A Novel Biodegradable Technology for Wool Fabric Restoration and Cotton Color Retention Based on Shikimic Acid and L-Arginine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Laundry Gel and Fabric Softener Samples
2.2. Wool Fabric Washing Procedure
2.3. SEM Studies
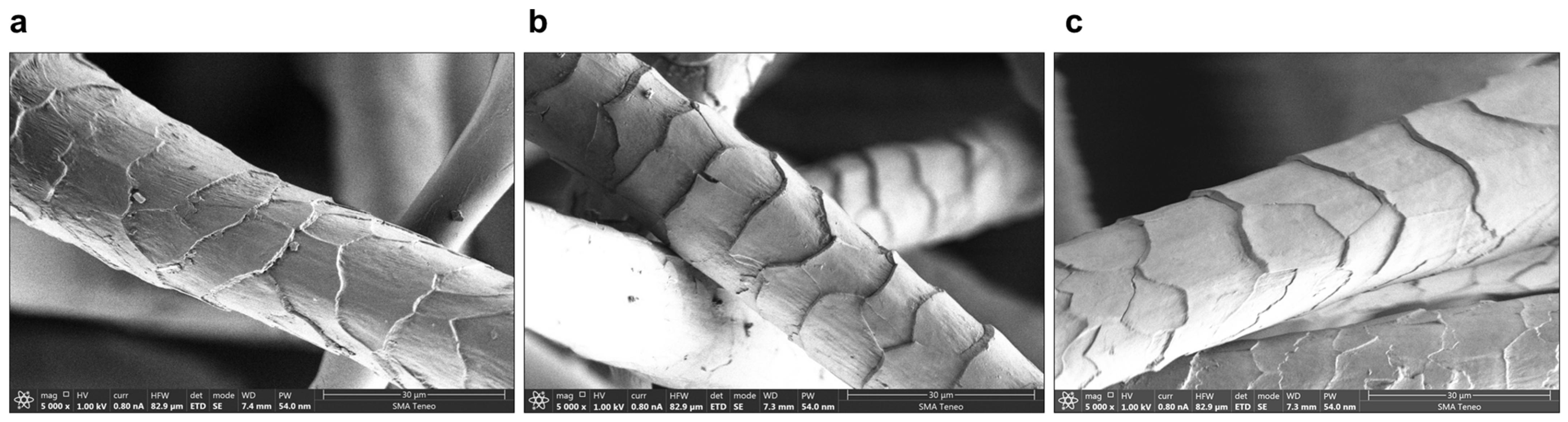
2.4. Fiber Damage Degree Scale
2.5. Color Retention of Cotton Fabric
3. Results
3.1. Evaluation of System Efficacy with a 0.25% Concentration of SA and L-Arginine
3.2. Evaluation of System Efficacy with a 0.001% and 0.01% Concentration of SA and L-Arginine
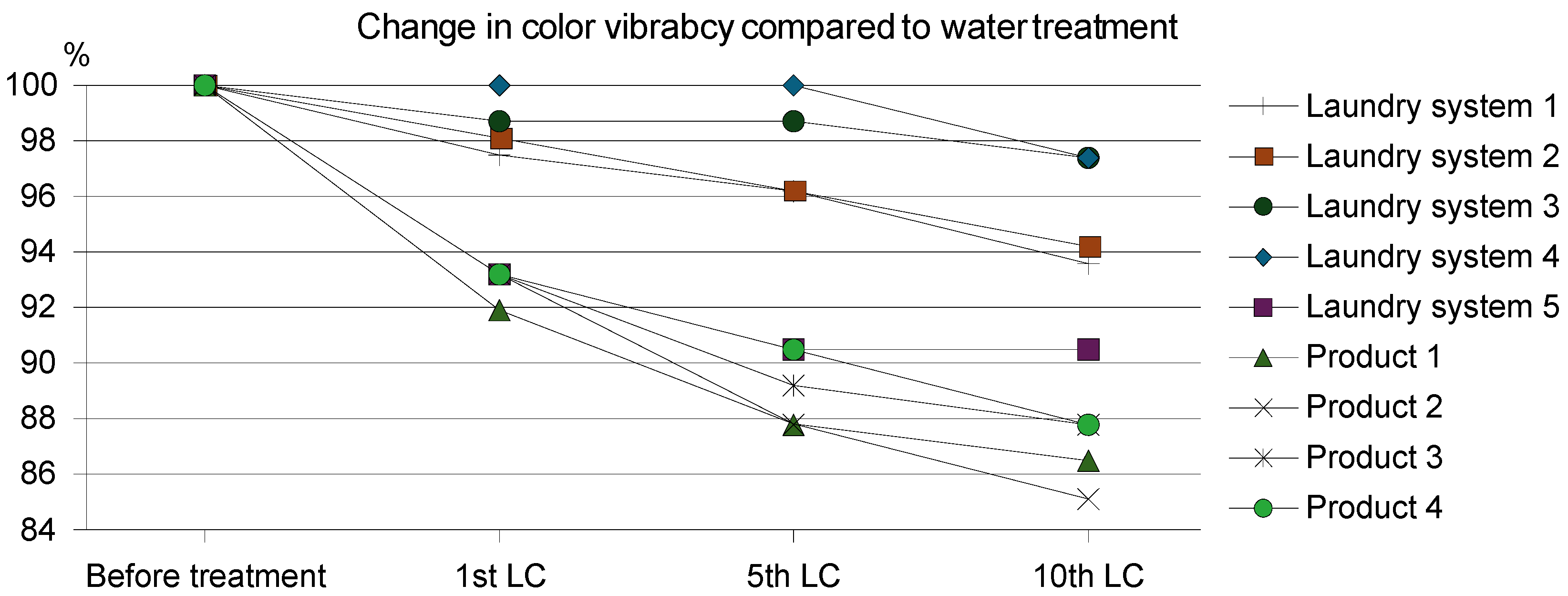
3.3. Evaluation of the Color Retention of Cotton Fabric
4. Discussion
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomšič, B.; Fink, R. Toward Sustainable Household Laundry. Washing Quality Vs. Environmental Impacts. Int. J. Environ. Health Res. 2023, 34, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Toledo, N.A.B.; Contreras, C.H.; Corredor, C.A.A.; Pérez, C.A.R. Effect of biodegradable detergents on water quality. NVEO 2021, 8, 12080–12095. [Google Scholar]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R. Evaluation of Microplastic Release Caused by Textile Washing Processes of Synthetic Fabrics. Environ. Pollut. 2017, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The Contribution of Washing Processes of Synthetic Clothes to Microplastic Pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Saravanja, A.; Pušić, T.; Dekanić, T. Microplastics in Wastewater by Washing Polyester Fabrics. Materials 2022, 15, 2683. [Google Scholar] [CrossRef]
