Abstract
Substitution of frozen-thawed food products for fresh ones is a significant authenticity issue being extensively investigated over the past few years by various conventional methods, but little success has been achieved. Fluorescence spectroscopy is a sensitive and selective spectroscopic technique that has been widely applied recently to deal with various food quality and authenticity issues. The technique is based on the excitation of certain photosensitive components (known as fluorophores) to fluoresce in the UV and visible spectral ranges. Fluorescence spectroscopy can be performed to obtain simple classical two-dimensional fluorescence spectra (excitation/emission), synchronous or three-dimensional excitation–emission matrices (excitation/emission/fluorescence signal). The technique can be used in front-face or right-angle configurations and can be even combined with hyperspectral imaging, requiring the use of multivariate data analysis to extract useful information. In this review, we summarize the recent progress in applications of fluorescence spectroscopy to differentiate truly fresh foods from frozen-thawed products. The basics of the technique will be briefly presented and some relevant examples, focusing especially on fish and meat products, will be given. It is believed that interdisciplinary collaboration between researchers working with data analysis and spectroscopy, as well as industry and regulatory authorities would help to overcome the current shortcomings, holding the great promise of fluorescence spectroscopy for fighting food fraud in the food industry.
Keywords:
seafood; meat; spectroscopy; excitation/emission; freshness; fraud; quality change; freezing; fluorophores 1. Introduction
Freshness is an important quality parameter for foods that are highly perishable, such as fish and meat products. To maintain the quality/freshness of such products, a wide range of traditional and innovative preservation and processing strategies has been applied [1,2,3,4,5,6,7,8,9,10]. Freezing is still the most common method used to retain freshness and extend the shelf life of many foods, including fish and meat and their products. However, it is well-established that freezing, frozen storage, and the following thawing process lead to some deteriorative quality changes, such as damage in cell structure, protein denaturation, texture, color, among others [11,12,13]. Although multiple novel freezing solutions [14,15,16,17,18] have been developed in recent years in order to reduce the damage caused by freezing and protect the sensory and nutritional quality of frozen foods, many consumers still prefer fresh products, and labeling frozen-thawed products as fresh is therefore illegal and considered as fraud [19,20,21].
To detect changes occurring during freezing, frozen storage, and thawing, and to discriminate fresh from frozen-thawed products, well-established traditional techniques, such as enzymatic methods and histological techniques, have been widely employed [22,23,24,25,26,27]. Other techniques based on sensory attributes and changes in proteins and physicochemical properties (such as electrophoretic analysis, protein and lipid oxidation, volatile compounds, water-holding capacity) have been also presented in recent publications [21,28,29,30]. Nevertheless, most of these techniques are destructive and not suitable for online applications. Therefore, there is a pressing need to move forward and to start thinking about alternative methods of determining the quality and authenticity of food products.
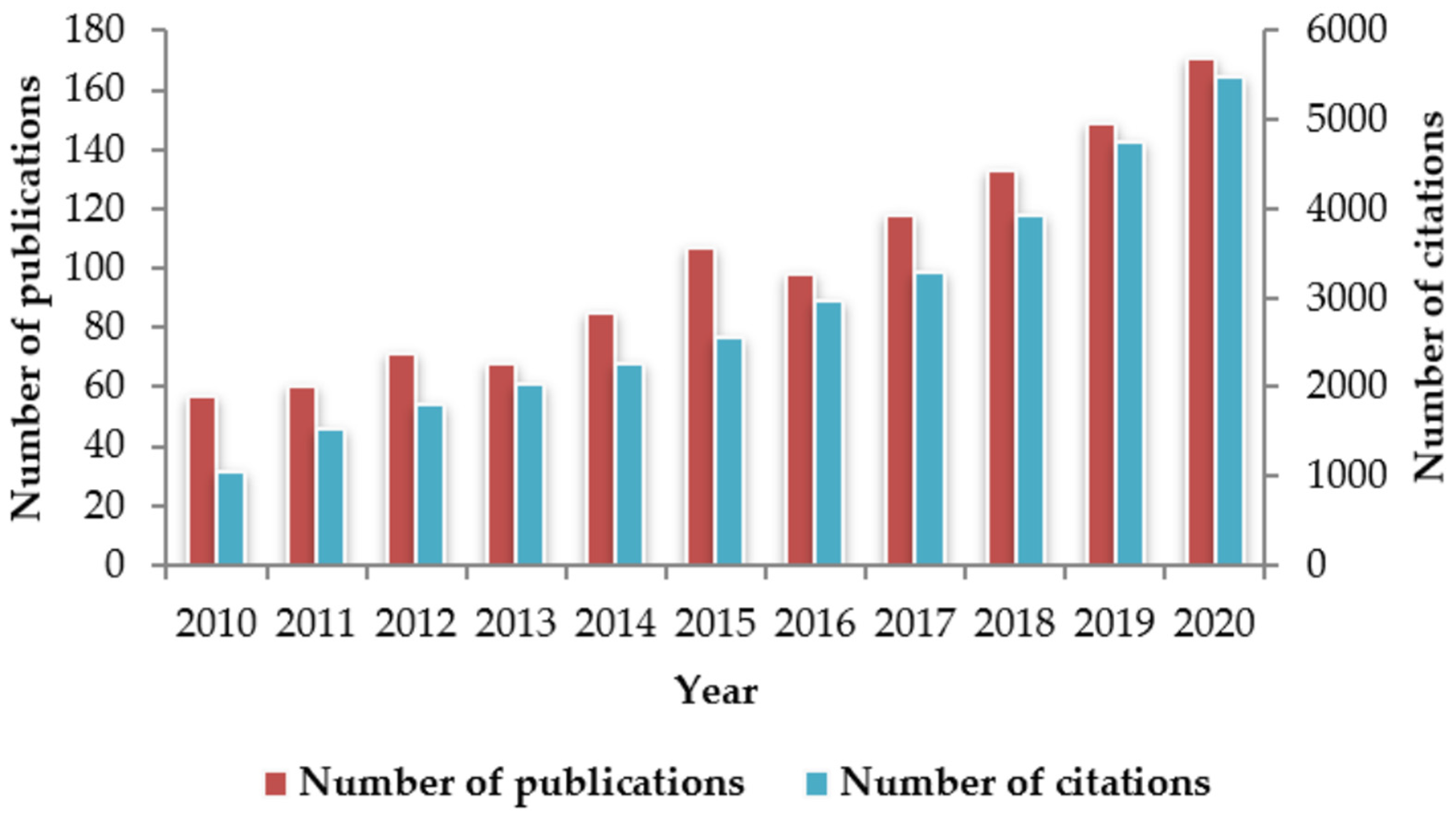
To respond to this need, a variety of emerging analytical techniques have been developed to overcome limitations associated with traditional methods [31,32,33,34,35,36,37,38,39,40,41,42,43]. Among the new techniques, spectroscopic ones have received a special attention and tested for a wide range of applications [2,20,44,45,46]. Indeed, the yearly number of publications on the use of spectroscopic techniques in the field of food quality and authenticity has tripled (increased from 57 to 171) during the last decade (Figure 1). Likewise, the number of citations has increased dramatically, going up from nearly 1000 citations in 2010 to more than 5000 in 2020. Several spectroscopic techniques have been used to differentiate fresh from frozen-thawed foods. These include visible and near-infrared spectroscopy [47,48,49,50,51,52,53,54,55], Fourier-transform infrared (FTIR) spectroscopy [56,57,58], and Raman spectroscopy [59,60]. These techniques have become even more interesting following the recent development of hyperspectral imaging (HSI). HSI has gained much attention over the past few years due to its ease of implementation in industrial settings as well as its capacity to combine spectroscopy and imaging in one system [61,62,63,64,65,66,67,68,69,70,71,72].
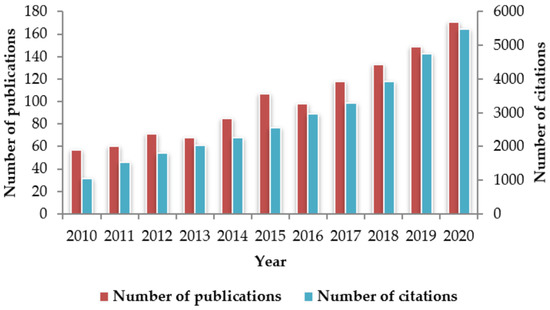
Figure 1.
Publications and citations number of scientific articles reporting on the use of spectroscopic techniques for the study of food quality and/or authenticity. Information taken from the database Scopus, with search criteria: Article title; spectroscopy AND Article title, Abstract, Keywords; food quality OR Article title, Abstract, Keywords; food authenticity. The data were obtained in June 2021.
