Light and Autofluorescence, Multitasking Features in Living Organisms
Abstract
:1. Early History
2. Plants and Algae
2.1. Pigments Involved in Light Harvesting and Photosynthesis
2.1.1. Chlorophyll Photophysical Properties
2.1.2. Chlorophyll as Photophysical Biomarker
2.1.3. Chlorophyll and Algae
2.2. Pigments Not Involved in Photosynthesis
2.2.1. Phenols and Polyphenols
2.2.2. Betalains
2.2.3. Additional Polyphenols
Catechins
Ferulate
Coumarins
2.2.4. Lignins
2.2.5. Other Photoactive Phytocompounds
3. Bacteria
4. Fungi
5. Autofluorescence in Animals
5.1. Arthropods
5.2. Fishes
5.3. Reptiles and Amphibians
5.4. Birds
5.5. Mammalians
6. Bioluminescence
7. Normal and Altered Cell and Tissues
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Acuña, A.U.; Amat-Guerri, F. Early History of Solution Fluorescence: The Lignum nephriticum of Nicolás Monardes. In Fluorescence of Supermolecules, Polymers, and Nanosystems; Berberan-Santos, M.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–20. [Google Scholar]
- Stokes, G.G. On the Change of Refrangibility of Light. Philos. Trans. R. Soc. Lond. 1852, 142, 463–562. [Google Scholar] [CrossRef]
- Kasten, F.H. Cell Structure and Function by Microspectrofluorometry; Elsevier: New York, NY, USA, 1989; pp. 3–50. ISBN 9780124177604. [Google Scholar]
- Klein, G.; Linser, H. Fluoreszenzanalytische Untersuchungen an Pflanzen. Osterr. Bot. Z. 1930, 79, 125–163. [Google Scholar] [CrossRef]
- Larcher, W. Schnellmethode Zur Unterscheidung Lebender von Toten Zellen Mit Hilfe Der Eigenfluoreszenz Pflanzlicher Zellsäfte. Mikroskopic 1953, 8, 299–304. [Google Scholar]
- Oppenheimer, H.R.; Jacoby, B. Usefulness of Autofluorescence Tests as Criterion of Life in Plant Tissues. Protoplasma 1961, 53, 220–226. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Frederich, N.; Nysten, B.; Muls, B.; Hofkens, J.; Habib Jiwan, J.L.; Jonas, A.M. Nano-Patterned Layers of a Grafted Coumarinic Chromophore. Photochem. Photobiol. Sci. 2008, 7, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N. Standards in Fluorescence Spectrometry: Ultraviolet Spectrometry Group; Chapman and Hall: London, UK, 1981; ISBN 9400959028. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ni, Z.; Lu, Q.; Huo, H.; Zhang, H. Estimation of Chlorophyll Fluorescence at Different Scales: A Review. Sensors 2019, 19, 3000. [Google Scholar] [CrossRef] [Green Version]
- Croce, A.C.; Santamaria, G.; de Simone, U.; Lucchini, F.; Freitas, I.; Bottiroli, G. Naturally-Occurring Porphyrins in a Spontaneous-Tumour Bearing Mouse Model. Photochem. Photobiol. Sci. 2011, 10, 1189–1195. [Google Scholar] [CrossRef]
- Lee, T.C.; Shih, T.H.; Huang, M.Y.; Lin, K.H.; Huang, W.D.; Yang, C.M. Eliminating Interference by Anthocyanins When Determining the Porphyrin Ratio of Red Plant Leaves. J. Photochem. Photobiol. B Biol. 2018, 187, 106–112. [Google Scholar] [CrossRef]
- Lightner, D.A. The Porphyrins; Academic Press: Cambridge, MA, USA, 1979; Volume 6, ISBN 9788578110796. [Google Scholar]
- Fălămas, A.; Porav, S.A.; Tosa, V. Investigations of the Energy Transfer in the Phycobilisome Antenna of Arthrospira Platensis Using Femtosecond Spectroscopy. Appl. Sci. 2020, 10, 4045. [Google Scholar] [CrossRef]
- Biswas, A.; Boutaghou, M.N.; Alvey, R.M.; Kronfel, C.M.; Cole, R.B.; Bryant, D.A.; Schluchter, W.M. Characterization of the Activities of the CpeY, CpeZ, and CpeS Bilin Lyases in Phycoerythrin Biosynthesis in Fremyella Diplosiphon Strain UTEX 481. J. Biol. Chem. 2011, 286, 35509–35521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, A.C.; Bottiroli, G. Autofluorescence Spectroscopy and Imaging: A Tool for Biomedical Research and Diagnosis. Eur. J. Histochem. 2014, 58, 320–337. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Tanaka, A. Chlorophyll Cycle Regulates the Construction and Destruction of the Light-Harvesting Complexes. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 968–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Vegetation Stress Detection through Chlorophyll a + b Estimation and Fluorescence Effects on Hyperspectral Imagery. J. Environ. Qual. 2002, 31, 1433–1441. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Lagorio, M.G.; Cordon, G.B.; Iriel, A. Reviewing the Relevance of Fluorescence in Biological Systems. Photochem. Photobiol. Sci. 2015, 14, 1538–1559. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Tran, L.H.; Jung, S. Perturbations in the Photosynthetic Pigment Status Result in Photooxidation-Induced Crosstalk between Carotenoid and Porphyrin Biosynthetic Pathways. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Gillbro, T.; Cogdell, R.J. Carotenoid Fluorescence. Chem. Phys. Lett. 1989, 158, 312–316. [Google Scholar] [CrossRef]
- Chen, C.; Gong, N.; Li, Z.; Sun, C.; Men, Z. Concentration Effect on Quenching of Chlorophyll a Fluorescence by All-Trans-β-Carotene in Photosynthesis. Molecules 2017, 22, 1585. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A. Non-Invasive Quantification of Foliar Pigments: Possibilities and Limitations of Reflectance- and Absorbance-Based Approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ospina Calvo, B.; Lagorio, M.G. Quantitative Effects of Pigmentation on the Re-Absorption of Chlorophyll a Fluorescence and Energy Partitioning in Leaves. Photochem. Photobiol. 2019, 95, 1360–1368. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Leiger, K.; Linnanto, J.M.; Freiberg, A. Establishment of the Qy Absorption Spectrum of Chlorophyll a Extending to Near-Infrared. Molecules 2020, 25, 3796. [Google Scholar] [CrossRef]
