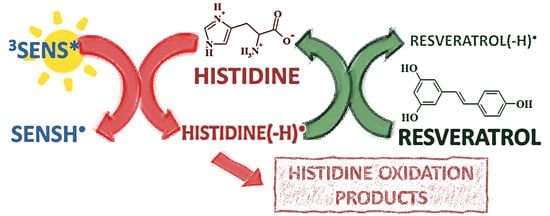
Unravelling the Photoprotection Capacity of Resveratrol on Histidine Oxidation
Abstract
:1. Introduction
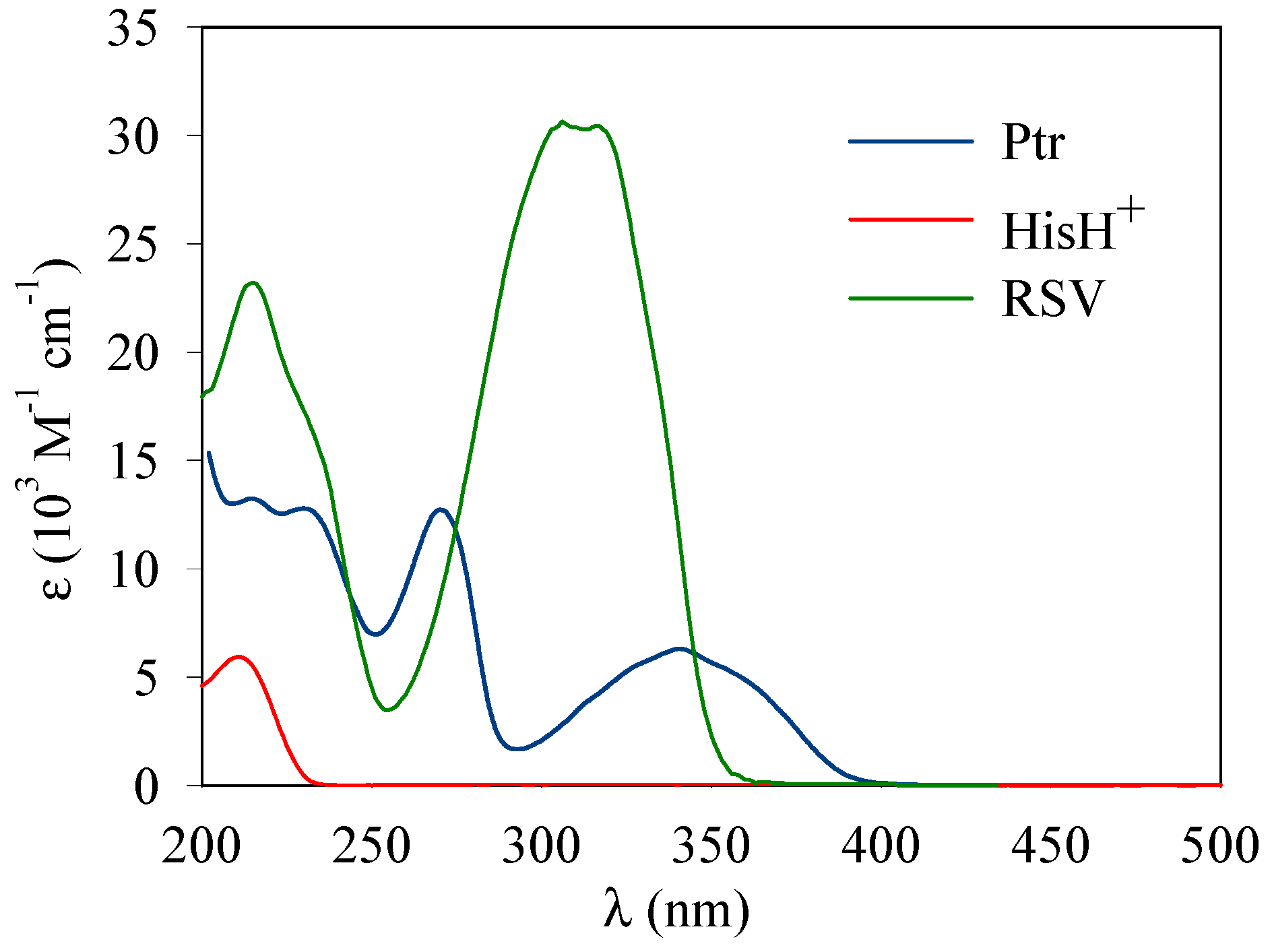
2. Materials and Methods
3. Results
3.1. Photosensitized Degradation of Histidine
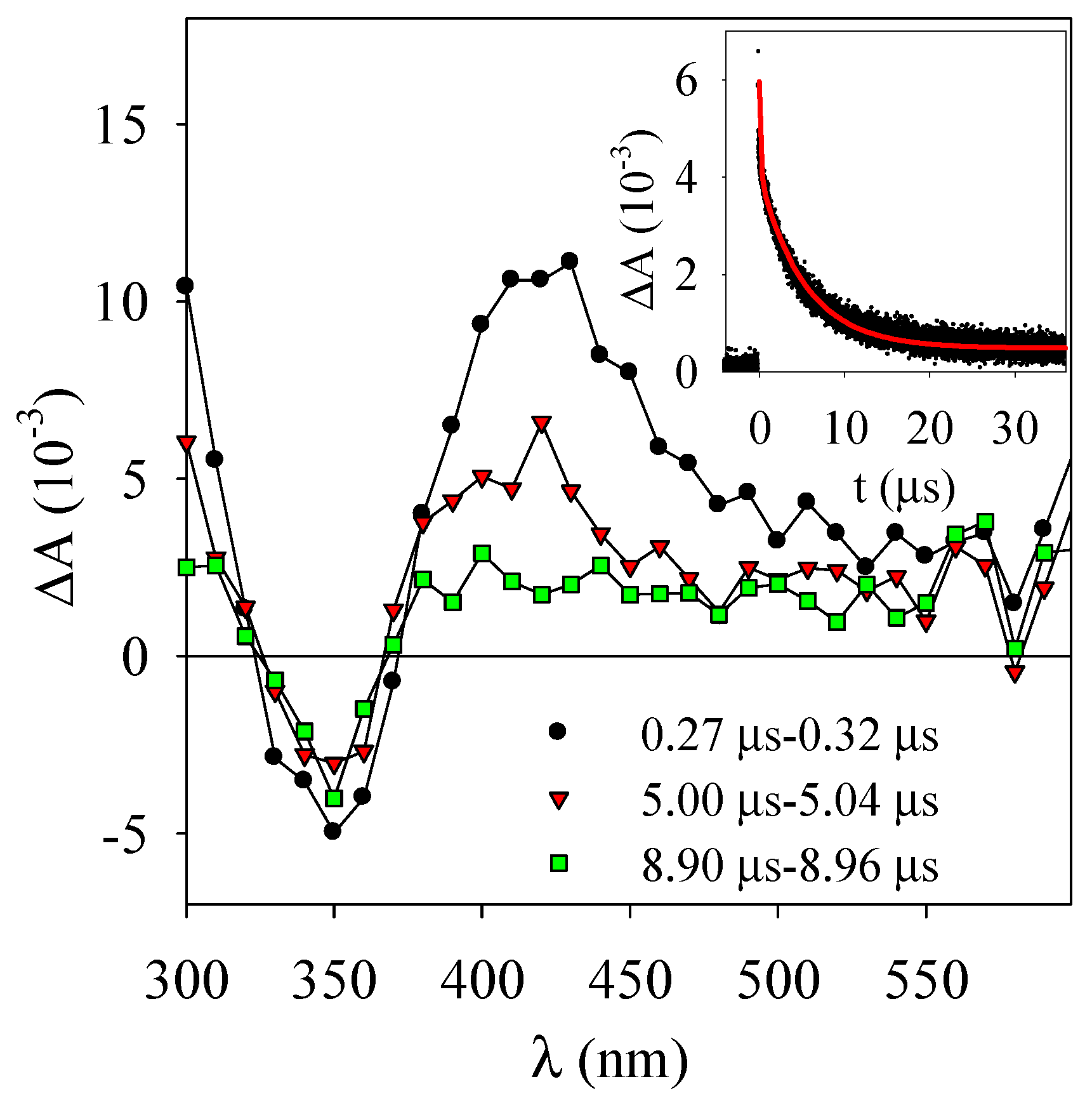
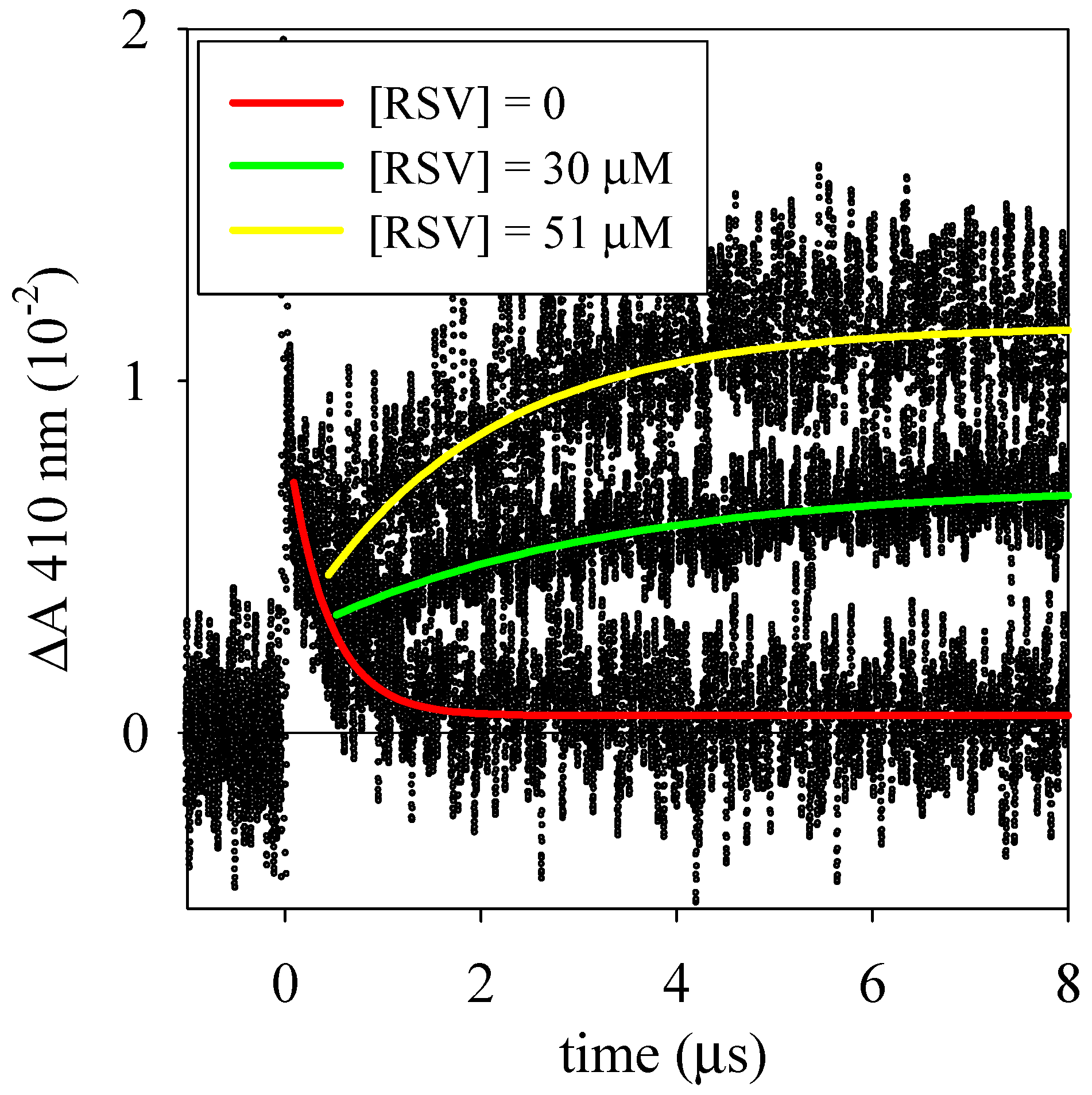
3.2. Laser Flash Photolysis Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langcake, P.; Pryce, R.J. The production of resveratrol by Wis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Harborne, J.B. Higher Plant-Lower Plant Interactions: Phytoalexins and Phytotoxins in Introduction to Ecological Biochemistry, 4th ed.; Academic Press: Cambridge, MA, USA, 1993; pp. 264–297. [Google Scholar]
- Colica, C.; Milanović, M.; Milić, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A systematic review on natural antioxidant properties of resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.M.S.; Cillard, J.; Friguet, B.; Cadenas, E.; Cadet, J.; Cayce, R.; Fishmann, A.; Liao, D.; Bulteau, A.L.; Derbré, F. The Oxygen Paradox, the French Paradox, and age-related diseases. GeroScience 2017, 39, 499–550. [Google Scholar] [CrossRef] [Green Version]
