, K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry 1976, 15, 1171–1180. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Brito, R.M.M.; Rosevear, P.R.; Putkey, J.A. The low-affinity Ca2+-binding sites in cardiac/slow skeletal muscle troponin C perform distinct functions: Site I alone cannot trigger contraction. Proc. Natl. Acad. Sci. USA 1990, 87, 9538–9542. [Google Scholar] [CrossRef]
- Marques, M.d.A.; Pinto, J.R.; Moraes, A.H.; Iqbal, A.; de Magalhães, M.T.Q.; Monteiro, J.; Pedrote, M.M.; Sorenson, M.M.; Silva, J.L.; de Oliveira, G.A.P. Allosteric Transmission along a Loosely Structured Backbone Allows a Cardiac Troponin C Mutant to Function with Only One Ca2+ Ion. J. Biol. Chem. 2017, 292, 2379–2394. [Google Scholar] [CrossRef]
- Collins, J.H. Myosin light chains and troponin C: Structural and evolutionary relationships revealed by amino acid sequence comparisons. J. Muscle Res. Cell Motil. 1991, 12, 3–25. [Google Scholar] [CrossRef]
- Houdusse, A.; Silver, M.; Cohen, C. A model of Ca2+-free calmodulin binding to unconventional myosins reveals how calmodulin acts as a regulatory switch. Structure 1996, 4, 1475–1490. [Google Scholar] [CrossRef]
- Gillis, T.E.; Marshall, C.R.; Tibbits, G.F. Functional and evolutionary relationships of troponin C. Physiol. Genom. 2007, 32, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Bethea, J.P.; Hetzel-Ebben, H.L.; Landim-Vieira, M.; Mayper, R.J.; Williams, R.L.; Kessler, L.E.; Ruiz, A.M.; Gargiulo, K.; Rose, J.S.M.; et al. Mandibular muscle troponin of the Florida carpenter ant Camponotus floridanus: Extending our insights into invertebrate Ca2+ regulation. J. Muscle Res. Cell Motil. 2021, 42, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Agianian, B.; Kržič, U.; Qiu, F.; Linke, W.A.; Leonard, K.; Bullard, B. A troponin switch that regulates muscle contraction by stretch instead of calcium. EMBO J. 2004, 23, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Lakey, A.; Agianian, B.; Hutchings, A.; Butcher, G.W.; Labeit, S.; Leonard, K.; Bullard, B. Troponin C in different insect muscle types: Identification of two isoforms in Lethocerus, Drosophila and Anopheles that are specific to asynchronous flight muscle in the adult insect. Biochem. J. 2003, 371, 811–821. [Google Scholar] [CrossRef]
- Collins, J.H.; Theibert, J.L.; Francois, J.-M.; Ashley, C.C.; Potter, J.D. Amino acid sequences and Ca2+-binding properties of two isoforms of barnacle troponin C. Biochemistry 1991, 30, 702–707. [Google Scholar] [CrossRef]
- van Eerd, J.-P.; Takahashi, K. The amino acid sequence of bovine cardiac troponin-C. Comparison with rabbit skeletal troponin-C. Biochem. Biophys. Res. Commun. 1975, 64, 122–127. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Troponin C from rabbit slow skeletal and cardiac muscle is the product of a single gene. Eur. J. Biochem. 1980, 103, 179–188. [Google Scholar] [CrossRef]
- Roher, A.; Leiska, N.; Spitz, W. The amino acid sequence of human cardiac troponin-C. Muscle Nerve 1986, 9, 73–77. [Google Scholar] [CrossRef]
- Wilkinson, J.M. The amino acid sequence of troponin C from chicken skeletal muscle. FEBS Lett. 1976, 70, 254–256. [Google Scholar] [CrossRef]
- Romero-Herrera, A.E.; Castillo, O.; Lehmann, H. Human skeletal muscle proteins. The primary structure of troponin C. J. Mol. Evol. 1976, 8, 251–270. [Google Scholar] [CrossRef]