- Vass, I.; Turcsányi, E.; Touloupakis, E.; Ghanotakis, D.; Petrouleas, V. The Mechanism of UV-A Radiation-Induced Inhibition of Photosystem II Electron Transport Studied by EPR and Chlorophyll Fluorescence. Biochemistry 2002, 41, 10200–10208. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Differences in Infectivity between Endosymbiotic Chlorella Variabilis Cultivated Outside Host Paramecium Bursaria for 50 Years and Those Immediately Isolated from Host Cells after One Year of Reendosymbiosis. Biol. Open 2016, 5, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Belyaeva, O.B.; Litvin, F.F. Spectral Dependence of Chlorophyll Biosynthesis Pathways in Plant Leaves. Biochemistry 2015, 80, 1716–1722. [Google Scholar] [CrossRef]
- Jia, M.; Li, D.; Colombo, R.; Wang, Y.; Wang, X.; Cheng, T.; Zhu, Y.; Yao, X.; Xu, C.; Ouer, G.; et al. Quantifying Chlorophyll Fluorescence Parameters from Hyperspectral Reflectance at the Leaf Scale under Various Nitrogen Treatment Regimes in Winter Wheat. Remote. Sens. 2019, 11, 2838. [Google Scholar] [CrossRef] [Green Version]
- Belyaeva, O.B.; Litvin, F.F. Pathways of Formation of Pigment Forms at the Terminal Photobiochemical Stage of Chlorophyll Biosynthesis. Biochemistry 2009, 74, 1535–1544. [Google Scholar] [CrossRef]
- Kim, M.S.; McMurtrey, J.E.; Mulchi, C.L.; Daughtry, C.S.T.; Chappelle, E.W.; Chen, Y.-R. Steady-State Multispectral Fluorescence Imaging System for Plant Leaves. Appl. Opt. 2001, 40, 157. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A Model of Leaf Optical Properties Spectra. Remote. Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Zubler, A.V.; Yoon, J.Y. Proximal Methods for Plant Stress Detection Using Optical Sensors and Machine Learning. Biosensors 2020, 10, 193. [Google Scholar] [CrossRef]
- Ko, S.S.; Jhong, C.M.; Shih, M.C. Blue Light Acclimation Reduces the Photoinhibition of Phalaenopsis Aphrodite (Moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of Leaf Chlorophyll a, b and Carotenoid Contents and Their Ratios Using Hyperspectral Reflectance. Remote. Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Agati, G.; Traversi, M.L.; Cerovic, Z.G. Chlorophyll Fluorescence Imaging for the Noninvasive Assessment of Anthocyanins in Whole Grape (Vitis Vinifera L.) Bunches. Photochem. Photobiol. 2008, 84, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Montero, R.; Pérez-Bueno, M.L.; Barón, M.; Florez-Sarasa, I.; Tohge, T.; Fernie, A.R.; Ouad, H.E.; Flexas, J.; Bota, J. Alterations in Primary and Secondary Metabolism in Vitis Vinifera ‘Malvasía de Banyalbufar’ upon Infection with Grapevine Leafroll-Associated Virus 3. Physiol. Plant. 2016, 157, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Kaniszewski, S.; Kowalski, A.; Dysko, J.; Agati, G. Application of a Combined Transmittance/Fluorescence Leaf Clip Sensor for the Nondestructive Determination of Nitrogen Status in White Cabbage Plants. Sensors 2021, 21, 482. [Google Scholar] [CrossRef]
- Janik-Zabrotowicz, E.; Arczewska, M.; Prochniewicz, P.; Swietlicka, I.; Terpilowski, K. Stability of Chlorophyll a Monomer Incorporated into Cremophor EL Nano-Micelles under Dark and Moderate Light Conditions. Molecules 2020, 25, 5059. [Google Scholar] [CrossRef]
- De Carvalho, A.G.A.; Olmo-García, L.; Gaspar, B.R.A.; Carrasco-Pancorbo, A.; Castelo-Branco, V.N.; Torres, A.G. Evaluating Quality Parameters, the Metabolic Profile, and Other Typical Features of Selected Commercial Extra Virgin Olive Oils from Brazil. Molecules 2020, 25, 4193. [Google Scholar] [CrossRef]
- Wang, H.; Wan, X. Effect of Chlorophyll Fluorescence Quenching on Quantitative Analysis of Adulteration in Extra Virgin Olive Oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Saleem, M.; Anser, M.R.; Khan, S.; Ullah, R.; Bilal, M. Validation of Fluorescence Spectroscopy to Detect Adulteration of Edible Oil in Extra Virgin Olive Oil (EVOO) by Applying Chemometrics. Appl. Spectrosc. 2018, 72, 1371–1379. [Google Scholar] [CrossRef]
- Bottiroli, G.; Croce, A.C. Optical Biopsy: A Promising Approach for Real-Time Liver Steatosis Grading. Liver Int. 2009, 29. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Routine Management of Microalgae Using Autofluorescence from Chlorophyll. Molecules 2019, 24, 4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmi, P.; Eskelinen, M.A.; Leppänen, M.T.; Pölönen, I. Rapid Quantification of Microalgae Growth with Hyperspectral Camera and Vegetation Indices. Plants 2021, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Fernández-Quintela, A.; Portillo, M.P. Anti-Obesity Effects of Microalgae. Int. J. Mol. Sci. 2020, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Lee, B.; Kim, C.Y. Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms. Antioxidants 2021, 10, 308. [Google Scholar] [CrossRef]