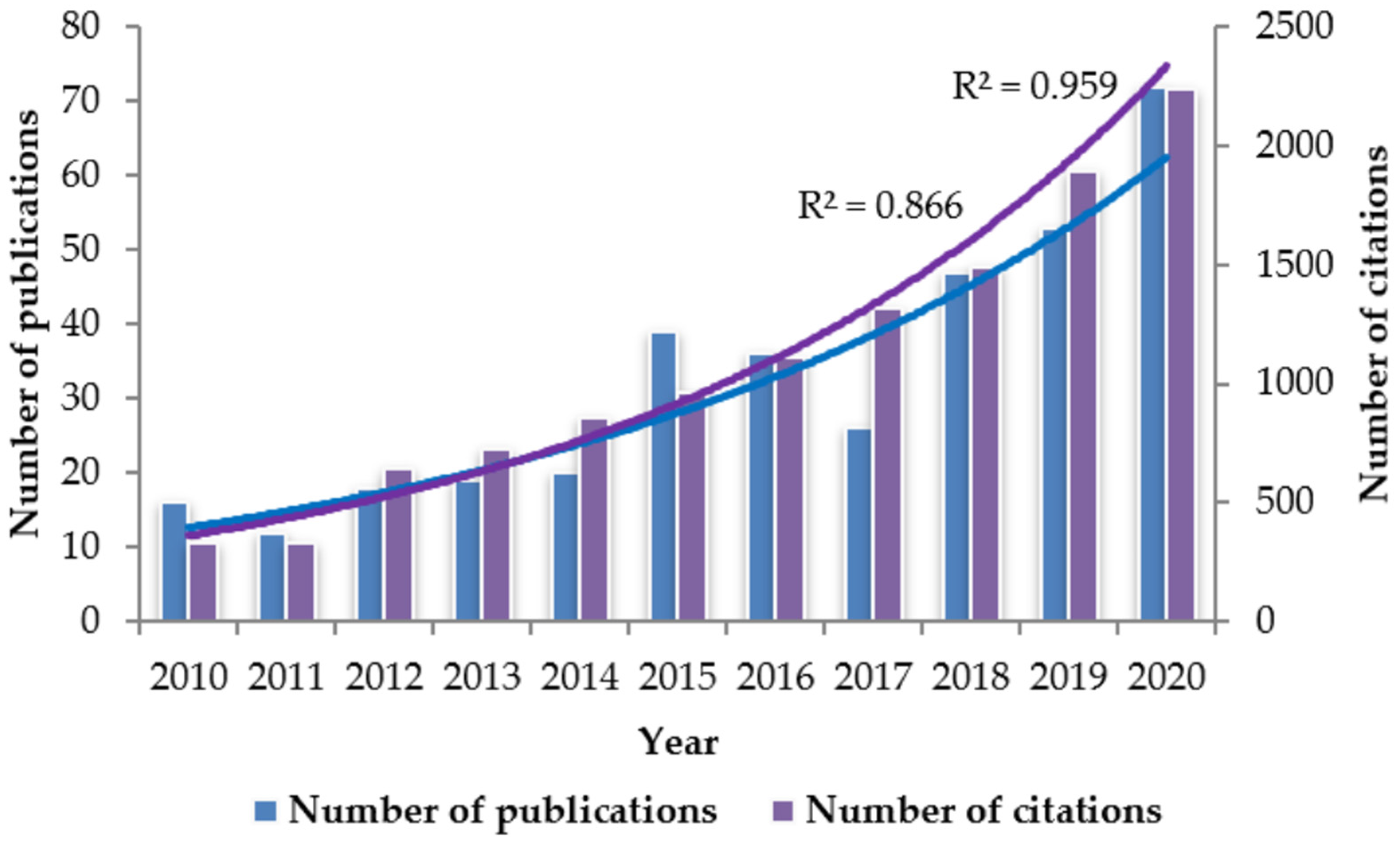
Fluorescence spectroscopy is a sensitive and selective spectroscopic technique that has undergone significant advancement in recent years due to, among other factors, the development of instrumentation and chemometric tools. Numerous studies have demonstrated that the technique can be applied as an effective method for detecting fraud and verifying authenticity in a wide range of food products [73,74,75,76,77,78,79]. As can be seen from Figure 2, the number of published papers and citations on the use of fluorescence spectroscopy for studying food quality and/or authenticity has increased exponentially during the last decade.
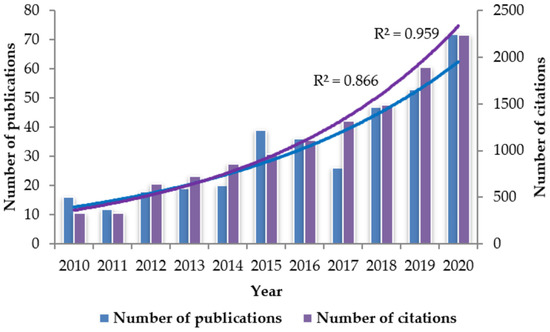
Figure 2.
Publications and citations number of scientific articles reporting on the use of fluorescence spectroscopy for studying food quality and/or authenticity. Information taken from the database Scopus, with search criteria: Article title, Abstract, Keywords; fluorescence spectroscopy AND Article title, Abstract, Keywords; food quality OR Article title, Abstract, Keywords; food authenticity. The data were obtained in June 2021.
The use of spectroscopic techniques for classification, authentication, and fraud detection in several food categories (e.g., milk, meats, oils, coffee, and juice) was reviewed in a general manner [80]. In another review paper [19], the focus was placed on fish and seafood products. Recently, we reviewed the application of conventional and spectroscopic techniques to detect fraudulent practices in food products of animal origin [20]. In these three review papers, the topic of discrimination between fresh and frozen-thawed food was addressed as a part of various other authenticity issues (such as substitution of species, production method, origin, etc.). Another review paper was proposed with the objective to report on the use of enzymatic, histological, and other traditional methods, as well as spectral techniques in conjunction with chemometric tools for the detection of frozen-thawed muscle food [21]. However, to date no review paper has been found in the literature that specifically and exclusively reports on the potential of fluorescence spectroscopy to distinguish this kind of fraud (i.e., labeling frozen-thawed food as fresh). More recently, an extensive and inspiring review paper has been published by Anna C. Croce to provide a general overview of the application of fluorescence in living organisms, including microorganisms, plants, and animals [81]. This review paper will provide an updated overview of practical applications of fluorescence spectroscopy in a specific research area, namely the discrimination between fresh and frozen-thawed foods, with a special focus to be placed on studies dealing with seafood and meat products, published over the last few years.
2. Principles and Fundamentals
Fluorescence refers to the emission of light by the sample (a substance or a molecule of interest) following the absorption of ultraviolet and visible light (usually from 200 to 800 nm). The technique is known to be highly sensitive, selective, and suitable for many matrices and purposes [37,78,79]. Fluorescence spectroscopy depends on the presence of molecules, called fluorophores (or lumiphores) in foods that emit the fluorescence. Several well-known fluorophores such as reduced nicotanamide adenine dinucleotide (NADH), tryptophan, collagen, vitamins (especially vitamin A and vitamin B2 or riboflavin, containing conjugated double bonds), and Maillard reaction products (resulting from the reaction of sugars with proteins) have been widely investigated in studies reporting on fish and meat. Each of these fluorophores has its own excitation/emission wavelength. Table 1 shows the characteristics of food-relevant intrinsic fluorophores that are commonly used in fluorescence studies. The focus in this review paper will be placed on endogenous (intrinsic) fluorophores (auto-fluorescence), although exogenous (extrinsic) fluorophores can also be used to label non-fluorescent or weak fluorescent samples or to stain cells. Fluorescence spectroscopy can be used in two main geometries; right-angle or front-face modes. The right-angle mode (i.e., collection of fluorescence at 90° to the incident excitation light.) is typically selected for diluted and clear samples, whereas front-face mode is performed by collecting the fluorescence off the front surface (the angle is less than 90°) of the solid samples [73,82]. In modern fluorometers, the angle between excitation and emission beam paths is easily changeable.

Table 1.
Examples of fluorescence properties of relevant fluorophores (or luminophores) in muscle foods.