- Strasburg, G.M.; Greaser, M.L.; Sundaralingam, M. X-ray diffraction studies of troponin-C crystals from rabbit and chicken skeletal muscles. J. Biol. Chem. 1980, 255, 3806–3808. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.T.; Tripet, B.P.; Hodges, R.S.; Sykes, B.D. Interaction of the second binding region of troponin I with the regulatory domain of skeletal muscle troponin C as determined by NMR spectroscopy. J. Biol. Chem. 1997, 272, 28494–28500. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.T.; Pearlstone, J.R.; Corson, D.C.; Gagné, S.M.; Smillie, L.B.; Sykes, B.D. Structure and interaction site of the regulatory domain of troponin-C when complexed with the 96-148 region of troponin-I. Biochemistry 1998, 37, 12419–12430. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.T.; Tripet, B.P.; Pearlstone, J.R.; Smillie, L.B.; Sykes, B.D. Defining the region of troponin-I that binds to troponin-C. Biochemistry 1999, 38, 5478–5489. [Google Scholar] [CrossRef]
- Li, M.X.; Spyracopoulos, L.; Sykes, B.D. Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry 1999, 38, 8289–8298. [Google Scholar] [CrossRef]
- Vassylyev, D.G.; Takeda, S.; Wakatsuki, S.; Maeda, K.; Maéda, Y. Crystal structure of troponin C in complex with troponin I fragment at 2.3-Å resolution. Proc. Natl. Acad. Sci. USA 1998, 95, 4847–4852. [Google Scholar] [CrossRef]
- Saijo, Y.; Takeda, S.; Scherer, A.; Kobayashi, T.; Maeda, Y.; Taniguchi, H.; Yao, M.; Wakatsuki, S. Production, crystallization, and preliminary X-ray analysis of rabbit skeletal muscle troponin complex consisting of troponin C and fragment (1-47) of troponin I. Protein Sci. 1997, 6, 916–918. [Google Scholar] [CrossRef]
- Findlay, W.A.; Sykes, B.D. 1H-NMR resonance assignments, secondary structure, and global fold of the TR1C fragment of turkey skeletal troponin C in the calcium-free state. Biochemistry 1993, 32, 3461–3467. [Google Scholar] [CrossRef]
- Li, M.X.; Gagné, S.M.; Tsuda, S.; Kay, C.M.; Smillie, L.B.; Sykes, B.D. Calcium binding to the regulatory N-domain of skeletal muscle troponin C occurs in a stepwise manner. Biochemistry 1995, 34, 8330–8340. [Google Scholar] [CrossRef]
- Spyracopoulos, L.; Li, M.X.; Sia, S.K.; Gagné, S.M.; Chandra, M.; Solaro, R.J.; Sykes, B.D. Calcium-induced structural transition in the regulatory domain of human cardiac troponin C. Biochemistry 1997, 36, 12138–12146. [Google Scholar] [CrossRef]
- Findlay, W.A.; Sönnichsen, F.D.; Sykes, B.D. Solution structure of the TR1C fragment of skeletal muscle troponin-C. J. Biol. Chem. 1994, 269, 6773–6778. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yanagisawa, H.; Wakabayashi, T. Cryo-EM structures of cardiac thin filaments reveal the 3D architecture of troponin. J. Struct. Biol. 2020, 209, 107450. [Google Scholar] [CrossRef] [PubMed]
- Risi, C.M.; Belknap, B.; White, H.D.; Dryden, K.; Pinto, J.R.; Chase, P.B.; Galkin, V.E. High-resolution cryo-EM structure of the junction region of the native cardiac thin filament in relaxed state. PNAS Nexus 2023, 2, pgac298. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Rivera, A.J.; Chase, P.B.; Smillie, L.B.; Sorenson, M.M. Regulation of skeletal muscle tension redevelopment by troponin C constructs with different Ca2+ affinities. Biophys. J. 1999, 76, 2664–2672. [Google Scholar] [CrossRef]