- Cho, J.A.; Baek, S.Y.; Cheong, S.H.; Kim, M.R. Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones. Nutrients 2020, 12, 1187. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, K.J.; Choi, J.; Koh, E.J.; Lee, B.Y. Spirulina Maxima Extract Reduces Obesity through Suppression of Adipogenesis and Activation of Browning in 3T3-L1 Cells and High-Fat Diet-Induced Obese Mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzorati, S.; Schievano, A.; Idà, A.; Verotta, L. Carotenoids, Chlorophylls and Phycocyanin from Spirulina: Supercritical CO2 and Water Extraction Methods for Added Value Products Cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira Platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonani, R.R. Recent Advances in Production, Purification and Applications of Phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, I.; Caycedo-Soler, F.; Harris, D.; Yochelis, S.; Huelga, S.F.; Plenio, M.B.; Adir, N.; Keren, N.; Paltiel, Y. Regulating the Energy Flow in a Cyanobacterial Light-Harvesting Antenna Complex. J. Phys. Chem. B 2017, 121, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, J.; Zheng, H.; Wu, X.; Wang, Y.; Liu, M.; Xiang, S.; Cao, L.; Ruan, R.; Liu, Y. Characterization of Additional Zinc Ions on the Growth, Biochemical Composition and Photosynthetic Performance from Spirulina Platensis. Bioresour. Technol. 2018, 269, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Celis-Plá, P.S.M.; Rodríguez-Rojas, F.; Méndez, L.; Moenne, F.; Muñoz, P.T.; Lobos, M.G.; Díaz, P.; Sánchez-Lizaso, J.L.; Brown, M.T.; Moenne, A.; et al. MAPK Pathway under Chronic Copper Excess in Green Macroalgae (Chlorophyta): Influence on Metal Exclusion/Extrusion Mechanisms and Photosynthesis. Int. J. Mol. Sci. 2019, 20, 4547. [Google Scholar] [CrossRef] [Green Version]
- Marcek Chorvatova, A.; Uherek, M.; Mateasik, A.; Chorvat, D. Time-Resolved Endogenous Chlorophyll Fluorescence Sensitivity to PH: Study on Chlorella Sp. Algae. Methods Appl. Fluoresc. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dong, J.; Li, B.; Xue, C.; Tetteh, P.A.; Li, D.; Gao, K.; Deng, X. Using a Freshwater Green Alga Chlorella Pyrenoidosa to Evaluate the Biotoxicity of Ionic Liquids with Different Cations and Anions. Ecotoxicol. Environ. Saf. 2020, 198. [Google Scholar] [CrossRef]
- Ma, J.; Jin, S.; Li, J.; He, Y.; Shang, W. Spatio-Temporal Variations and Driving Forces of Harmful Algal Blooms in Chaohu Lake: A Multi-Source Remote Sensing Approach. Remote Sens. 2021, 13, 427. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Wang, L. Satellite Solar-Induced Chlorophyll Fluorescence Reveals Heat Stress Impacts on Wheat Yield in India. Remote Sens. 2020, 12, 3277. [Google Scholar] [CrossRef]
- Li, J.; Pei, Y.; Zhao, S.; Xiao, R.; Sang, X.; Zhang, C. A Review of Remote Sensing for Environmental Monitoring in China. Remote Sens. 2020, 12, 1130. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rojas, F.; Celis-Plá, P.S.M.; Méndez, L.; Moenne, F.; Muñoz, P.T.; Lobos, M.G.; Díaz, P.; Sánchez-Lizaso, J.L.; Brown, M.T.; Moenne, A.; et al. MAPK Pathway under Chronic Copper Excess in Green Macroalgae (Chlorophyta): Involvement in the Regulation of Detoxification Mechanisms. Int. J. Mol. Sci. 2019, 20, 4546. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. ISBN 9783642221446. [Google Scholar]
- Weston, L.A.; Mathesius, U. Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Future J. Pharm. Sci. 2021, 7. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Berland, H.; Albert, N.W.; Stavland, A.; Jordheim, M.; McGhie, T.K.; Zhou, Y.; Zhang, H.; Deroles, S.C.; Schwinn, K.E.; Jordan, B.R.; et al. Auronidins Are a Previously Unreported Class of Flavonoid Pigments that Challenges when Anthocyanin Biosynthesis Evolved in Plants. Proc. Natl. Acad. Sci. USA 2019, 116, 20232–20239. [Google Scholar] [CrossRef] [Green Version]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; van der Kooi, C.J. Coloration of Flowers by Flavonoids and Consequences of PH Dependent Absorption. Front. Plant Sci. 2021, 11, 2148. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Yoshioka, S.; Kishimoto, S.; Nakayama, M.; Douzono, M.; Tanaka, Y.; Aida, R. Generation of Blue Chrysanthemums by Anthocyanin B-Ring Hydroxylation and Glucosylation and Its Coloration Mechanism. Sci. Adv. 2017, 3, e1602785. [Google Scholar] [CrossRef] [Green Version]
- Noda, N. Recent Advances in the Research and Development of Blue Flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the Rose Flavonoid Biosynthetic Pathway Successfully Generated Blue-Hued Flowers Accumulating Delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F. Flower Colour and Cytochromes P450. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Li, Q.; Zhou, Q.; Liu, P. Subcellular Localization and Vesicular Structures of Anthocyanin Pigmentation by Fluorescence Imaging of Black Rice (Oryza Sativa L.) Stigma Protoplast. Plants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Conejero, G.; Torregrosa, L.; Cheynier, V.; Terrier, N.; Ageorges, A. In Vivo Grapevine Anthocyanin Transport Involves Vesicle-Mediated Trafficking and the Contribution of AnthoMATE Transporters and GST. Plant J. 2011, 67, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Sytar, O.; Zivcak, M.; Abdelly, C.; Cerda, A.; Brestic, M. Anthocyanins of Coloured Wheat Genotypes in Specific Response to Salstress. Molecules 2018, 23, 1518. [Google Scholar] [CrossRef] [Green Version]