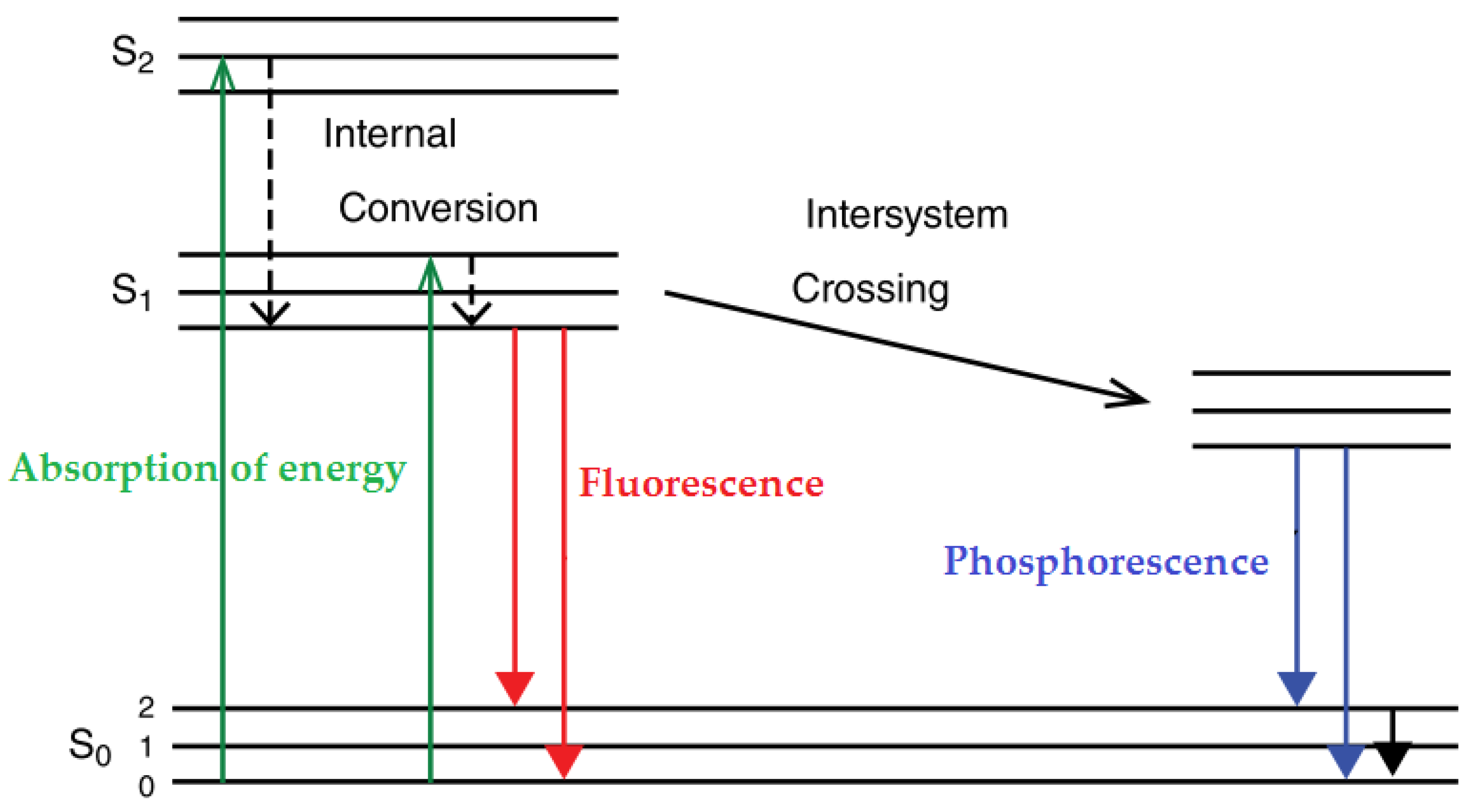
A Jablonski diagram is often used to better interpret the phenomenon of fluorescence. Indeed, this diagram illustrates the electronic energy levels of a fluorophore and the transitions that occur between the different electronic and vibrational states upon the absorption of excitation light and the following emission of fluorescence [108]. The molecules are usually at the lowest vibrational energy level of the singlet electronic ground state S0. The process starts with the absorption of the appropriate energy (electromagnetic radiations), which occurs fast (in less than 10−15 s), provoking the transition from the S0 to one of the various excited electronic states (S1 or S2). Then, vibrational relaxation (or internal conversion) can occur within 10−13 to 10−11 s, moving the excited molecule to the lower vibrational state (S1). After this internal conversion from S2 to S1, an additional vibrational relaxation occurs within S1, relaxing the molecule into the lowest vibrational level of S1. Next, depending on the time, the molecule can return to one of the various vibrational levels of the ground electronic state, emitting fluorescence (within 10−10 to 10−7 s) or further vibrational relaxations and intersystem crossing can occur at longer times (more than 10−6 s), leading to another phenomenon related to fluorescence, called phosphorescence (Figure 3). The main difference between fluorescence and phosphorescence is that the fluorescence occurs within singlet states (spin-allowed transition) on time scales much shorter than phosphorescence occurring between singlet and triplet states (a spin-forbidden transition) [77,79,108,109].
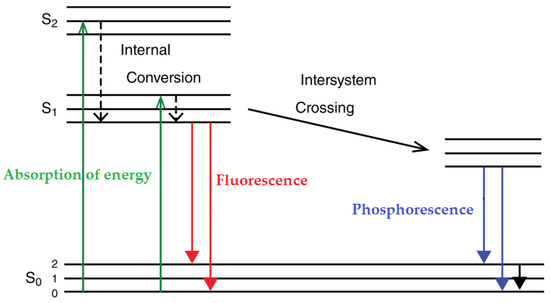
Figure 3.
Jablonski diagram illustrating the fluorescence and phosphorescence process [108,109].
In most muscle food studies, fluorescence is characterized by wavelength and intensity (which will be the focus of this review paper), but other applications related to lifetime and polarization can also be found in the literature [79,81,108]. Several fluorescence types can be distinguished, but the excitation–emission spectrum is the most commonly used. In this two-dimensional classical type (or traditional, single-scan method), fluorescence intensity can be shown in a two-dimensional plot as a function of a fixed excitation wavelength (emission scan) or a fixed emission wavelength (excitation scan). Fluorescence excitation spectrum of a fluorophore is close to its absorption spectrum, while the emission spectrum is shifted to longer wavelengths than the excitation spectrum, due to Stokes shift (the difference between the position of the band maxima of the absorption and emission spectra). For example, to obtain the emission spectrum of tryptophan, the excitation wavelength is often fixed at 290 nm and the emission fluorescence is collected between 305 nm and 420 nm. Despite the simplicity of this fluorescence type, it is used for fluorescence monitoring when the scanned sample is known to contain only one single fluorophore. However, it is well-established that most food products are considered complex multi-fluorophore systems due to their content of several fluorophores. Therefore, other fluorescence spectroscopic methods have been suggested and investigated in recent years. Synchronous fluorescence spectroscopy is performed by scanning both the excitation and emission wavelengths simultaneously and a constant wavelength interval Δλ is kept between them. Thus, the synchronous fluorescence spectroscopy is a plot of the variations in fluorescence intensity as a function of either the emission wavelength or the excitation wavelength for a fixed Δλ. Although technique is advantageous over the conventional classical methods, being able to characterize several fluorophores from a single measurement, rare studies have applied this technique [73,76].
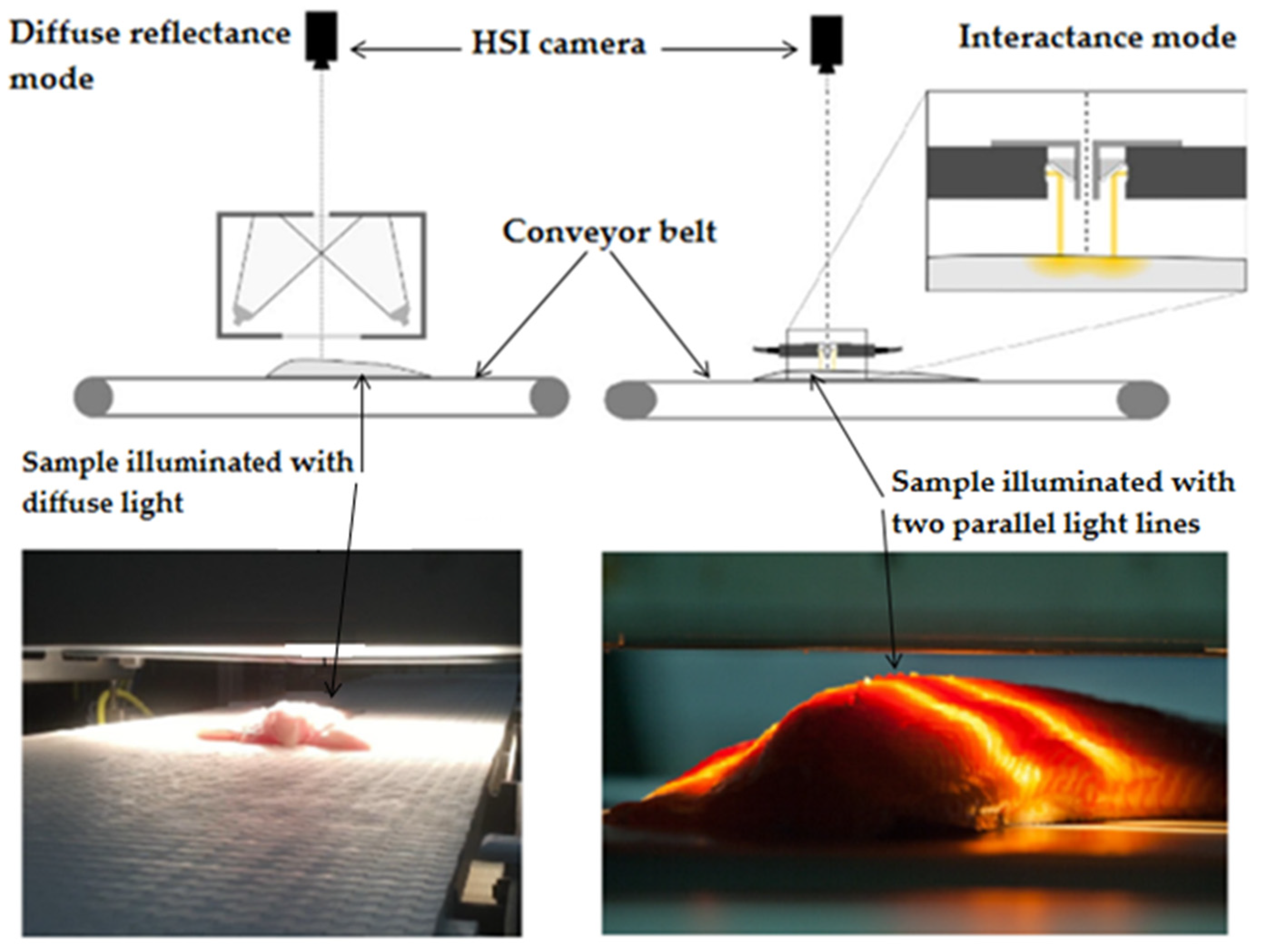
Most importantly, three-dimensional excitation–emission matrices (EEM), also known as fluorescence fingerprint or fluorescence landscape have been widely investigated recently, enabling the simultaneous detection of multiple fluorophores. EEM spectra are obtained by acquiring the emission spectra at multiple excitation wavelengths. Thus, EEM spectra consist of all the excitation and emission spectra over the scanned range of wavelengths and can be presented as a three-dimensional plot, with the fluorescence intensity (Z-axis) plotted as a function of both the excitation (X-axis) and the emission (Y-axis) wavelengths. One drawback related to this measurement mode is the long time (usually between 30 and 40 min depending on the measurement parameters) required to record spectra, although the modern fluorometers are capable of collecting EEM spectra in a much shorter time range. One of the main limitations of the aforementioned techniques is the high dependence of the spectra on composition and the physical properties of the analyzed samples. Indeed, the heterogeneity of most food matrices, especially muscle foods such as fish and meat, requires both spectral and geometric information to be obtained. Therefore, in recent years, spectroscopic techniques have been further expanded into hyperspectral imaging (HSI) [63,110,111]. HSI allows acquiring a full spectrum for every pixel of an image, giving three-dimensional data (two spatial and one spectral dimensions) simultaneously. Moreover, the technique is rapid, non-destructive, and can be compatible with applications in industrial settings, such as monitoring products on a conveyer belt (Figure 4). In recent years, significant evolution and development in HSI have been seen in terms of image-sensing modes (e.g., interactance, diffuse reflectance, and transmittance), detector performance, illumination sources, and data analysis and algorithms. In addition to HSI, a simplified version, called multispectral imaging, generating a smaller number of spectral bands compared to HSI, has also been extensively used in recent years [112,113]. Although there has been a surge in the use of this technique with infrared and Raman spectroscopy [114,115], little has been published regarding the use of hyperspectral fluorescence imaging technique, and no study has been published yet about the use of fluorescence spectroscopy in EEM mode coupled with HSI.