- Gulati, J.; Babu, A.; Su, H.; Zhang, Y.F. Identification of the regions conferring calmodulin-like properties to troponin C. J. Biol. Chem. 1993, 268, 11685–11690. [Google Scholar] [CrossRef]
- Pearlstone, J.R.; Borgford, T.; Chandra, M.; Oikawa, K.; Kay, C.M.; Herzberg, O.; Moult, J.; Herklotz, A.; Reinach, F.C.; Smillie, L.B. Construction and characterization of a spectral probe mutant of troponin C: Application to analyses of mutants with increased Ca2+ affinity. Biochemistry 1992, 31, 6545–6553. [Google Scholar] [CrossRef]
- da Silva, E.F.; Sorenson, M.M.; Smillie, L.B.; Barrabin, H.; Scofano, H.M. Comparison of calmodulin and troponin C with and without its amino-terminal helix (residues 1-11) in the activation of erythrocyte Ca2+-ATPase. J. Biol. Chem. 1993, 268, 26220–26225. [Google Scholar] [CrossRef]
- Babu, A.; Orr, G.; Gulati, J. Calmodulin supports the force-generating function in desensitized muscle fibers. J. Biol. Chem. 1988, 263, 15485–15491. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Reinoso, T.R.; Landim-Vieira, M.; Shi, Y.; Johnston, J.R.; Chase, P.B.; Parvatiyar, M.S.; Landstrom, A.P.; Pinto, J.R.; Tadros, H.J. A Comprehensive Guide to Genetic Variants and Post-Translational Modifications of Cardiac Troponin C. J. Muscle Res. Cell Motil. 2021, 42, 323–342. [Google Scholar] [CrossRef]
- Willott, R.H.; Gomes, A.V.; Chang, A.N.; Parvatiyar, M.S.; Pinto, J.R.; Potter, J.D. Mutations in Troponin that cause HCM, DCM AND RCM: What can we learn about thin filament function? J. Mol. Cell. Cardiol. 2010, 48, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Tadros, H.J.; Life, C.S.; Garcia, G.; Pirozzi, E.; Jones, E.G.; Datta, S.; Parvatiyar, M.S.; Chase, P.B.; Allen, H.D.; Kim, J.J.; et al. Meta-analysis of cardiomyopathy-associated variants in troponin genes identifies loci and intragenic hot spots that are associated with worse clinical outcomes. J. Mol. Cell. Cardiol. 2020, 142, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Na, I.; Kong, M.J.; Straight, S.; Pinto, J.R.; Uversky, V.N. Troponins, intrinsic disorder, and cardiomyopathy. Biol. Chem. 2016, 397, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, A.P.; Parvatiyar, M.S.; Pinto, J.R.; Marquardt, M.L.; Bos, J.M.; Tester, D.J.; Ommen, S.R.; Potter, J.D.; Ackerman, M.J. Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J. Mol. Cell. Cardiol. 2008, 45, 281–288. [Google Scholar] [CrossRef]
- Pinto, J.R.; Parvatiyar, M.S.; Jones, M.A.; Liang, J.; Ackerman, M.J.; Potter, J.D. A functional and structural study of troponin C mutations related to hypertrophic cardiomyopathy. J. Biol. Chem. 2009, 284, 19090–19100. [Google Scholar] [CrossRef]
- Swindle, N.; Tikunova, S.B. Hypertrophic cardiomyopathy-linked mutation D145E drastically alters calcium binding by the C-domain of cardiac troponin C. Biochemistry 2010, 49, 4813–4820. [Google Scholar] [CrossRef]
- Pinto, J.R.; Reynaldo, D.P.; Parvatiyar, M.S.; Dweck, D.; Liang, J.; Jones, M.A.; Sorenson, M.M.; Potter, J.D. Strong cross-bridges potentiate the Ca2+ affinity changes produced by hypertrophic cardiomyopathy cardiac troponin C mutants in myofilaments: A fast kinetic approach. J. Biol. Chem. 2011, 286, 1005–1013. [Google Scholar] [CrossRef]