- Wiltshire, E.J.; Eady, C.C.; Collings, D.A. Induction of Anthocyanin in the Inner Epidermis of Red Onion Leaves by Environmental Stimuli and Transient Expression of Transcription Factors. Plant Cell Rep. 2017, 36, 987–1000. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional Roles of Flavonoids in Photoprotection: New Evidence, Lessons from the Past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Roshchina, V.; Shvirst, N.E.; Kuchin, A. The Autofluorescence Response of Flower Cells from Saintpaulia Ionantha as the Biosensor Reaction to Ozone. Comput. Biol. Bioinform. 2017, 4, 60–66. [Google Scholar] [CrossRef]
- Cisterna, B.; Boschi, F.; Croce, A.C.; Podda, R.; Zanzoni, S.; Degl’Innocenti, D.; Bernardi, P.; Costanzo, M.; Marzola, P.; Covi, V.; et al. Ozone Treatment of Grapes during Withering for Amarone Wine: A Multimodal Imaging and Spectroscopic Analysis. Microsc. Microanal. 2018, 24, 564–573. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Muleke, E.M.; Fan, L.; Wang, Y.; Xu, L.; Zhu, X.; Zhang, W.; Cao, Y.; Karanja, B.K.; Liu, L. Coordinated Regulation of Anthocyanin Biosynthesis Genes Confers Varied Phenotypic and Spatial-Temporal Anthocyanin Accumulation in Radish (Raphanus sativus L.). Front. Plant Sci. 2017, 8, 1243. [Google Scholar] [CrossRef] [Green Version]
- Moruzzi, M.; Klöting, N.; Blüher, M.; Martinelli, I.; Tayebati, S.K.; Gabrielli, M.G.; Roy, P.; Micioni Di Bonaventura, M.V.; Cifani, C.; Lupidi, G.; et al. Tart Cherry Juice and Seeds Affect Pro-Inflammatory Markers in Visceral Adipose Tissue of High-Fat Diet Obese Rats. Molecules 2021, 26, 1403. [Google Scholar] [CrossRef]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive Compounds and Antioxidant Capacity in Anthocyanin-Rich Carrots: A Comparison between the Black Carrot and the Apulian Landrace “Polignano” Carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-o-galactoside: A Natural Compound with Multiple Health Benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef] [PubMed]
- Vidot, K.; Devaux, M.F.; Alvarado, C.; Guyot, S.; Jamme, F.; Gaillard, C.; Siret, R.; Lahaye, M. Phenolic Distribution in Apple Epidermal and Outer Cortex Tissue by Multispectral Deep-UV Autofluorescence Cryo-Imaging. Plant Sci. 2019, 283, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Scalisi, A.; Pelliccia, D.; O’Connell, M.G. Maturity Prediction in Yellow Peach (Prunus Persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors 2020, 20, 6555. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Oliveira, J.; De Freitas, V.; Mateus, N. Fluorescence Approach for Measuring Anthocyanins and Derived Pigments in Red Wine. J. Agric. Food Chem. 2013, 61, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Tuccio, L.; Cavigli, L.; Rossi, F.; Dichala, O.; Katsogiannos, F.; Kalfas, I.; Agati, G. Fluorescence-Sensor Mapping for the in Vineyard Non-Destructive Assessment of Crimson Seedless Table Grape Quality. Sensors 2020, 20, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sytar, O.; Zivcak, M.; Neugart, S.; Brestic, M. Assessment of Hyperspectral Indicators Related to the Content of Phenolic Compounds and Multispectral Fluorescence Records in Chicory Leaves Exposed to Various Light Environments. Plant Physiol. Biochem. 2020, 154, 429–438. [Google Scholar] [CrossRef]
- Zivcak, M.; Brückova, K.; Sytar, O.; Brestic, M.; Olsovska, K.; Allakhverdiev, S.I. Lettuce Flavonoids Screening and Phenotyping by Chlorophyll Fluorescence Excitation Ratio. Planta 2017, 245, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Monica Giusti, M. Monitoring the Interaction between Thermally Induced Whey Protein and Anthocyanin by Fluorescence Quenching Spectroscopy. Foods 2021, 10, 310. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Crăciunescu, O.; Aprodu, I.; Bolea, C.A.; Iosăgeanu, A.; Petre, B.A.; Bahrim, G.E.; Oancea, A.; Stănciuc, N. Tailoring the Health-Promoting Potential of Protein Hydrolysate Derived from Fish Wastes and Flavonoids from Yellow Onion Skins: From Binding Mechanisms to Microencapsulated Functional Ingredients. Biomolecules 2020, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Vergara, C.; Pino, M.T.; Zamora, O.; Parada, J.; Pérez, R.; Uribe, M.; Kalazich, J. Microencapsulation of Anthocyanin Extracted from Purple Flesh Cultivated Potatoes by Spray Drying and Its Effects on in Vitro Gastrointestinal Digestion. Molecules 2020, 25, 722. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent Developments in Emerging Technologies for Beetroot Pigment Extraction and Its Food Applications. Food Chem. 2021, 356. [Google Scholar] [CrossRef]
- Guerrero-Rubio, M.A.; Escribano, J.; García-Carmona, F.; Gandía-Herrero, F. Light Emission in Betalains: From Fluorescent Flowers to Biotechnological Applications. Trends Plant Sci. 2020, 25, 159–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Botany: Floral Fluorescence Effect. Nature 2005, 437, 334. [Google Scholar] [CrossRef]
- Harris, N.N.; Javellana, J.; Davies, K.M.; Lewis, D.H.; Jameson, P.E.; Deroles, S.C.; Calcott, K.E.; Gould, K.S.; Schwinn, K.E. Betalain Production Is Possible in Anthocyanin-Producing Plant Species given the Presence of DOPA-Dioxygenase and L-DOPA. BMC Plant Biol. 2012, 12. [Google Scholar] [CrossRef] [Green Version]