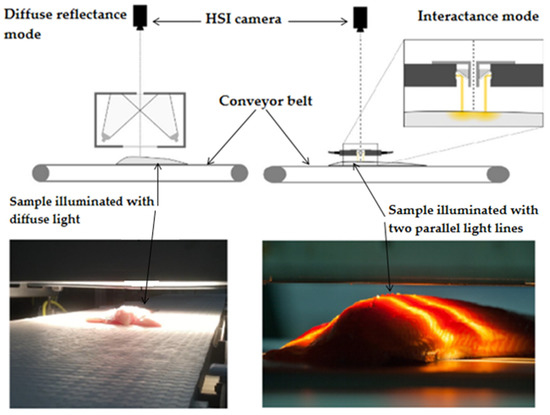
Figure 4.
Hyperspectral imaging (visible and near-infrared) operating in diffuse reflectance and interactance mode [2,45].
3. Examples of Applications
Traditional methods, based on the changes in color, texture, and other physicochemical properties (e.g., lipid and protein oxidation) have been widely applied. Histological measurements are among the most used conventional methods that describe changes in muscle structure and microstructure. Several studies reported shrinkage and appearance of spaces between fibers and the formation of ice crystals and large gaps between muscle fibers during freezing and frozen storage [22,24]. Numerous studies investigated the possibility of using chromatographic techniques and mass spectrometry methods to discriminate between fresh and frozen-thawed fish and meat [23,40,116]. Enzymatic techniques, electrophoretic approaches, and other biochemical parameters have been widely applied to achieve this purpose [117,118,119]. Table 2 shows examples of some of these techniques and approaches.

Table 2.
Examples of the main conventional methods used to differentiate fresh from frozen-thawed muscle foods and to examine quality changes occurring during freezing.
However, these targeted methods cannot be applied for a large number of samples, and the modern food industry requires analytical techniques that are rapid and can be used online of the production, such as spectroscopic techniques. Most of these techniques are characterized by fast and robust performance, low requirements for sample preparation, high throughput, and low cost [124,125]. The basic idea behind the application of spectroscopic methods to discriminate between fresh and frozen-thawed muscle foods is modifications in the optical characteristics (i.e., absorption and scattering) of the muscle tissue upon freezing, frozen storage, and thawing. The following section will present some examples that show the potential of different methods of fluorescence spectroscopy in this context.
Classical two-dimensional single-scan fluorescence spectroscopy was used to discriminate between fresh and frozen-thawed sea bass fillets based on the emission spectra of tryptophan, NADH, and riboflavin [85]. The position of the maximum peak of emission varied as a function of the storage conditions of the samples (fresh, chilled/frozen, or frozen/chilled). Canonical correlation analysis showed high correlations between fluorescence data and some physicochemical traditional measurements. Factorial discriminant analysis (a multivariate method that allows the testing of hypotheses) was performed to compare the discriminant ability of each fluorophore, and the results showed that 85.90% of the samples were correctly classified when using the fluorescence of tryptophan. Even more promising results were obtained using NADH and riboflavin (88.46%), while the best results were obtained when the factorial discriminant analysis was applied to the concatenated emission spectra of these fluorophores, giving a correct classification rate of about 95%. Laser-induced fluorescence is a variant of fluorescence spectroscopy where the usual lamp excitation is replaced by a laser source. This technique was applied using laser light at 266 nm for the excitation and scanning fluorescence between 400 and 800 nm to discriminate between fresh, chilled, and frozen-thawed chicken [126]. Three emission peaks were observed at 440, 535, and 700 nm and were attributed to the amino acids, collagen, and porphyrin, respectively. Laser-induced red fluorescence intensity was higher for fresh chicken samples compared to the frozen ones, indicating a decrease in the porphyrin levels during the frozen storage. On the contrary, the fluorescence intensity of collagen was higher in the frozen samples, confirming tenderness and texture cohesion changes during freezing and storage. PCA showed good discrimination between the three groups of samples, which was validated by measuring the optical absorption and scattering coefficients by an integrating sphere and modeled by a combination of the Beer–Lambert law and the Kubelka–Munk model. In another study, the fluorescence of the myofibrillar protein of largemouth bass (Micropterus salmoides) was used to investigate changes in protein oxidation and microstructure after 3 freezing and thawing cycles [121]. Emission spectra of tryptophan were obtained between 300–400 nm after excitation at 280 nm. The results showed a shift of the maximum emission of tryptophan (around 330 nm) to shorter wavelengths (blue shift) in the frozen samples compared to the fresh ones. In addition, a decrease in the fluorescence intensity was observed in the frozen samples due to the formation of ice crystals during the freezing process, leading to protein aggregation and tryptophan residue embedding. The authors demonstrated that the use of herring antifreeze protein minimizes the frozen-thawed damage and maintains the stability of the tertiary conformation of the proteins more effectively. However, it should be stressed that the fluorescence spectra in this study were obtained on the extracted proteins, which implies more time for the extraction step and the following measurements.
To evaluate the tertiary structure changes in proteins of pork patties with different fat addition under freeze-thaw cycles, two-dimensional classical fluorescence spectra were recorded in the wavelength range between 300–400 nm after excitation at 283 nm [123]. In this study, the maximum emission of tryptophan was shifted to longer wavelengths (red shift) after 5 freeze-thaw cycles. This red shift was explained by the exposure of tryptophan residues to the polar environment when subjecting the patties to multiple freeze-thaw cycles. Moreover, a significant decrease in fluorescence intensity was observed with increasing freeze-thaw cycles due to protein partial unfolding. In a previous study, protein structure changes in porcine longissimus muscle induced by multiple freeze-thaw cycles were evaluated by various methods, including fluorescence spectroscopy [127]. Again, a red shift in the maximum emission peak of tryptophan and a decrease in the fluorescence intensity were observed in the frozen-thawed samples compared with the fresh ones (control), which was explained by protein unfolding and a possible oxidation of tryptophan.