- Albury, A.N.J.; Swindle, N.; Swartz, D.R.; Tikunova, S.B. Effect of hypertrophic cardiomyopathy-linked troponin C mutations on the response of reconstituted thin filaments to calcium upon troponin I phosphorylation. Biochemistry 2012, 51, 3614–3621. [Google Scholar] [CrossRef]
- Martins, A.S.; Parvatiyar, M.S.; Feng, H.-Z.; Bos, J.M.; Gonzalez-Martinez, D.; Vukmirovic, M.; Turna, R.S.; Sanchez-Gonzalez, M.A.; Badger, C.-D.; Zorio, D.A.R.; et al. In Vivo Analysis of Troponin C Knock-In (A8V) Mice: Evidence that TNNC1 is a Hypertrophic Cardiomyopathy Susceptibility Gene. Circ. Cardiovasc. Genet. 2015, 8, 653–664. [Google Scholar] [CrossRef]
- Kathuria, S.V.; Chan, Y.H.; Nobrega, R.P.; Özen, A.; Matthews, C.R. Clusters of isoleucine, leucine, and valine side chains define cores of stability in high-energy states of globular proteins: Sequence determinants of structure and stability. Protein Sci. 2016, 25, 662–675. [Google Scholar] [CrossRef]
- Zot, H.G.; Hasbun, J.E.; Michell, C.A.; Landim-Vieira, M.; Pinto, J.R. Enhanced troponin I binding explains the functional changes produced by the hypertrophic cardiomyopathy mutation A8V of cardiac troponin C. Arch. Biochem. Biophys. 2016, 601, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Baxley, T.; Johnson, D.; Pinto, J.R.; Chalovich, J.M. Troponin C Mutations Partially Stabilize the Active State of Regulated Actin and Fully Stabilize the Active State When Paired with Δ14 TnT. Biochemistry 2017, 56, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Johnston, J.R.; Karam, T.; Wang, L.; Singh, R.K.; Pinto, J.R. Myosin Rod Hypophosphorylation and CB Kinetics in Papillary Muscles from a TnC-A8V KI Mouse Model. Biophys. J. 2017, 112, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.M.; Rayani, K.; Singh, G.; Lotfalisalmasi, B.; Tieleman, D.P.; Tibbits, G.F. Changes in the dynamics of the cardiac troponin C molecule explain the effects of Ca2+-sensitizing mutations. J. Biol. Chem. 2017, 292, 11915–11926. [Google Scholar] [CrossRef]
- Veltri, T.; Landim-Vieira, M.; Parvatiyar, M.S.; Gonzalez-Martinez, D.; Dieseldorff Jones, K.M.; Michell, C.A.; Dweck, D.; Landstrom, A.P.; Chase, P.B.; Pinto, J.R. Hypertrophic cardiomyopathy cardiac troponin C mutations differentially affect slow skeletal and cardiac muscle regulation. Front. Physiol. 2017, 8, 221. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, D.; Johnston, J.R.; Landim-Vieira, M.; Ma, W.; Antipova, O.; Awan, O.; Irving, T.C.; Chase, P.B.; Pinto, J.R. Structural and functional impact of troponin C-mediated Ca2+ sensitization on myofilament lattice spacing and cross-bridge mechanics in mouse cardiac muscle. J. Mol. Cell. Cardiol. 2018, 123, 26–37. [Google Scholar] [CrossRef]
- Johnston, J.R.; Landim-Vieira, M.; Marques, M.A.; de Oliveira, G.A.P.; Gonzalez-Martinez, D.; Moraes, A.H.; He, H.; Iqbal, A.; Wilnai, Y.; Birk, E.; et al. The intrinsically disordered C terminus of troponin T binds to troponin C to modulate myocardial force generation. J. Biol. Chem. 2019, 294, 20054–20069. [Google Scholar] [CrossRef]
- Dieseldorff Jones, K.M.; Vied, C.; Valera, I.C.; Chase, P.B.; Parvatiyar, M.S.; Pinto, J.R. Sexual dimorphism in cardiac transcriptome associated with a troponin C murine model of hypertrophic cardiomyopathy. Physiol. Rep. 2020, 8, e14396. [Google Scholar] [CrossRef]