- Polturak, G.; Grossman, N.; Vela-Corcia, D.; Dong, Y.; Nudel, A.; Pliner, M.; Levy, M.; Rogachev, I.; Aharoni, A. Engineered Gray Mold Resistance, Antioxidant Capacity, and Pigmentation in Betalain-Producing Crops and Ornamentals. Proc. Natl. Acad. Sci. USA 2017, 114, 9062–9067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monago-Maraña, O.; Durán-Merás, I.; Galeano-Díaz, T.; Muñoz De La Peña, A. Fluorescence Properties of Flavonoid Compounds. Quantification in Paprika Samples Using Spectrofluorimetry Coupled to Second Order Chemometric Tools. Food Chem. 2016, 196, 1058–1065. [Google Scholar] [CrossRef]
- Ruoff, K.; Karoui, R.; Dufour, E.; Luginbbuhl, W.; Bosset, J.O.; Bogdanov, S.; Amado, R. Authentication of the Botanical Origin of Honey by Front-Face Fluorescence Spectroscopy: A Preliminary Study. J. Agric. Food Chem. 2005, 53, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Fukui, H.; Oishi, M.; Sakuma, M.; Kawakami, M.; Tsukioka, J.; Goto, K.; Hirai, N. Biocommunication between Plants and Pollinating Insects through Fluorescence of Pollen and Anthers. J. Chem. Ecol. 2018, 44, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Kurup, R.; Johnson, A.J.; Sankar, S.; Hussain, A.A.; Kumar, C.S.; Sabulal, B. Fluorescent Prey Traps in Carnivorous Plants. Plant Biol. 2013, 15, 611–615. [Google Scholar] [CrossRef]
- Bark, K.M.; Yeom, J.E.; Yang, J.I.; Yang, I.J.; Park, C.H.; Park, H.R. Spectroscopic Studies on the Oxidation of Catechin in Aqueous Solution. Bull. Korean Chem. Soc. 2011, 32, 3443–3447. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Kim, T.E.; Shin, K.H. Quantitative Analysis of Four Catechins from Green Tea Extract in Human Plasma Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Pharmacokinetic Studies. Molecules 2018, 23, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casale, M.; Pasquini, B.; Hooshyari, M.; Orlandini, S.; Mustorgi, E.; Malegori, C.; Turrini, F.; Ortiz, M.C.; Sarabia, L.A.; Furlanetto, S. Combining Excitation-Emission Matrix Fluorescence Spectroscopy, Parallel Factor Analysis, Cyclodextrin-Modified Micellar Electrokinetic Chromatography and Partial Least Squares Class-Modelling for Green Tea Characterization. J. Pharm. Biomed. Anal. 2018, 159, 311–317. [Google Scholar] [CrossRef]
- Du, C.; Ma, C.; Gu, J.; Li, L.; Zhu, C.; Chen, L.; Wang, T.; Chen, G. Rapid Determination of Catechin Content in Black Tea by Fluorescence Spectroscopy. J. Spectrosc. 2020, 2020. [Google Scholar] [CrossRef]
- Karamac, M.; Koleva, L.; Kancheva, V.D.; Amarowicz, R. The Structure-Antioxidant Activity Relationship of Ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, L.; Williams, N. Imaging and Spectroscopy of Natural Fluorophores in Pine Needles. Plants 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atta, B.M.; Saleem, M.; Ali, H.; Bilal, M.; Fayyaz, M. Application of Fluorescence Spectroscopy in Wheat Crop: Early Disease Detection and Associated Molecular Changes. J. Fluoresc. 2020, 30, 801–810. [Google Scholar] [CrossRef]
- Bowers, A.G. Phytophotodermatitis. Am. J. Contact Dermat. 1999, 10, 89–93. [Google Scholar] [CrossRef]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef]
- Wozniak, L.; Połaska, M.; Marszalek, K.; Skapska, S. Photosensitizing Furocoumarins: Content in Plant Matrices and Kinetics of Supercritical Carbon Dioxide Extraction. Molecules 2020, 25, 3805. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.; Radotić, K.; Kalauzi, A.; Djikanović, D.; Jeremić, M. Quantification of Compression Wood Severity in Tracheids of Pinus Radiata Using Confocal Fluorescence Imaging and Spectral Deconvolution. J. Struct. Biol. 2010, 169, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Malachowska, E.; Dubowik, M.; Boruszewski, P.; Lojewska, J.; Przybysz, P. Influence of Lignin Content in Cellulose Pulp on Paper Durability. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P.; et al. Wood-Lignin: Supply, Extraction Processes and Use as Bio-Based Material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Auxenfans, T.; Terryn, C.; Paës, G. Seeing Biomass Recalcitrance through Fluorescence. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as Pigments Responsible for Visible Fluorescence in Flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. A Novel Method Using High-Performance Liquid Chromatography with Fluorescence Detection for the Determination of Betaxanthins. J. Chromatogr. A 2005, 1078, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and Pharmacological Properties of Hydroxychloroquine and Chloroquine in Treatment of Systemic Lupus Erythematosus, Rheumatoid Arthritis and Related Diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an Old Anti-Malarial Drug in a Modern World: Role in the Treatment of Malaria. Malar. J. 2011, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liles, N.W.; Page, E.E.; Liles, A.L.; Vesely, S.K.; Raskob, G.E.; George, J.N. Diversity and Severity of Adverse Reactions to Quinine: A Systematic Review. Am. J. Hematol. 2016, 91, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, A.C.; Bottiroli, G.; Supino, R.; Favini, E.; Zuco, V.; Zunino, F. Subcellular Localization of the Camptothecin Analogues, Topotecan and Gimatecan. Biochem. Pharmacol. 2004, 67, 1035–1045. [Google Scholar] [CrossRef]