- Prata, J.C.; Patrício Silva, A.L.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; An, L.; Ding, J.; Xu, Y. Atmospheric Microfibers Dominated by Natural and Regenerated Cellulosic Fibers: Explanations from the Textile Engineering Perspective. Environ. Pollut. 2022, 317, 120771. [Google Scholar] [CrossRef]
- Santonicola, S.; Volgare, M.; Rossi, F.; Castaldo, R.; Cocca, M.; Colavita, G. Detection of Fibrous Microplastics and Natural Microfibers in Fish Species (Engraulis Encrasicolus, Mullus Barbatus and Merluccius Merluccius) for Human Consumption from the Tyrrhenian Sea. Chemosphere 2024, 363, 142778. [Google Scholar] [CrossRef]
- Zallmann, M.; Smith, P.; Tang, M.L.K.; Tang, M.L.K.; Spelman, L.; Cahill, J.L.; Wortmann, G.; Katelaris, C.H.; Allen, K.J.; Allen, K.J. Debunking the Myth of Wool Allergy: Reviewing the Evidence for Immune and Non-immune Cutaneous Reactions. Acta Derm. Venereol. 2017, 97, 906–915. [Google Scholar] [CrossRef]
- Kılınç, M.; Korkmaz, G.; Kılınç, N.; Kut, D. The Use of Wool Fiber in Technical Textiles and Recent Developments. In The Wool Handbook; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Wool Market’s Production Statistics|IWTO. Available online: https://iwto.org/resources/statistics/ (accessed on 10 June 2024).