- de Feria, A.E.; Kott, A.E.; Becker, J.R. Sarcomere mutation negative hypertrophic cardiomyopathy is associated with ageing and obesity. Open Heart 2021, 8, e001560. [Google Scholar] [CrossRef]
- Pinto, J.R.; Siegfried, J.D.; Parvatiyar, M.S.; Li, D.; Norton, N.; Jones, M.A.; Liang, J.; Potter, J.D.; Hershberger, R.E. Functional characterization of TNNC1 rare variants identified in dilated cardiomyopathy. J. Biol. Chem. 2011, 286, 34404–34412. [Google Scholar] [CrossRef]
- Hespe, S.; Waddell, A.; Asatryan, B.; Owens, E.; Thaxton, C.; Adduru, M.-L.; Anderson, K.; Brown, E.E.; Hoffman-Andrews, L.; Jordan, E.; et al. Genes Associated with Hypertrophic Cardiomyopathy: A Reappraisal by the ClinGen Hereditary Cardiovascular Disease Gene Curation Expert Panel. J. Am. Coll. Cardiol. 2025, 85, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Juárez, C.K.; Sequeira, V.; van den Boogaard, M.; Veerman, C.C.; Hoetjes, N.J.; Poel, E.; Tanck, M.W.T.; Lekanne Deprez, R.H.; Vermeer, A.M.C.; van der Velden, J.; et al. Tropomyosin-troponin complex in inherited cardiomyopathies. Heart Rhythm 2024, 21, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Tikunova, S.B.; Thuma, J.; Davis, J.P. Mouse Models of Cardiomyopathies Caused by Mutations in Troponin C. Int. J. Mol. Sci. 2023, 24, 12349. [Google Scholar] [CrossRef] [PubMed]
- Keyt, L.K.; Duran, J.M.; Bui, Q.M.; Chen, C.; Miyamoto, M.I.; Silva Enciso, J.; Tardiff, J.C.; Adler, E.D. Thin filament cardiomyopathies: A review of genetics, disease mechanisms, and emerging therapeutics. Front. Cardiovasc. Med. 2022, 9, 972301. [Google Scholar] [CrossRef]
- Hassoun, R.; Budde, H.; Mannherz, H.G.; Lodi, M.; Fujita-Becker, S.; Laser, K.T.; Gartner, A.; Klingel, K.; Mohner, D.; Stehle, R.; et al. De Novo Missense Mutations in TNNC1 and TNNI3 Causing Severe Infantile Cardiomyopathy Affect Myofilament Structure and Function and Are Modulated by Troponin Targeting Agents. Int. J. Mol. Sci. 2021, 22, 9625. [Google Scholar] [CrossRef]
- Tobacman, L.S.; Cammarato, A. Cardiomyopathic troponin mutations predominantly occur at its interface with actin and tropomyosin. J. Gen. Physiol. 2021, 153, e202012815. [Google Scholar] [CrossRef]
- Ploski, R.; Rydzanicz, M.; Ksiazczyk, T.M.; Franaszczyk, M.; Pollak, A.; Kosinska, J.; Michalak, E.; Stawinski, P.; Ziolkowska, L.; Bilinska, Z.T.; et al. Evidence for troponin C (TNNC1) as a gene for autosomal recessive restrictive cardiomyopathy with fatal outcome in infancy. Am. J. Med. Genet. Part A 2016, 170, 3241–3248. [Google Scholar] [CrossRef]
- Patsalis, C.; Kyriakou, S.; Georgiadou, M.; Ioannou, L.; Constantinou, L.; Soteriou, V.; Jossif, A.; Evangelidou, P.; Sismani, C.; Kypri, E.; et al. Investigating TNNC1 gene inheritance and clinical outcomes through a comprehensive familial study. Am. J. Med. Genet. Part A 2025, 197, e63838. [Google Scholar] [CrossRef]
- Veltri, T.; de Oliveira, G.A.P.; Bienkiewicz, E.A.; Palhano, F.L.; Marques, M.A.; Moraes, A.H.; Silva, J.L.; Sorenson, M.M.; Pinto, J.R. Amide hydrogens reveal a temperature-dependent structural transition that enhances site-II Ca2+-binding affinity in a C-domain mutant of cardiac troponin C. Sci. Rep. 2017, 7, 691. [Google Scholar] [CrossRef]