- Guamán Ortiz, L.M.; Croce, A.L.; Aredia, F.; Sapienza, S.; Fiorillo, G.; Syeda, T.M.; Buzzetti, F.; Lombardi, P.; Scovassi, A.I. Effect of New Berberine Derivatives on Colon Cancer Cells. Acta Biochim. Biophys. Sin. 2015, 47, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Raizman, R.; Little, W.; Smith, A.C. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics 2021, 11, 280. [Google Scholar] [CrossRef]
- Hurley, C.M.; McClusky, P.; Sugrue, R.M.; Clover, J.A.; Kelly, J.E. Efficacy of a Bacterial Fluorescence Imaging Device in an Outpatient Wound Care Clinic: A Pilot Study. J. Wound Care 2019, 28, 438–443. [Google Scholar] [CrossRef]
- Stiehl, J.B. Bacterial Autofluorescence Digital Imaging Guides Treatment in Stage 4 Pelvic Pressure Injuries: A Preliminary Case Series. Diagnostics 2021, 11, 839. [Google Scholar] [CrossRef]
- Baird, F.J.; Wadsworth, M.P.; Hill, J.E. Evaluation and Optimization of Multiple Fluorophore Analysis of a Pseudomonas Aeruginosa Biofilm. J. Microbiol. Methods 2012, 90, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.L.S.; Shalita, A.R.; Poh-Fitzpatrick, M.B. Comparative Studies of Porphyrin Production in Propionibacterium Acnes and Propionibacterium Granulosum. J. Bacteriol. 1978, 133, 811–815. [Google Scholar] [CrossRef] [Green Version]
- König, K.; Meyer, H.; Schneckenburger, H.; Rück, A. The Study of Endogenous Porphyrins in Human Skin and Their Potential for Photodynamic Therapy by Laser Induced Fluorescence Spectroscopy. Lasers Med Sci. 1993, 8, 127–132. [Google Scholar] [CrossRef]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium Acnes by Its Endogenic Porphyrins after Illumination with High Intensity Blue Light. FEMS Immunol. Med Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Lennon, A.M.; Buchalla, W.; Brune, L.; Zimmermann, O.; Gross, U.; Attin, T. The Ability of Selected Oral Microorganisms to Emit Red Fluorescence. Caries 2005, 40, 2–5. [Google Scholar] [CrossRef]
- König, K.; Flemming, G.; Hibst, R. Laser-Induced Autofluorescence Spectroscopy of Dental Caries. Cell. Mol. Biol. 1998, 44, 1293–1300. [Google Scholar] [PubMed]
- Kang, S.M.; de Josselin de Jong, E.; Higham, S.M.; Hope, C.K.; Kim, B. Il Fluorescence Fingerprints of Oral Bacteria. J. Biophotonics 2020, 13. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Roberts, T.A.; Moore, G.; Ward, J.M.; Muller, J.-P. Fluorescence Characterization of Clinically-Important Bacteria. PLoS ONE 2013, 8, e75270. [Google Scholar] [CrossRef] [Green Version]
- Ammor, M.S. Recent Advances in the Use of Intrinsic Fluorescence for Bacterial Identification and Characterization. J. Fluoresc. 2007, 17, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Himmelsbach, D.S.; Barton, F.E.; Fedorka-Cray, P.J. Fluorescence Spectroscopy for Rapid Detection and Classification of Bacterial Pathogens. Appl. Spectrosc. 2009, 63, 1251–1255. [Google Scholar] [CrossRef]
- Li, W.H.; Sheng, G.P.; Liu, X.W.; Yu, H.Q. Characterizing the Extracellular and Intracellular Fluorescent Products of Activated Sludge in a Sequencing Batch Reactor. Water Res. 2008, 42, 3173–3181. [Google Scholar] [CrossRef]
- Tian, J.H.; Yan, C.; Nasir, Z.A.; Alcega, S.G.; Tyrrel, S.; Coulon, F. Real Time Detection and Characterisation of Bioaerosol Emissions from Wastewater Treatment Plants. Sci. Total. Environ. 2020, 721. [Google Scholar] [CrossRef]
- Chen, W.J.; Kuo, T.Y.; Hsieh, F.C.; Chen, P.Y.; Wang, C.S.; Shih, Y.L.; Lai, Y.M.; Liu, J.R.; Yang, Y.L.; Shih, M.C. Involvement of Type VI Secretion System in Secretion of Iron Chelator Pyoverdine in Pseudomonas taiwanensis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Knaus, H.; Blab, G.A.; Jerre van Veluw, G.; Gerritsen, H.C.; Wösten, H.A.B. Label-Free Fluorescence Microscopy in Fungi. Fungal Biol. Rev. 2013, 27, 60–66. [Google Scholar] [CrossRef]
- Lin, S.J.; Tan, H.Y.; Kuo, C.J.; Wu, R.J.; Wang, S.H.; Chen, W.L.; Jee, S.H.; Dong, C.Y. Multiphoton Autofluorescence Spectral Analysis for Fungus Imaging and Identification. Appl. Phys. Lett. 2009, 95, 43703. [Google Scholar] [CrossRef]
- Arcangeli, C.; Yu, W.; Cannistraro, S.; Gratton, E. Two-Photon Autofluorescence Microscopy and Spectroscopy of Antarctic Fungus: New Approach for Studying Effects of UV-B Irradiation. Biopolym. Biospectrosc. Sect. 2000, 57, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Graus, M.S.; Neumann, A.K.; Timlin, J.A. Hyperspectral Fluorescence Microscopy Detects Autofluorescent Factors That Can Be Exploited as a Diagnostic Method for Candida Species Differentiation. J. Biomed. Opt. 2017, 22, 016002. [Google Scholar] [CrossRef] [Green Version]
- Knaus, H.; Blab, G.A.; Agronskaia, A.V.; van den Heuvel, D.J.; Gerritsen, H.C.; Wösten, H.A.B. Monitoring the Metabolic State of Fungal Hyphae and the Presence of Melanin by Nonlinear Spectral Imaging. Appl. Environ. Microbiol. 2013, 79, 6345–6350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Mansoldo, F.R.P.; Firpo, R.; da Cardoso, V.S.; Queiroz, G.N.; Cedrola, S.M.L.; de Godoy, M.G.; Vermelho, A.B. New Method for Rapid Identification and Quantification of Fungal Biomass Using Ergosterol Autofluorescence. Talanta 2020, 219, 121238. [Google Scholar] [CrossRef]