It should be stressed that the use of fluorescence spectroscopy in two-dimensional classical mode (at only one excitation wavelength) limits its potential since most of the food matrices contain various fluorophores that should be excited at different wavelengths. Therefore, the fluorescence spectroscopy in most of the previously discussed studies was used as a complementary tool. Interestingly, the use of fluorescence spectroscopy in the EEM mode has been accelerated in recent years in muscle foods and other food products. This technique allows overcoming the aforementioned limitation by operating over a broad wavelength range, thus enabling the detection of several fluorophores simultaneously [97,98,99]. For example, fluorescence spectra in EEM mode, recorded at the wavelength range of 250–800 nm for both the excitation and the emission were obtained on horse mackerel (Trachurus japonicus) to evaluate the freshness of frozen whole fish and fillets, and different traditional freshness indicators (K, K1, P, H, G, and log10G) were estimated on the same samples [96]. Various spectral pre-treatment schemes (including normalization, smoothing, and derivatives that were applied to reduce random noise and improve the measurement sensitivity) and multivariate analyses (partial least squares regression models built on original and pre-treated datasets) were investigated to find the most effective combinations of excitation–emission wavelengths that enable building robust multivariate calibration models. The developed algorithm showed that the excitation wavelength at 390 nm gives the highest prediction accuracy of the different freshness indicators. Similar results were obtained on the same fish species using the same fluorescence method [94,95,97,98]. Recently, EEM fluorescence spectra, collected in the wavelength range of 250–700 nm for both the excitation and the emission light were used to evaluate changes in post-mortem freshness of frozen shrimp [99]. In this study, excitation at the wavelength of 330 nm and emission wavelength range of 380–610 nm were found to be the most efficient to build partial least squares regression models of pH and K-value in frozen shrimp. Most interestingly, the selected excitation and emission wavelengths were used to visualize the spatial-temporal distribution of these freshness indices (i.e., pH and K-value) as influenced by the post-mortem ice storage period. The technique was also used to observe changes in protein of pork patties during frozen storage [122]. As for fish and other seafood, the spectra of meat showed also a decrease in the fluorescence intensity and a shift in the emission maximum in the frozen samples, compared to the fresh ones. The results were explained by protein aggregation, oxidation, and the exposure of the tryptophan residues and their polar environment caused by freezing impact.
When EEM spectra are collected, pre-treatment techniques, decomposition methods such as parallel factorial analysis (PARAFAC), methods for selecting the most effective feature-related excitation and emission wavelengths and other advanced statistical methods are necessary to extract information from such a multi-way complex dataset. The PARAFAC model is usually used to decompose the fluorescence data into several components, corresponding to the distinct fluorophores present in the samples. For example, PARAFAC algorithm was applied recently on EEM to establish a rapid and non-destructive approach for evaluation of beef freshness [105]. In this study, the PARAFAC modeling successfully decomposed the EEM spectra into three factors where the factor 1 primarily showed the peaks of amino acids and collagen, the factor 2 represented the conjugated Schiff base, whereas the factor 3 demonstrated the fluorescence of NADH and vitamins. In another study, sparse regression, which is a common approach used in various scientific settings as a feature selection technique, was employed on EEM spectra for selecting relevant excitation and emission fluorescence wavelengths [128]. The models were successfully tested for predicting the count of viable bacteria on the surface of porcine meat and predicting freshness of frozen fish.
Another challenge related to fluorescence spectroscopy, especially the EEM technique, is the lack of standard protocols for measurement conditions. A quick look at the literature reveals considerable variability in terms of spectra acquisition settings such as excitation and emission wavelength range, slit band pass and bandwidth, step increment, and scan speed and integration time. A typical EEM scans the sample across the excitation wavelengths from about 200–800 nm, and across the emission wavelengths from 250–800 nm. Over this wide excitation and emission wavelength range, many fluorophores (e.g., aromatic amino acids, NADH, nucleic acids, pigments, vitamins, collagen, and oxidation products) can be covered. The band pass slits on both the excitation and the emission side is an important parameter that directly affects the fluorescence intensity and refers to the physical slit size at the entrance and exit of a monochromator of a fluorometer instrument. The step increment represents spacing between two adjacent measurement points, while the integration time determines the measurement speed. All the aforementioned parameter should be selected carefully in order to optimize the signal-to-noise ratio.
4. Challenges and Future Trends
Differentiation between fresh and frozen-thawed food products is a topic of great interest, not only to food researchers but also to consumers, food technologists, and governmental authorities. A wide range of varieties of conventional and unconventional techniques has been investigated for this purpose with varying degrees of success. Although fluorescence spectroscopy has shown promising potential to tackle the problem of fraud related to frozen-thawed food products sold as fresh, some challenges are still unresolved and need to be addressed in order to reach the implantation stage of this technique in the food industry.
First, important amounts of data are usually generated from fluorescence spectroscopy measurements that must be efficiently preprocessed and transformed in order to extract the maximum useful information from these complex data. Thus, the development of new fluorescence analytical method, or more generally a new spectroscopic technique, should synchronize with the advance in data preprocessing and processing strategies. Recently, a new generation of chemometric tools based on artificial intelligence, particularly machine learning techniques, multi-block data analysis, and data modeling approaches have emerged and gained significant emphasis.
Second, it should be highlighted that the effect of various experimental conditions used in the published studies must be taken into consideration. This huge variability either related to instrumentation (acquisition mode, integration time, etc.) or to the scanned samples (different sizes and shapes of samples) makes a direct comparison of the different published results quite challenging. Therefore, future studies should focus on the standardization of measurement settings, guidelines, instruments, calibration procedures, and data analysis. Third, the findings of the review reveal the widespread application of hyperspectral imaging with the traditional spectroscopic techniques, but limited attention has been paid to the hyperspectral fluorescence imaging technique. If calibrations and corrections are quite straightforward and often performed automatically in lab commercial spectrofluorimeters, these procedures become more complex under distant probing conditions (as in the case of hyperspectral fluorescence imaging), which might explain the scarcity of hyperspectral fluorescence imaging studies. Extensive and widespread use of this technique in practical applications requires much more research and development as well as and continuous innovations. Another interesting avenue for future research is to study the possibility of transferring such technology to novel applications by developing different miniaturized and robotic analytical techniques, smart sensors, and autonomous analytical platforms.
Finally, to enhance the analytical power of spectroscopic techniques, close collaboration between the different actors involved in this field, including particularly food industry companies, data analysts, spectroscopists, robotics and sensors developers, and regulatory authorities, will provide interesting perspectives on further opportunities of these techniques.
5. Conclusions