- Neyra Recky, J.R.; Gaspar Tosato, M.; Serrano, M.P.; Thomas, A.H.; Dántola, M.L.; Lorente, C. Evidence of the effectiveness of Resveratrol in the prevention of guanine one-electron oxidation: Possible benefits in cancer prevention. Phys. Chem. Chem. Phys. 2019, 21, 16190–16197. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Simoes Ribeiro, M.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Neyra Recky, J.R.; Serrano, M.P.; Dántola, M.L.; Lorente, C. Oxidation of tyrosine: Antioxidant mechanism of L-DOPA disclosed. Free Radic. Biol. Med. 2021, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Muralla, R.; Sweeney, C.; Stepansky, A.; Leustek, T.; Meinke, D. Genetic dissection of histidine biosynthesis in Arabidopsis. Plant. Physiol. 2007, 144, 890–903. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.K.; Hägglund, P.M.; Max Møller, I.; Davies, M.J.; Bjerrum, M.J. Copper ion/H2O2 oxidation of Cu/Zn-Superoxide dismutase: Implications for enzymatic activity and antioxidant action. Redox Biol. 2019, 26, 101262. [Google Scholar] [CrossRef]
- Guerra, W.D.; Odella, E.; Urrutia, M.N.; Liddell, P.A.; Moore, T.A.; Moore, A.L. Models to study photoinduced multiple proton coupled electron transfer processes. J. Porphyr. Phthalocyanines 2021, 25, 674–682. [Google Scholar] [CrossRef]
- Uchida, K. Histidine and lysine as targets of oxidative modification. Amino Acids 2003, 25, 249–257. [Google Scholar] [CrossRef]
- Tsentalovich Yu, P.; Lopez, J.J.; Hore, P.J.; Sagdeev, R.Z. Mechanisms of reactions of flavin mononucleotide triplet with aromatic amino acids. Spectrochim. Acta Part A 2002, 58, 2043–2050. [Google Scholar] [CrossRef]
- Agon, V.V.; Bubb, W.A.; Wright, A.; Hawkins, C.L.; Davies, M.J. Sensitizer-mediated photooxidation of histidine residues: Evidence for the formation of reactive side-chain peroxides. Free Radic. Biol. Med. 2006, 40, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Oliveros, E.; Thomas, A.H.; Lorente, C. Histidine oxidation photosensitized by pterin: pH dependent mechanism. J. Photochem. Photobiol. B Biol. 2015, 153, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Matheson, I.B.C.; Lee, J. Chemical reaction rates of amino acids with singlet oxygen. Photochem. Photobiol. 1979, 29, 879–881. [Google Scholar] [CrossRef]