- Reismann, A.W.A.F.; Atanasova, L.; Zeilinger, S.; Schütz, G.J. Single-Molecule Localization Microscopy to Study Protein Organization in the Filamentous Fungus Trichoderma atroviride. Molecules 2020, 25, 3199. [Google Scholar] [CrossRef]
- Margo, C.E.; Bombardier, T. The Diagnostic Value of Fungal Autofluorescence. Surv. Ophthalmol. 1985, 29, 374–376. [Google Scholar] [CrossRef]
- Chua, S.C.J.H.; Tan, H.Q.; Engelberg, D.; Lim, L.H.K. Alternative Experimental Models for Studying Influenza Proteins, Host-Virus Interactions and Anti-Influenza Drugs. Pharmaceuticals 2019, 12, 147. [Google Scholar] [CrossRef]
- Zhao, R.Y. Yeast for Virus Research. Microb. Cell 2017, 4, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D.; Murray, D.B.; Aon, M.A.; Cortassa, S.; Roussel, M.R.; Beckmann, M.; Poole, R.K. Temporal Metabolic Partitioning of the Yeast and Protist Cellular Networks: The Cell Is a Global Scale-Invariant (Fractal or Self-Similar) Multioscillator. J. Biomed. Opt. 2018, 24, 1. [Google Scholar] [CrossRef]
- Maltas, J.; Amer, L.; Long, Z.; Palo, D.; Oliva, A.; Folz, J.; Urayama, P. Autofluorescence from NADH Conformations Associated with Different Metabolic Pathways Monitored Using Nanosecond-Gated Spectroscopy and Spectral Phasor Analysis. Anal. Chem. 2015. [Google Scholar] [CrossRef]
- Duysens, L.N.M.; Kronenberg, G.H.M. The Fluorescence Spectrum of the Complex of Reduced Phosphopyridine Nucleotide and Alcohol Dehydrogenase from Yeast. Biochim. Biophys. Acta 1957, 26, 437–438. [Google Scholar] [CrossRef]
- Chance, B.; Thorell, B. Localization and Kinetics of Reduced Pyridine Nucleotide in Living Cells by Microfluorometry. J. Biol. Chem. 1959, 234, 3044–3050. [Google Scholar] [CrossRef]
- Chance, B.; Ernster, L.; Garland, P.B.; Lee, C.P.; Light, P.A.; Ohnishi, T.; Ragan, C.I.; Wong, D. Flavoproteins of the Mitochondrial Respiratory Chain. Proc. Natl. Acad. of Sci. USA 1967, 57, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Mayevsky, A.; Rogatsky, G.G. Mitochondrial Function In Vivo Evaluated by NADH Fluorescence: From Animal Models to Human Studies. Am. J. Physiol. Cell Physiol. 2007, 292. [Google Scholar] [CrossRef]
- Mayevsky, A.; Barbiro-Michaely, E. Shedding Light on Mitochondrial Function by Real Time Monitoring of NADH Fluorescence: II: Human Studies. J. Clin. Monit. Comput. 2013, 27, 125–145. [Google Scholar] [CrossRef]
- Mayevsky, A.; Walden, R.; Pewzner, E.; Deutsch, A.; Heldenberg, E.; Lavee, J.; Tager, S.; Kachel, E.; Raanani, E.; Preisman, S.; et al. Mitochondrial Function and Tissue Vitality: Bench-to-Bedside Real-Time Optical Monitoring System. J. Biomed. Opt. 2011, 16, 067004. [Google Scholar] [CrossRef] [Green Version]
- Blacker, T.S.; Duchen, M.R. Investigating Mitochondrial Redox State Using NADH and NADPH Autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Chance, B.; Estabrook, R.W.; Ghosh, A. Damped Sinusoidal Oscillatons of Cytoplasmic Reduced Pyridine. Proc. Natl. Acad. Sci. USA 1964, 51, 1244–1251. [Google Scholar] [CrossRef] [Green Version]
- Mochan, E.; Pye, E.K. Respiratory Oscillations in Adapting Yeast Cultures. Nat. New Biol. 1973, 242, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Cortassa, S.; O’Rourke, B.; Aon, M.A. What Yeast and Cardiomyocytes Share: Ultradian Oscillatory Redox Mechanisms of Cellular Coherence and Survival. Integr. Biol. 2012, 4, 65–74. [Google Scholar] [CrossRef]
- Chance, B.; Thorell, B. Fluorescence Measurements of Mitochondrial Pyridine Nucleotide in Aerobiosis and Anaerobiosis. Nature 1959, 184, 931–934. [Google Scholar] [CrossRef]
- Cockayne, E.A.I. The Distribution of Fluorescent Pigments in Lepidoptera. Trans. R. Entomol. Soc. Lond. 1924, 72, 1–19. [Google Scholar] [CrossRef]
- Franceschini, N.; Kirschfeld, K.; Minke, B. Fluorescence of Photoreceptor Cells Observed in Vivo. Science 1981, 213, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Rost, F.W.D.; Hales, D.F. Fluorescent Markings in Some Australian Butterflies. Aust. Entomol. Mag. 1988, 15, 91–94. [Google Scholar]
- Kumazawa, K.; Tabata, H. A Three-Dimensional Fluorescence Analysis of the Wings of Male Morpho Sulkowskyi and Papilio Xuthus Butterflies. Zool. Sci. 2001, 18, 1073–1079. [Google Scholar] [CrossRef]
- Umebachi, Y. Yellow Pigments in the Wings of Papilio Xuthus (Papilionid Butterfly). Acta Vitaminol. Enzymol. 1975, 29, 219–222. [Google Scholar] [PubMed]
- Wilts, B.D.; Trzeciak, T.M.; Vukusic, P.; Stavenga, D.G. Papiliochrome II Pigment Reduces the Angle Dependency of Structural Wing Colouration in Nireus Group Papilionids. J. Exp. Biol. 2012, 215, 796–805. [Google Scholar] [CrossRef] [Green Version]
- Wilts, B.D.; Ijbema, N.; Stavenga, D.G. Pigmentary and Photonic Coloration Mechanisms Reveal Taxonomic Relationships of the Cattlehearts (Lepidoptera: Papilionidae: Parides). BMC Evol. Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneron, J.P.; Kertész, K.; Vértesy, Z.; Rassart, M.; Lousse, V.; Bálint, Z.; Biró, L.P. Correlated Diffraction and Fluorescence in the Backscattering Iridescence of the Male Butterfly Troides Magellanus (Papilionidae). Phys. Rev. E 2008, 78, 021903. [Google Scholar] [CrossRef] [Green Version]
- De Vidal, B.C. Butterfly Scale Form Birefringence Related to Photonics. Micron 2011, 42, 801–807. [Google Scholar] [CrossRef]