Unceasing efforts have been made to establish a reliable method of detection of frozen-thawed food products. In this review, we have shed some light on the recent progress made in this context. Fluorescence spectroscopy seems to be a powerful tool to achieve this goal. In particular, in the last years, the development of hyperspectral imaging has given a new impulse, not only to fluorescence spectroscopy but also to other spectroscopic techniques. However, additional research efforts are still required to overcome the few limitations of fluorescence spectroscopy, develop more robust analytical procedures, and find new applications in the food industry. It is expected that more collaboration between different disciplines will take place in the coming years, leading to the rapid acquisition of data and real-time monitoring, the emergence of more advanced and flexible fluorometers, and miniaturization of instrumentation for portable use.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Ojha, S.; Tiwari, B.; Rustad, T.; Nilsen, H.; Heia, K.; Cozzolino, D.; El-Din Bekhit, A.; Biancolillo, A.; Wold, J.P. Monitoring thermal and non-thermal treatments during processing of muscle foods: A comprehensive review of recent technological advances. Appl. Sci. 2020, 10, 6802. [Google Scholar] [CrossRef]
- Meijer, G.W.; Lähteenmäki, L.; Stadler, R.H.; Weiss, J. Issues surrounding consumer trust and acceptance of existing and emerging food processing technologies. Crit. Rev. Food Sci. Nutr. 2021, 61, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lan, R.; Zhang, B.; Erdogdu, F.; Wang, S. A comprehensive review on recent developments of radio frequency treatment for pasteurizing agricultural products. Crit. Rev. Food Sci. Nutr. 2020, 61, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and applications of non-thermal technologies and alternative chemical compounds in meat and fish. Crit. Rev. Food Sci. Nutr. 2021, 61, 1163–1183. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Oliveira, M.; Burgess, C.M.; Cropotova, J.; Rustad, T.; Sun, D.-W.; Tiwari, B.K. Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. LWT 2021, 150, 111957. [Google Scholar] [CrossRef]
- Egelandsdal, B.; Abie, S.M.; Bjarnadottir, S.; Zhu, H.; Kolstad, H.; Bjerke, F.; Martinsen, Ø.G.; Mason, A.; Münch, D. Detectability of the degree of freeze damage in meat depends on analytic-tool selection. Meat Sci. 2019, 152, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Y.; Sun, D.W. Biomimetic modification of freezing facility surfaces to prevent icing and frosting during freezing for the food industry. Trends Food Sci. Technol. 2021, 111, 581–594. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Zhang, M.; Adhikari, B.; Sun, J. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3620–3631. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Zhan, X.; Sun, D.W.; Zhu, Z.; Wang, Q.J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2925–2938. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Wang, H.; Xia, X.; Kong, B. Ultrasound-assisted immersion freezing reduces the structure and gel property deterioration of myofibrillar protein from chicken breast. Ultrason. Sonochem. 2020, 67, 105137. [Google Scholar] [CrossRef]
- James, C.; Purnell, G.; James, S.J. A Review of Novel and Innovative Food Freezing Technologies. Food Bioprocess Technol. 2015, 8, 1616–1634. [Google Scholar] [CrossRef]
- Ghidini, S.; Varrà, M.O.; Zanardi, E. Approaching Authenticity Issues in Fish and Seafood Products by Qualitative Spectroscopy and Chemometrics. Molecules 2019, 24, 1812. [Google Scholar] [CrossRef] [Green Version]
- Hassoun, A.; Måge, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.-Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in Animal Origin Food Products: Advances in Emerging Spectroscopic Detection Methods over the Past Five Years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef]
- Hassoun, A.; Shumilina, E.; Di Donato, F.; Foschi, M.; Simal-Gandara, J.; Biancolillo, A. Emerging techniques for differentiation of fresh and frozen-thawed seafoods: Highlighting the potential of spectroscopic techniques. Molecules 2020, 25, 4472. [Google Scholar] [CrossRef]
- Strateva, M.; Penchev, G.; Stratev, D. Histological, Physicochemical and Microbiological Changes in the Carp (Cyprinus carpio) Muscles after Freezing. J. Aquat. Food Prod. Technol. 2021, 30, 324–338. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Pavlovic, R.; Nobile, M.; Di Cesare, F.; Malandra, R.; Pessina, D.; Panseri, S. Discrimination between fresh and frozen-thawed fish involved in food safety and fraud protection. Foods 2020, 9, 1896. [Google Scholar] [CrossRef] [PubMed]
- Pezzolato, M.; Baioni, E.; Maurella, C.; Varello, K.; Meistro, S.; Balsano, A.; Bozzetta, E. Distinguishing between fresh and frozen-thawed smoked salmon: Histology to detect food adulteration in high-value products. J. Food Prot. 2020, 83, 52–55. [Google Scholar] [CrossRef]
- Akbarabadi, M.; Mohsenzadeh, M.; Housaindokht, M.R. Ribose-induced maillard reaction as an analytical method for detection of adulteration and differentiation of chilled and frozen-thawed minced veal. Food Sci. Anim. Resour. 2020, 40, 350–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinacci, L.; Armani, A.; Guidi, A.; Nucera, D.; Shvartzman, D.; Miragliotta, V.; Coli, A.; Giannessi, E.; Stornelli, M.R.; Fronte, B.; et al. Histological discrimination of fresh and frozen/thawed fish meat: European hake (Merluccius merluccius) as a possible model for white meat fish species. Food Control 2018, 92, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Škorpilová, T.; Šístková, I.; Adamcová, M.; Pohůnek, V.; Kružík, V.; Ševčík, R. Measuring citrate synthase activity as an enzymatic approach to the differentiation of chilled and frozen/thawed meat. Meat Sci. 2019, 158, 107856. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Jin, Z.; Cheng, Q.; Qian, M.; Zhu, B.; Dong, X. Sensory evaluation of fresh/frozen mackerel products: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3504–3530. [Google Scholar] [CrossRef]
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. Quality Index Method for fish quality control: Understanding the applications, the appointed limits and the upcoming trends. Trends Food Sci. Technol. 2021, 111, 333–345. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.-W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Valdés, A.; Beltrán, A.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Analytical methods combined with multivariate analysis for authentication of animal and vegetable food products with high fat content. Trends Food Sci. Technol. 2018, 77, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Franceschelli, L.; Berardinelli, A.; Dabbou, S.; Ragni, L.; Tartagni, M. Sensing Technology for Fish Freshness and Safety: A Review. Sensors 2021, 21, 1373. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Parrish, C.A.; Adedeji, A. Emerging nondestructive approaches for meat quality and safety evaluation—A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3438–3463. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.; Stella, R.; Negro, A.; Bragolusi, M.; Miano, B.; Arcangeli, G.; Biancotto, G.; Piro, R.; Tata, A. New strategies for the differentiation of fresh and frozen/thawed fish: A rapid and accurate non-targeted method by ambient mass spectrometry and data fusion (part A). Food Control 2021, 130, 108364. [Google Scholar] [CrossRef]
- Chung, W.Y.; Le, G.T.; Tran, T.V.; Nguyen, N.H. Novel proximal fish freshness monitoring using batteryless smart sensor tag. Sens. Actuators B Chem. 2017, 248, 910–916. [Google Scholar] [CrossRef]
- Ma, Q.; Lu, X.; Wang, W.; Hubbe, M.A.; Liu, Y.; Mu, J.; Wang, J.; Sun, J.; Rojas, O.J. Recent developments in colorimetric and optical indicators stimulated by volatile base nitrogen to monitor seafood freshness. Food Packag. Shelf Life 2021, 28, 100634. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bertani, F.R.; Mencattini, A.; Gerardino, A.; Martinelli, E.; Weesepoel, Y.; van Ruth, S. The spectral treasure house of miniaturized instruments for food safety, quality and authenticity applications: A perspective. Trends Food Sci. Technol. 2021, 110, 841–848. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Jha, P.K.; Tavakoli, J.; Daraei-Garmakhany, A.; Xanthakis, E.; Le-Bail, A. Review on identification, underlying mechanisms and evaluation of freezing damage. J. Food Eng. 2019, 255, 50–60. [Google Scholar] [CrossRef]
- Mirzaee-Ghaleh, E.; Taheri-Garavand, A.; Ayari, F.; Lozano, J. Identification of Fresh-Chilled and Frozen-Thawed Chicken Meat and Estimation of their Shelf Life Using an E-Nose Machine Coupled Fuzzy KNN. Food Anal. Methods 2020, 13, 678–689. [Google Scholar] [CrossRef]
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food Bioprocess Technol. 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Hassoun, A.; Heia, K.; Lindberg, S.; Nilsen, H. Spectroscopic Techniques for Monitoring Thermal Treatments in Fish and Other Seafood: A Review of Recent Developments and Applications. Foods 2020, 6, 767. [Google Scholar] [CrossRef]
- Hassoun, A.; Gudjónsdóttir, M.; Prieto, M.A.; Garcia-Oliveira, P.; Simal-Gandara, J.; Marini, F.; Di Donato, F.; D’Archivio, A.A.; Biancolillo, A. Application of novel techniques for monitoring quality changes in meat and fish products during traditional processing processes: Reconciling novelty and tradition. Processes 2020, 8, 988. [Google Scholar] [CrossRef]
- Pu, H.; Sun, D.W.; Ma, J.; Liu, D.; Cheng, J. hu Using Wavelet Textural Features of Visible and Near Infrared Hyperspectral Image to Differentiate Between Fresh and Frozen–Thawed Pork. Food Bioprocess Technol. 2014, 7, 3088–3099. [Google Scholar] [CrossRef]