- Marques, M.A.; Landim-Vieira, M.; Moraes, A.H.; Sun, B.; Johnston, J.R.; Dieseldorff Jones, K.M.; Cino, E.A.; Parvatiyar, M.S.; Valera, I.C.; Silva, J.L.; et al. Anomalous structural dynamics of minimally frustrated residues in cardiac troponin C triggers hypertrophic cardiomyopathy. Chem. Sci. 2021, 12, 7308–7323. [Google Scholar] [CrossRef]
- Varughese, J.F.; Baxley, T.; Chalovich, J.M.; Li, Y. A computational and experimental approach to investigate bepridil binding with cardiac troponin. J. Phys. Chem. B 2011, 115, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- van de Locht, M.; Donkervoort, S.; de Winter, J.M.; Conijn, S.; Begthel, L.; Kusters, B.; Mohassel, P.; Hu, Y.; Medne, L.; Quinn, C.; et al. Pathogenic variants in TNNC2 cause congenital myopathy due to an impaired force response to calcium. J. Clin. Investig. 2021, 131, e145700. [Google Scholar] [CrossRef]
- Rodriguez Garcia, M.C.; Schmeckpeper, J.; Landim-Vieira, M.; Coscarella, I.L.; Fang, X.; Ma, W.; Spran, P.A.; Yuan, S.; Qi, L.; Rahimi Kahmini, A.; et al. Disruption of Z-disc function promotes mechanical dysfunction in human myocardium: Evidence for a dual myofilament modulatory role by alpha-actinin 2. Int. J. Mol. Sci. 2023, 24, 14572. [Google Scholar] [CrossRef] [PubMed]
- Landim-Vieira, M.; Childers, M.C.; Wacker, A.L.; Rodriguez Garcia, M.; He, H.; Singh, R.; Brundage, E.A.; Johnston, J.R.; Whitson, B.A.; Chase, P.B.; et al. Post-translational modification patterns on β-myosin heavy chain are altered in ischemic and non-ischemic human hearts. eLife 2022, 11, e74919. [Google Scholar] [CrossRef] [PubMed]
- Sevrieva, I.; Knowles, A.C.; Kampourakis, T.; Sun, Y.-B. Regulatory domain of troponin moves dynamically during activation of cardiac muscle. J. Mol. Cell. Cardiol. 2014, 75, 181–187. [Google Scholar] [CrossRef]
- Sevrieva, I.R.; Kampourakis, T.; Irving, M. Structural changes in troponin during activation of skeletal and heart muscle determined in situ by polarised fluorescence. Biophys. Rev. 2024, 16, 753–772. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Blackwell, L.A.; Schroy, R.K.; Cleland, B.M.; Risi, C.M.; Parvatiyar, M.S.; Pinto, J.R.; Galkin, V.E.; Chase, P.B. A Central Role for Troponin C Amino-Terminal α-Helix in Vertebrate Thin Filament Ca2+-Activation. Physchem 2025, 5, 16. https://doi.org/10.3390/physchem5020016
Shi Y, Blackwell LA, Schroy RK, Cleland BM, Risi CM, Parvatiyar MS, Pinto JR, Galkin VE, Chase PB. A Central Role for Troponin C Amino-Terminal α-Helix in Vertebrate Thin Filament Ca2+-Activation. Physchem. 2025; 5(2):16. https://doi.org/10.3390/physchem5020016
Chicago/Turabian StyleShi, Yun, Lauren A. Blackwell, Ryan K. Schroy, B. Max Cleland, Cristina M. Risi, Michelle S. Parvatiyar, Jose R. Pinto, Vitold E. Galkin, and P. Bryant Chase. 2025. "A Central Role for Troponin C Amino-Terminal α-Helix in Vertebrate Thin Filament Ca2+-Activation" Physchem 5, no. 2: 16. https://doi.org/10.3390/physchem5020016
APA StyleShi, Y., Blackwell, L. A., Schroy, R. K., Cleland, B. M., Risi, C. M., Parvatiyar, M. S., Pinto, J. R., Galkin, V. E., & Chase, P. B. (2025). A Central Role for Troponin C Amino-Terminal α-Helix in Vertebrate Thin Filament Ca2+-Activation. Physchem, 5(2), 16. https://doi.org/10.3390/physchem5020016