- Erdogan, U.H.; Seki, Y.; Selli, F. Wool fibres. Handb. Nat. Fibres 2020, 1, 257–278. [Google Scholar]
- Feughelman, M. Natural Protein Fibers. J. Appl. Polym. Sci. 2002, 83, 489–507. [Google Scholar] [CrossRef]
- Jones, L.N. Hair Structure Anatomy and Comparative Anatomy1. Clin. Dermatol. 2001, 19, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Livestock Production Management—Structure of Wool and Its Differentiation from Hair Fibre. Available online: https://sites.google.com/site/viveklpm/wool/structure-of-wool-and-its-differentiation-from-hair-fibre (accessed on 10 June 2024).
- Popescu, C.; Höcker, H. Hair—The Most Sophisticated Biological Composite Material. Chem. Soc. Rev. 2007, 36, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, F. The Structure and Properties of Wool and Hair Fibres. In Handbook of Textile Fibre Structure; Woodhead Publishing: Cambridge, UK, 2009; pp. 108–145. [Google Scholar]
- Dupres, V.; Langevin, D.; Guenoun, P.; Checco, A.; Luengo, G.S.; Leroy, F. Wetting and Electrical Properties of the Human Hair Surface: Delipidation Observed at the Nanoscale. J. Colloid Interface Sci. 2007, 306, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, S.; Tanamachi, H.; Ishikawa, K. Degradation of Hair Surface: Importance of 18-MEA and Epicuticle. Cosmetics 2019, 6, 31. [Google Scholar] [CrossRef]
- Silva, C.J.S.M.; Prabaharan, M.; Gübitz, G.; Cavaco-Paulo, A. Treatment of Wool Fibres with Subtilisin and Subtilisin-peg. Enzym. Microb. Technol. 2005, 36, 917–922. [Google Scholar] [CrossRef]
- Oshimura, E.; Abe, H.; Oota, R. Hair and Amino Acids: The Interactions and the Effects. J. Cosmet. Sci. 2007, 58, 347–358. [Google Scholar]
- Arai, M.; Suzuki, T.; Kaneko, Y.; Miyake, M.; Nishikawa, N. Properties of Aggregates of Amide Guanidine Type Cationic Surfactant with 1-hexadecanol Adsorbed on Hair. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 132, pp. 1005–1008. [Google Scholar]
- Ivanov, K.; Stoimenova, A.; Obreshkova, D.; Saso, L. Biotechnology in the Production of Pharmaceutical Industry Ingredients: Amino Acids. Biotechnol. Equip. 2013, 27, 3620–3626. [Google Scholar] [CrossRef]
- Rathinamoorthy, R. Performance Analysis of Pyridine N-Oxide as Dye Transfer Inhibitor in Household Laundry. Fibers Polym. 2019, 20, 1218–1225. [Google Scholar] [CrossRef]
- Cotton, L.; Hayward, A.S.; Lant, N.J.; Blackburn, R.S. Improved Garment Longevity and Reduced Microfibre Release Are Important Sustainability Benefits of Laundering in Colder and Quicker Washing Machine Cycles. Dye. Pigment. 2020, 177, 108120. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Zheng, C.; Zhou, T.; Sun, J. Synthesis of Acid Dyes Containing Polyetheramine Moieties and Their Low-temperature Dyeing Properties on Wool Fiber. J. Appl. Polym. Sci. 2018, 135, 45793. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Salama, M.; Kantouch, A.A.M. Wool Micro Powder as a Metal Ion Exchanger for the Removal of Copper and Zinc. Desalination Water Treat. 2015, 56, 1010–1019. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, A.R.; Baek, J.-H.; Kim, H.O.; Shin, M.K.; Koh, J.-S. Twelve-point Scale Grading System of Scanning Electron Microscopic Examination to Investigate Subtle Changes in Damaged Hair Surface. Ski. Res. Technol. 2016, 22, 406–411. [Google Scholar] [CrossRef]