- Pennisi, F.; Giraudo, A.; Cavallini, N.; Esposito, G.; Merlo, G.; Geobaldo, F.; Acutis, P.L.; Pezzolato, M.; Savorani, F.; Bozzetta, E. Differentiation between Fresh and Thawed Cephalopods Using NIR Spectroscopy and Multivariate Data Analysis. Foods 2021, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Nevado, J.M.; Garrido-Varo, A.; De Pedro-Sanz, E.; Tejerina-Barrado, D.; Pérez-Marín, D.C. Non-destructive Near Infrared Spectroscopy for the labelling of frozen Iberian pork loins. Meat Sci. 2021, 175, 108440. [Google Scholar] [CrossRef] [PubMed]
- Fengou, L.-C.; Lianou, A.; Tsakanikas, P.; Mohareb, F.; Nychas, G.-J.E. Detection of Meat Adulteration Using Spectroscopy-Based Sensors. Foods 2021, 10, 861. [Google Scholar] [CrossRef]
- Atanassova, S.; Stoyanchev, T.; Yorgov, D.; Nachev, V. Differentiation of fresh and frozen-thawed poultry breast meat by near infrared spectroscopy. Bulg. J. Agric. Sci. 2018, 24, 162–168. [Google Scholar]
- Wang, W.L.; Chen, W.H.; Tian, H.Y.; Liu, Y. Detection of Frozen-Thawed Cycles for Frozen Tilapia (Oreochromis) Fillets Using Near Infrared Spectroscopy. J. Aquat. Food Prod. Technol. 2018, 27, 609–618. [Google Scholar] [CrossRef]
- Reis, M.M.; Martínez, E.; Saitua, E.; Rodríguez, R.; Pérez, I.; Olabarrieta, I. Non-invasive differentiation between fresh and frozen/thawed tuna fillets using near infrared spectroscopy (Vis-NIRS). LWT Food Sci. Technol. 2017, 78, 129–137. [Google Scholar] [CrossRef]
- Reis, M. Near infrared spectroscopy (Vis-NIRS) applied to differentiation between chilled and frozen/thawed meat. NIR News 2017, 28, 10–15. [Google Scholar] [CrossRef]
- Barbin, D.F.; Sun, D.W.; Su, C. NIR hyperspectral imaging as non-destructive evaluation tool for the recognition of fresh and frozen-thawed porcine longissimus dorsi muscles. Innov. Food Sci. Emerg. Technol. 2013, 18, 226–236. [Google Scholar] [CrossRef]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and Quality Assessment of Meat Products by Fourier-Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2020, 13, 66–91. [Google Scholar] [CrossRef]
- Zhao, M.; Downey, G.; O’Donnell, C.P. Detection of adulteration in fresh and frozen beefburger products by beef offal using mid-infrared ATR spectroscopy and multivariate data analysis. Meat Sci. 2014, 96, 1003–1011. [Google Scholar] [CrossRef]
- Grunert, T.; Stephan, R.; Ehling-Schulz, M.; Johler, S. Fourier Transform Infrared Spectroscopy enables rapid differentiation of fresh and frozen/thawed chicken. Food Control 2016, 60, 361–364. [Google Scholar] [CrossRef]
- Velioğlu, H.M.; Temiz, H.T.; Boyaci, I.H. Differentiation of fresh and frozen-thawed fish samples using Raman spectroscopy coupled with chemometric analysis. Food Chem. 2015, 172, 283–290. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Sun, D.W. Raman spectroscopic techniques for detecting structure and quality of frozen foods: Principles and applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–17. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Pu, H.-B.; Chen, X.; Liu, Y.; Zhang, H.; Li, J.-L. Integration of classifiers analysis and hyperspectral imaging for rapid discrimination of fresh from cold-stored and frozen-thawed fish fillets. J. Food Eng. 2015, 161, 33–39. [Google Scholar] [CrossRef]
- Shan, J.; Wang, X.; Russel, M.; Zhao, J.; Zhang, Y. Comparisons of Fish Morphology for Fresh and Frozen-Thawed Crucian Carp Quality Assessment by Hyperspectral Imaging Technology. Food Anal. Methods 2018, 11, 1701–1710. [Google Scholar] [CrossRef]
- Retz, S.; Porley, V.E.; von Gersdorff, G.; Hensel, O.; Crichton, S.; Sturm, B. Effect of maturation and freezing on quality and drying kinetics of beef. Dry. Technol. 2017, 35, 2002–2014. [Google Scholar] [CrossRef]
- Anderssen, K.E.; Stormo, S.K.; Skåra, T.; Skjelvareid, M.H.; Heia, K. Predicting liquid loss of frozen and thawed cod from hyperspectral imaging. LWT 2020, 133, 110093. [Google Scholar] [CrossRef]
- Guo, W.; Li, X.; Xie, T. Method and system for nondestructive detection of freshness in Penaeus vannamei based on hyperspectral technology. Aquaculture 2021, 538, 736512. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Zhang, H.; Nie, P. Hyperspectral imaging (HSI) technology for the non-destructive freshness assessment of pearl gentian grouper under different storage conditions. Sensors 2021, 21, 583. [Google Scholar] [CrossRef]
- Özdoğan, G.; Lin, X.; Sun, D.W. Rapid and noninvasive sensory analyses of food products by hyperspectral imaging: Recent application developments. Trends Food Sci. Technol. 2021, 111, 151–165. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, A.; Shi, P.; Cai, L. Rapid evaluation of freshness of largemouth bass under different thawing methods using hyperspectral imaging. Food Control 2021, 125, 108023. [Google Scholar] [CrossRef]
- Antequera, T.; Caballero, D.; Grassi, S.; Uttaro, B.; Perez-Palacios, T. Evaluation of fresh meat quality by Hyperspectral Imaging (HSI), Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI): A review. Meat Sci. 2021, 172, 108340. [Google Scholar] [CrossRef]
- Saha, D.; Manickavasagan, A. Machine learning techniques for analysis of hyperspectral images to determine quality of food products: A review. Curr. Res. Food Sci. 2021, 4, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pu, H.; Sun, D.W. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Feng, C.-H.; Makino, Y.; Yoshimura, M.; Rodríguez-Pulido, F.J. Estimation of adenosine triphosphate content in ready-to-eat sausages with different storage days, using hyperspectral imaging coupled with R statistics. Food Chem. 2018, 264, 419–426. [Google Scholar] [CrossRef]
- Hassoun, A.; Sahar, A.; Lakhal, L.; Aït-Kaddour, A. Fluorescence spectroscopy as a rapid and non-destructive method for monitoring quality and authenticity of fish and meat products: Impact of different preservation conditions. LWT 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Sahar, A.; Hitzmann, B. Fluorescence Spectroscopy for the Monitoring of Food Processes. Adv. Biochem. Eng. Biotechnol. 2017, 161, 121–152. [Google Scholar] [CrossRef] [PubMed]
- Faassen, S.M.; Hitzmann, B. Fluorescence spectroscopy and chemometric modeling for bioprocess monitoring. Sensors 2015, 15, 10271–10291. [Google Scholar] [CrossRef] [Green Version]
- Samokhvalov, A. Analysis of various solid samples by synchronous fluorescence spectroscopy and related methods: A review. Talanta 2020, 216, 120944. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Fluorescence spectroscopy and imaging instruments for food quality evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 491–533. ISBN 9780128142189. [Google Scholar]
- Kumar, K.; Tarai, M.; Mishra, A.K. Unconventional steady-state fluorescence spectroscopy as an analytical technique for analyses of complex-multifluorophoric mixtures. TrAC Trends Anal. Chem. 2017, 97, 216–243. [Google Scholar] [CrossRef]
- Colaruotolo, L.A.; Peters, E.; Corradini, M.G. Novel luminescent techniques in aid of food quality, product development, and food processing. Curr. Opin. Food Sci. 2021, 42, 148–156. [Google Scholar] [CrossRef]
- Esteki, M.; Shahsavari, Z.; Simal-Gandara, J. Use of spectroscopic methods in combination with linear discriminant analysis for authentication of food products. Food Control 2018, 91, 100–112. [Google Scholar] [CrossRef]
- Croce, A.C. Light and Autofluorescence, Multitasking Features in Living Organisms. Photochem 2021, 1, 7. [Google Scholar] [CrossRef]
- Mousdis, G.; Mellou, F. Vibrational and Fluorescence Spectroscopy. Food Authentication 2017, 298–324. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.S.; Lee, W.-H.; Cho, B.-K. Determination of the total volatile basic nitrogen (TVB-N) content in pork meat using hyperspectral fluorescence imaging. Sens. Actuators B Chem. 2018, 259, 532–539. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, W.; Cao, A.; Cao, M.; Li, J. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (red drum). Ultrason. Sonochem. 2019, 55, 96–104. [Google Scholar] [CrossRef]
- Karoui, R.; Hassoun, A.; Ethuin, P. Front face fluorescence spectroscopy enables rapid differentiation of fresh and frozen-thawed sea bass (Dicentrarchus labrax) fillets. J. Food Eng. 2017, 202, 89–98. [Google Scholar] [CrossRef]
- Tan, M.; Xie, J. Exploring the effect of dehydration on water migrating property and protein changes of large yellow croaker (Pseudosciaena crocea) during frozen storage. Foods 2021, 10, 784. [Google Scholar] [CrossRef]
- Durek, J.; Bolling, J.S.; Knorr, D.; Schwägele, F.; Schlüter, O. Effects of different storage conditions on quality related porphyrin fluorescence signatures of pork slices. Meat Sci. 2012, 90, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Heia, K.; Lindberg, S.-K.; Nilsen, H. Performance of Fluorescence and Diffuse Reflectance Hyperspectral Imaging for Characterization of Lutefisk: A Traditional Norwegian Fish Dish. Molecules 2020, 25, 1191. [Google Scholar] [CrossRef] [Green Version]
- Airado-Rodríguez, D.; Skaret, J.; Wold, J.P. Assessment of the quality attributes of cod caviar paste by means of front-face fluorescence spectroscopy. J. Agric. Food Chem. 2010, 58, 5276–5285. [Google Scholar] [CrossRef] [PubMed]