- Lorente, C.; Serrano, M.P.; Vignoni, M.; Dántola, M.L.; Thomas, A.H. A model to understand type I oxidations of biomolecules photosensitized by pterins. J. Photochem. Photobiol. 2021, 7, 100045. [Google Scholar] [CrossRef]
- Lorente, C.; Petroselli, G.; Dántola, M.L.; Oliveros, E.; Thomas, A.H. Electron transfer initiated reactions photoinduced by pterins. Pteridines 2011, 22, 111–119. [Google Scholar] [CrossRef]
- Oliveros, E.; Dántola, M.L.; Vignoni, M.; Thomas, A.H.; Lorente, C. Production and quenching of reactive oxygen species by pterin derivatives, an intriguing class of biomolecules. Pure Appl. Chem. 2011, 83, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Braun, A.M.; Maurette, M.T.; Oliveros, E. Photochemical Technology; Wiley: Chichester, UK; New York, NY, USA, 1991; Chapter 2. [Google Scholar]
- Kuhn, H.J.; Braslavsky, S.E.; Schmidt, R. Chemical actinometry (IUPAC technical report). Pure Appl. Chem. 2004, 76, 2105–2146. [Google Scholar] [CrossRef]
- Serrano, M.P.; Lorente, C.; Borsarelli, C.D.; Thomas, A.H. Unravelling the degradation mechanism of purine nucleotides photosensitized by pterins: The role of charge-transfer steps. ChemPhysChem 2015, 16, 2244–2252. [Google Scholar] [CrossRef]
- Castaño, C.; Serrano, M.P.; Lorente, C.; Borsarelli, C.D.; Thomas, A.H. Quenching of the singlet and triplet excited states of pterin by amino acids. Photochem. Photobiol. 2019, 95, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, L.O.; Castaño, C.; Dántola, M.L.; Lhiaubet-Vallet, V.; Miranda, M.A.; Marin, M.L.; Thomas, A.H. A novel synthetic approach to tyrosine dimers based on pterin photosensitization. Dyes Pigm. 2017, 147, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Džeba, I.; Pedzinski, T.; Mihaljevi, B. Photophysical and photochemical properties of resveratrol. J. Photochem. Photobiol. A Chem. 2015, 299, 118–124. [Google Scholar] [CrossRef]

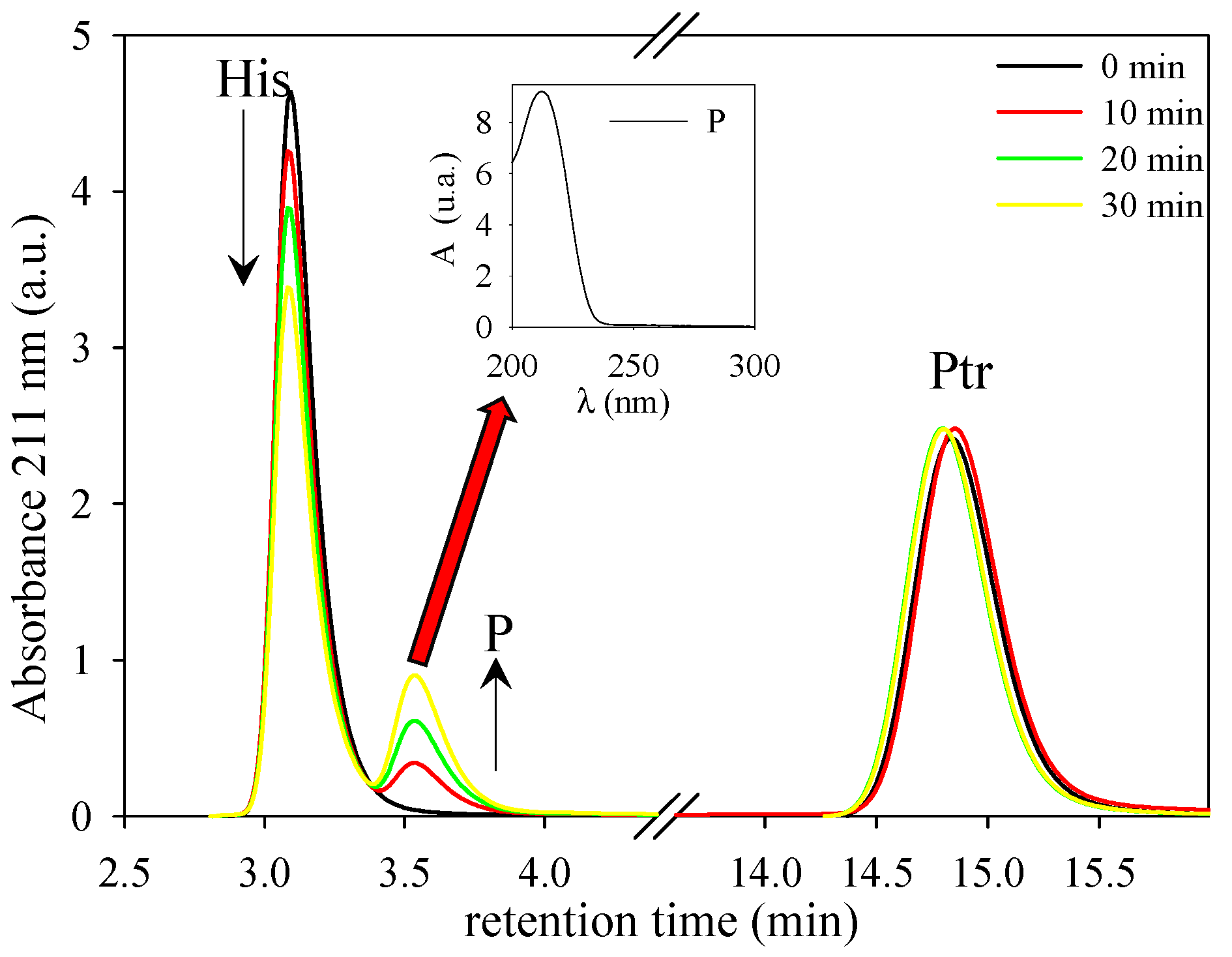




| [RSV] μM | [His] μM | fHis | fRSV | fO2 |
|---|---|---|---|---|
| -- | 100 | 0.21 | 0 | 0.56 |
| 15 μM | 100 | 0.19 | 0.09 | 0.51 |
| 45 μM | 100 | 0.16 | 0.24 | 0.43 |
| -- | 700 | 0.65 | 0 | 0.25 |
| 5 μM | 700 | 0.64 | 0.01 | 0.24 |
| 15 μM | 700 | 0.62 | 0.04 | 0.24 |
| 30 μM | 700 | 0.60 | 0.08 | 0.23 |