- Swift, J.A.; Smith, J.R. Microscopical Investigations on the Epicuticle of Mammalian Keratin Fibres. J. Microsc. 2001, 204, 203–211. [Google Scholar] [CrossRef]
- Kang, T.J.; Kim, M.S. Effects of Silicone Treatments on the Dimensional Properties of Wool Fabric. Text. Res. J. 2001, 71, 295–300. [Google Scholar] [CrossRef]
- Forzano, I.; Avvisato, R.; Varzideh, F.; Jankauskas, S.S.; Cioppa, A.; Mone, P.; Salemme, L.; Kansakar, U.; Tesorio, T.; Trimarco, V.; et al. L-Arginine in Diabetes: Clinical and Preclinical Evidence. Cardiovasc. Diabetol. 2023, 22, 89. [Google Scholar] [CrossRef]
- Grasemann, H.; Kurtz, F.; Ratjen, F. Inhaled L-Arginine Improves Exhaled Nitric Oxide and Pulmonary Function in Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 208–212. [Google Scholar] [CrossRef]
- Garcia-Bravo, B.; Pérez Bernal, A.; Garcia-Hernandez, M.J.; Camacho, F. Occupational Contact Dermatitis from Anethole in Food Handlers. Contact Dermat. 1997, 37, 38. [Google Scholar] [CrossRef]
- Poon, T.S.C.; Freeman, S. Cheilitis Caused by Contact Allergy to Anethole in Spearmint Flavoured Toothpaste. Australas. J. Dermatol. 2006, 47, 300–301. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Yang, W.-K.; Lee, A.Y.; Kim, D.-S.; Nho, K.J.; Kim, Y.S.; Kim, H.K. Topical Application of an Ethanol Extract Prepared from Illicium Verum Suppresses Atopic Dermatitis in NC/Nga Mice. J. Ethnopharmacol. 2012, 144, 151–159. [Google Scholar] [CrossRef]

| Compounds | Concentration, wt.% |
|---|---|
| Purified water | up to 100 |
| Tetrasodium glutamate diacetate, 40% solution | 1.60 |
| Trisodium citrate dihydrate | 0.80 |
| Sodium laureth sulfate, 70% solution | 7.50 |
| Decyl glucoside, 15–50% solution | 3.50 |
| Lauryl glucoside, 15–50% solution | 3.50 |
| Fatty alcohol ethoxylate, 7 mol | 3.20 |
| Glycerin | 3.00 |
| Beta cyclodextrin | 0.10 |
| Cocamidopropyl betaine, 40–45% solution | 1.50 |
| 1,2-benzisothiazol-3(2H)-one, potassium hydroxide, 2.5–5% solution | 0.10 |
| N-(3-aminopropyl)-N-dodecylpropane-1,3-diamine, 2.5–5% solution | |
| Potassium hydroxide, 1–2.5% solution | |
| 2-Pyridinethiol-1-oxide sodium salt, 0.5–1% solution | |
| Sodium carboxymethyl inulin | 0.5 |
| Sodium chloride | 2.8 |
| Citric acid monohydrate | 0.25 |
| Potassium cocoate | 2.00 |
| Protease (Subtilisin), 1–2.5% solution | 0.30 |
| Cellulase, 0.1–1% solution | |
| Lipase, 0.1–1% solution | |
| Alpha-amylase, 0.1–1% solution Mannanase, 0.1–1% solution Pectinase, 0.1–1% solution | |
| Shikimic acid | 0.001–0.25 |
| L-arginine | 0.001–0.25 |
| Compounds | Concentration, wt.% |
|---|---|
| Purified water | up to 100 |
| Triethanolamine ammonium methyl sulfate di-alkyl ester | 13.50 |
| Glycerin | 3.00 |
| 3-acetyl-6-methyl-2H-pyran-2,4(3H)-Dione | 0.02–0.03 |
| Benzoic acid | 0.04 |
| Benzyl alcohol | 0.24–0.25 |
| Calcium lactate | 0.05 |
| Shikimic acid | 0.00-0.25 |
| L-arginine | 0.00-0.25 |
| Fragrance and essential oils | 0.40 |
| Compounds | Concentration, wt.% |
|---|---|
| Purified water | Up to 100 |
| Sodium laureth sulfate, 70% solution | 7.50 |
| Decyl glucoside, 51–55% solution | 3.50 |
| Fatty alcohol ethoxylate, 7 mol | 3.20 |
| Glycerin | 3.00 |
| Methyl glycine diacetic acid trisodium salt, 40% solution | 1.60 |