- Airado-Rodríguez, D.; Høy, M.; Skaret, J.; Wold, J.P. From multispectral imaging of autofluorescence to chemical and sensory images of lipid oxidation in cod caviar paste. Talanta 2014, 122, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ertbjerg, P. On the origin of thaw loss: Relationship between freezing rate and protein denaturation. Food Chem. 2019, 299, 125104. [Google Scholar] [CrossRef]
- Nian, L.; Cao, A.; Cai, L.; Ji, H.; Liu, S. Effect of vacuum impregnation of red sea bream (Pagrosomus major) with herring AFP combined with CS@Fe3O4 nanoparticles during freeze-thaw cycles. Food Chem. 2019, 291, 139–148. [Google Scholar] [CrossRef]
- Qin, J.; Vasefi, F.; Hellberg, R.S.; Akhbardeh, A.; Isaacs, R.B.; Yilmaz, A.G.; Hwang, C.; Baek, I.; Schmidt, W.F.; Kim, M.S. Detection of fish fillet substitution and mislabeling using multimode hyperspectral imaging techniques. Food Control 2020, 114, 107234. [Google Scholar] [CrossRef]
- Elmasry, G.; Nagai, H.; Moria, K.; Nakazawa, N.; Tsuta, M.; Sugiyama, J.; Okazaki, E.; Nakauchi, S. Freshness estimation of intact frozen fish using fluorescence spectroscopy and chemometrics of excitation-emission matrix. Talanta 2015, 143, 145–156. [Google Scholar] [CrossRef]
- Shibata, M.; ElMasry, G.; Moriya, K.; Rahman, M.M.; Miyamoto, Y.; Ito, K.; Nakazawa, N.; Nakauchi, S.; Okazaki, E. Smart technique for accurate monitoring of ATP content in frozen fish fillets using fluorescence fingerprint. LWT 2018, 92, 258–264. [Google Scholar] [CrossRef]
- ElMasry, G.; Nakazawa, N.; Okazaki, E.; Nakauchi, S. Non-invasive sensing of freshness indices of frozen fish and fillets using pretreated excitation-emission matrices. Sens. Actuators B Chem. 2016, 228, 237–250. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shibata, M.; ElMasry, G.; Nakazawa, N.; Nakauchi, S.; Hagiwara, T.; Osako, K.; Okazaki, E. Expeditious prediction of post-mortem changes in frozen fish meat using three-dimensional fluorescence fingerprints. Biosci. Biotechnol. Biochem. 2019, 83, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.V.; Rahman, M.M.; Nakazawa, N.; Okazaki, E.; Nakauchi, S. Visualize the quality of frozen fish using fluorescence imaging aided with excitation-emission matrix. Opt. Express 2018, 26, 22954–22964. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bui, M.V.; Shibata, M.; Nakazawa, N.; Rithu, M.N.A.; Yamashita, H.; Sadayasu, K.; Tsuchiyama, K.; Nakauchi, S.; Hagiwara, T.; et al. Rapid noninvasive monitoring of freshness variation in frozen shrimp using multidimensional fluorescence imaging coupled with chemometrics. Talanta 2021, 224, 121871. [Google Scholar] [CrossRef]
- Omwange, K.A.; Al Riza, D.F.; Sen, N.; Shiigi, T.; Kuramoto, M.; Ogawa, Y.; Kondo, N.; Suzuki, T. Fish freshness monitoring using UV-fluorescence imaging on Japanese dace (Tribolodon hakonensis) fisheye. J. Food Eng. 2020, 287, 110111. [Google Scholar] [CrossRef]
- Liao, Q.; Suzuki, T.; Shirataki, Y.; Kuramoto, M.; Kondo, N. Freshness related fluorescent compound changes in Japanese dace fish (Tribolodon hakonensis) eye fluid during storage. Eng. Agric. Environ. Food 2018, 11, 95–100. [Google Scholar] [CrossRef]
- Risso, S.J.; Crovetto, C.; Ávila, A.; Gutiérrez, M.I. Fluorescence of Extracts from Argentine Hake (Merluccius hubbsi) Muscle and Its Correlation with Quality Indices During Chilled Storage. J. Aquat. Food Prod. Technol. 2017, 26, 215–223. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sugiyama, J.; Tsuta, M.; Fujita, K.; Shibata, M.; Kokawa, M.; Oshita, S.; Oto, N. Prediction of Aerobic Plate Count on Beef Surface Using Fluorescence Fingerprint. Food Bioprocess Technol. 2014, 7, 1496–1504. [Google Scholar] [CrossRef]
- Mita Mala, D.; Yoshimura, M.; Kawasaki, S.; Tsuta, M.; Kokawa, M.; Trivittayasil, V.; Sugiyama, J.; Kitamura, Y. Fiber optics fluorescence fingerprint measurement for aerobic plate count prediction on sliced beef surface. LWT Food Sci. Technol. 2016, 68, 14–20. [Google Scholar] [CrossRef]
- Liu, H.; Saito, Y.; Riza, D.F.; Kondo, N.; Yang, X.; Han, D. Rapid evaluation of quality deterioration and freshness of beef during low temperature storage using three-dimensional fluorescence spectroscopy. Food Chem. 2019, 287, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Oto, N.; Oshita, S.; Makino, Y.; Kawagoe, Y.; Sugiyama, J.; Yoshimura, M. Non-destructive evaluation of ATP content and plate count on pork meat surface by fluorescence spectroscopy. Meat Sci. 2013, 93, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ji, Z.; Liu, X.; Shi, C.; Yang, X. Non-destructive determination of chemical and microbial spoilage indicators of beef for freshness evaluation using front-face synchronous fluorescence spectroscopy. Food Chem. 2020, 321, 126628. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Fukasawa, A.; Hirano, M.; Ide, T. Low-light photodetectors for fluorescence microscopy. Appl. Sci. 2021, 11, 2773. [Google Scholar] [CrossRef]
- Tan, J.Y.; Ker, P.J.; Lau, K.Y.; Hannan, M.A.; Tang, S.G.H. Applications of Photonics in Agriculture Sector: A Review. Molecules 2019, 24, 2025. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Sun, D.-W.; Pu, H.; Cheng, J.-H.; Wei, Q. Advanced Techniques for Hyperspectral Imaging in the Food Industry: Principles and Recent Applications. Annu. Rev. Food Sci. Technol. 2019, 10, 197–220. [Google Scholar] [CrossRef]
- Reis, M.M.; Van Beers, R.; Al-Sarayreh, M.; Shorten, P.; Yan, W.Q.; Saeys, W.; Klette, R.; Craigie, C. Chemometrics and hyperspectral imaging applied to assessment of chemical, textural and structural characteristics of meat. Meat Sci. 2018, 144, 100–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.-J.E. Data mining derived from food analyses using non-invasive/non-destructive analytical techniques; determination of food authenticity, quality & safety in tandem with computer science disciplines. Trends Food Sci. Technol. 2016, 50, 11–25. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.J.E. Rapid detection of frozen-then-thawed minced beef using multispectral imaging and Fourier transform infrared spectroscopy. Meat Sci. 2018, 135, 142–147. [Google Scholar] [CrossRef]
- Silva, S.; Guedes, C.; Rodrigues, S.; Teixeira, A. Non-destructive imaging and spectroscopic techniques for assessment of carcass and meat quality in sheep and goats: A review. Foods 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, J. A Review of Hyperspectral Imaging for Chicken Meat Safety and Quality Evaluation: Application, Hardware, and Software. Compr. Rev. Food Sci. Food Saf. 2019, 18, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Guglielmetti, C.; Manfredi, M.; Brusadore, S.; Sciuto, S.; Esposito, G.; Ubaldi, P.G.; Magnani, L.; Gili, S.; Marengo, E.; Acutis, P.L.; et al. Two-dimensional gel and shotgun proteomics approaches to distinguish fresh and frozen-thawed curled octopus (Eledone cirrhosa). J. Proteom. 2018, 186, 1–7. [Google Scholar] [CrossRef]
- Ethuin, P.; Marlard, S.; Delosière, M.; Carapito, C.; Delalande, F.; Van Dorsselaer, A.; Dehaut, A.; Lencel, V.; Duflos, G.; Grard, T. Differentiation between fresh and frozen-thawed sea bass (Dicentrarchus labrax) fillets using two-dimensional gel electrophoresis. Food Chem. 2015, 176, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Marlard, S.; Doyen, P.; Grard, T. Rapid Multiparameters Approach to Differentiate Fresh Skinless Sea Bass (Dicentrarchus labrax) Fillets from Frozen-Thawed Ones. J. Aquat. Food Prod. Technol. 2019, 28, 253–262. [Google Scholar] [CrossRef]
- Šimoniová, A.; Rohlík, B.A.; Škorpilová, T.; Petrová, M.; Pipek, P. Differentiation between fresh and thawed chicken meats. Czech J. Food Sci. 2013, 31, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.B.; Zhou, G.H. Effect of multiple freeze-thaw cycles on the quality of chicken breast meat. Food Chem. 2015, 173, 808–814. [Google Scholar] [CrossRef]
- Nian, L.; Cao, A.; Cai, L. Investigation of the antifreeze mechanism and effect on quality characteristics of largemouth bass (Micropterus salmoides) during F-T cycles by hAFP. Food Chem. 2020, 325, 126918. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Wang, B.; Pan, N.; Xia, X. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef]
- Pan, N.; Hu, Y.; Li, Y.; Ren, Y.; Kong, B.; Xia, X. Changes in the thermal stability and structure of myofibrillar protein from quick-frozen pork patties with different fat addition under freeze-thaw cycles. Meat Sci. 2021, 175, 108420. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Li, H.; Shen, J.; Wu, Y. Nontargeted Detection Methods for Food Safety and Integrity. Annu. Rev. Food Sci. Technol. 2019, 10, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Abdel-Salam, Z.; Abdel-Harith, M. Discrimination between fresh, chilled, and frozen/ thawed chicken based on its skin’s spectrochemical and optical properties. Anal. Methods 2020, 12, 2093–2101. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Higashi, H.; ElMasry, G.M.; Nakauchi, S. Sparse regression for selecting fluorescence wavelengths for accurate prediction of food properties. Chemom. Intell. Lab. Syst. 2016, 154, 29–37. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).