| [HisH+] = 1000 μM | |||
|---|---|---|---|
| [RSV] = 0 | -- | 0.60 | 0.40 |
| [RSV] = 30 μM | 3.0 | 0.55 | -- |
| [RSV] = 35 μM | 2.5 | 0.55 | -- |
| [RSV] = 40 μM | 2.4 | 0.54 | -- |
| [RSV] = 45 μM | 2.2 | 0.53 | -- |
| [RSV] = 51 μM | 1.7 | 0.52 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neyra Recky, J.R.; Dántola, M.L.; Lorente, C. Unravelling the Photoprotection Capacity of Resveratrol on Histidine Oxidation. Photochem 2021, 1, 209-219. https://doi.org/10.3390/photochem1020012
Neyra Recky JR, Dántola ML, Lorente C. Unravelling the Photoprotection Capacity of Resveratrol on Histidine Oxidation. Photochem. 2021; 1(2):209-219. https://doi.org/10.3390/photochem1020012
Chicago/Turabian StyleNeyra Recky, Jael R., M. Laura Dántola, and Carolina Lorente. 2021. "Unravelling the Photoprotection Capacity of Resveratrol on Histidine Oxidation" Photochem 1, no. 2: 209-219. https://doi.org/10.3390/photochem1020012
APA StyleNeyra Recky, J. R., Dántola, M. L., & Lorente, C. (2021). Unravelling the Photoprotection Capacity of Resveratrol on Histidine Oxidation. Photochem, 1(2), 209-219. https://doi.org/10.3390/photochem1020012