| Sodium hydroxide, 0.3–1% solution | 1.60 |
| Beta cyclodextrin | 0.10 |
| Cocamidopropyl betaine, 40–45% solution | 1.50 |
| Trisodium citrate dihydrate | 0.8 |
| 1,2-benzisothiazol-3(2H)-one, potassium hydroxide, 2.5–5% solution | 0.10 |
| N-(3-aminopropyl)-N-dodecylpropane-1,3-diamine, 2.5–5% solution | |
| Potassium hydroxide, 1–2.5% solution | |
| 2-Pyridinethiol-1-oxide sodium salt, 0.5–1% solution | |
| Sodium carboxymethyl inulin | 0.5 |
| Sodium chloride | 2.8 |
| Citric acid monohydrate | 0.25 |
| Potassium cocoate | 2.00 |
| Protease (Subtilisin), 1–2.5% solution | 0.30 |
| Lipase, 0.1–1% solution | |
| Alpha-amylase, 0.1–1% solution Mannanase, 0.1–1% solution Pectinase, 0.1–1% solution Cellulase, 0.1–1% solution | |
| Cellulase, 1.1–3.5% solution | 0.28 |
| Sample | Laundry Gel | Fabric Softener | ||
|---|---|---|---|---|
| SA, wt.% | L-Arginine, wt.% | SA, wt.% | L-Arginine, wt.% | |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0.25 | 0.25 | 0 | 0 |
| 3 | 0 | 0 | 0.25 | 0.25 |
| 4 | 0.25 | 0.25 | 0.25 | 0.25 |
| 5 | 0 | 0 | 0.01 | 0.01 |
| 6 | 0.01 | 0.01 | 0.01 | 0.01 |
| 7 | 0 | 0 | 0.001 | 0.001 |
| 8 | 0.001 | 0.001 | 0.001 | 0.001 |
| Sample Number | Mean Value of Scale Structure of Wool Damage | Decrease in the Scale Structure of Wool Damage Compared to the Control *, % |
|---|---|---|
| Before treatment | 2.44 ± 0.63 | - |
| After treatment with protease | 2.23 ± 0.63 | - |
| 1 | 1.93 ± 0.62 | 13.65 |
| 2 | 1.46 ± 0.69 | 34.87 |
| 3 | 2.20 ± 0.63 | 1.49 |
| 4 | 1.80 ± 0.63 | 19.40 |
| 5 | 1.71 ± 0.56 | 29.67 |
| 6 | 1.80 ± 0.63 | 26.15 |
| 7 | 1.53 ± 0.77 | 13.65 |
| 8 | 1.68 ± 0.67 | 30.90 |
| The Name of the Laundry System | CF after 1st LC, % * | CF after 5th LC, % * | CF after 10th LC, % * |
|---|---|---|---|
| Laundry System 1 | 2.5 | 3.8 | 6.4 |
| Laundry System 2 | 1.9 | 3.8 | 5.8 |
| Laundry System 3 | 1.3 | 1.3 | 2.6 |
| Laundry System 4 | 0 | 0 | 2.6 |
| Product 1 | 6.8 | 12.2 | 14.9 |
| Product 2 | 8.1 | 12.2 | 13.5 |
| Product 3 | 6.8 | 10.8 | 12.2 |
| Product 4 | 6.8 | 9.5 | 12.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latypova, T.; Kosovskaya, D.; Lovygin, M.; Evseev, G.; Olkhovskaya, M.; Filatov, V. A Novel Biodegradable Technology for Wool Fabric Restoration and Cotton Color Retention Based on Shikimic Acid and L-Arginine. Textiles 2024, 4, 549-560. https://doi.org/10.3390/textiles4040032
Latypova T, Kosovskaya D, Lovygin M, Evseev G, Olkhovskaya M, Filatov V. A Novel Biodegradable Technology for Wool Fabric Restoration and Cotton Color Retention Based on Shikimic Acid and L-Arginine. Textiles. 2024; 4(4):549-560. https://doi.org/10.3390/textiles4040032
Chicago/Turabian StyleLatypova, Taisiia, Darya Kosovskaya, Mikhail Lovygin, Grigoriy Evseev, Mariya Olkhovskaya, and Viktor Filatov. 2024. "A Novel Biodegradable Technology for Wool Fabric Restoration and Cotton Color Retention Based on Shikimic Acid and L-Arginine" Textiles 4, no. 4: 549-560. https://doi.org/10.3390/textiles4040032
APA StyleLatypova, T., Kosovskaya, D., Lovygin, M., Evseev, G., Olkhovskaya, M., & Filatov, V. (2024). A Novel Biodegradable Technology for Wool Fabric Restoration and Cotton Color Retention Based on Shikimic Acid and L-Arginine. Textiles, 4(4), 549-560. https://doi.org/10.3390/textiles4040032