- Zobl, S.; Wilts, B.D.; Salvenmoser, W.; Pölt, P.; Gebeshuber, I.C.; Schwerte, T. Orientation-Dependent Reflection of Structurally Coloured Butterflies. Biomimetics 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thayer, R.C.; Allen, F.I.; Patel, N.H. Structural Color in Junonia Butterflies Evolves by Tuning Scale Lamina Thickness. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Wilts, B.D.; Matsushita, A.; Arikawa, K.; Stavenga, D.G. Spectrally Tuned Structural and Pigmentary Coloration of Birdwing Butterfly Wing Scales. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, Q.; Chen, W.; Xie, W.; Wang, Y.; Wang, M.; You, T.; Pan, G. Biomimetic Design of Photonic Materials for Biomedical Applications. Acta Biomater. 2021, 121, 143–179. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Tabarrant, T.; Lucas, S.; Su, B.-L.; Vukusic, P.; Deparis, O. Vapor Sensing with a Natural Photonic Cell. Opt. Express 2016, 24, 12267. [Google Scholar] [CrossRef]
- Mouchet, S.R.; van Hooijdonk, E.; Welch, V.L.; Louette, P.; Colomer, J.F.; Su, B.L.; Deparis, O. Liquid-Induced Colour Change in a Beetle: The Concept of a Photonic Cell. Sci. Rep. 2016, 6, 19322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouchet, S.R.; Verstraete, C.; Mara, D.; Van Cleuvenbergen, S.; Finlayson, E.D.; Van Deun, R.; Deparis, O.; Verbiest, T.; Maes, B.; Vukusic, P.; et al. Nonlinear Optical Spectroscopy and Two-Photon Excited Fluorescence Spectroscopy Reveal the Excited States of Fluorophores Embedded in a Beetle’s Elytra. Interface Focus 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, S.O.; Weis-Fogh, T. Resilin. A Rubberlike Protein in Arthropod Cuticle. Adv. Insect Physiol. 1964, 2, 1–65. [Google Scholar] [CrossRef]
- Michels, J.; Gorb, S.N. Detailed Three-Dimensional Visualization of Resilin in the Exoskeleton of Arthropods Using Confocal Laser Scanning Microscopy. J. Microsc. 2012, 245, 1–16. [Google Scholar] [CrossRef]
- Büsse, S.; Gorb, S.N. Material Composition of the Mouthpart Cuticle in a Damselfly Larva (Insecta: Odonata) and Its Biomechanical Significance. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Bäumler, F.; Gorb, S.N.; Büsse, S. Comparative Morphology of the Thorax Musculature of Adult Anisoptera (Insecta: Odonata): Functional Aspects of the Flight Apparatus. Arthropod Struct. Dev. 2018, 47, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, F.; Büsse, S. Resilin in the Flight Apparatus of Odonata (Insecta)-Cap Tendons and Their Biomechanical Importance for Flight. Biol. Lett. 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentzold, S.; Marion-Poll, F.; Grabe, V.; Burse, A. Autofluorescence-Based Identification and Functional Validation of Antennal Gustatory Sensilla in a Specialist Leaf Beetle. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Young, R.G.; Tappel, A.L. Fluorescent Pigment and Pentane Production by Lipid Peroxidation in Honey Bees, Apis Mellifera. Exp. Gerontol. 1978, 13, 457–459. [Google Scholar] [CrossRef]
- Chio, K.S.; Reiss, U.; Fletcher, B.; Tappel, A.L. Peroxidation of Subcellular Organelles: Formation of Lipofuscinlike Fluorescent Pigments. Science 1969, 166, 1535–1536. [Google Scholar] [CrossRef]
- Sparks, J.S.; Schelly, R.C.; Smith, W.L.; Davis, M.P.; Tchernov, D.; Pieribone, V.A.; Gruber, D.F. The Covert World of Fish Biofluorescence: A Phylogenetically Widespread and Phenotypically Variable Phenomenon. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Brauwer, M.; Hobbs, J.P.A.; Ambo-Rappe, R.; Jompa, J.; Harvey, E.S.; McIlwain, J.L. Biofluorescence as a Survey Tool for Cryptic Marine Species. Conserv. Biol. 2018, 32, 706–715. [Google Scholar] [CrossRef]
- Meadows, M.G.; Anthes, N.; Dangelmayer, S.; Alwany, M.A.; Gerlach, T.; Schulte, G.; Sprenger, D.; Theobald, J.; Michiels, N.K. Red Fluorescence Increases with Depth in Reef Fishes, Supporting a Visual Function, Not UV Protection. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef]
- Kashimoto, R.; Hisata, K.; Shinzato, C.; Satoh, N.; Shoguchi, E. Expansion and Diversification of Fluorescent Protein Genes in Fifteen Acropora Species during the Evolution of Acroporid Corals. Genes 2021, 12, 397. [Google Scholar] [CrossRef]
- Macel, M.L.; Ristoratore, F.; Locascio, A.; Spagnuolo, A.; Sordino, P.; D’Aniello, S. Sea as a Color Palette: The Ecology and Evolution of Fluorescence. Zool. Lett. 2020, 6. [Google Scholar] [CrossRef]
- Kumagai, A.; Ando, R.; Miyatake, H.; Greimel, P.; Kobayashi, T.; Hirabayashi, Y.; Shimogori, T.; Miyawaki, A. XA Bilirubin-Inducible Fluorescent Protein from Eel Muscle. Cell 2013, 153, 1602–1611. [Google Scholar] [CrossRef] [Green Version]
- Krivoshik, S.R.; Guarnaccia, A.M.; Fried, D.B.; Gruber, D.F.; Gaffney, J.P. Disrupting Fluorescence by Mutagenesis in a Green Fluorescent Fatty Acid Binding Protein from a Marine Eel. Protein J. 2020, 39, 145–151. [Google Scholar] [CrossRef]
- Gruber, D.F.; Gaffney, J.P.; Mehr, S.; Desalle, R.; Sparks, J.S.; Platisa, J.; Pieribone, V.A. Adaptive Evolution of Eel Fluorescent Proteins from Fatty Acid Binding Proteins Produces Bright Fluorescence in the Marine Environment. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Wucherer, M.F.; Michiels, N.K. Regulation of Red Fluorescent Light Emission in a Cryptic Marine Fish. Front. Zool. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Harant, U.K.; Santon, M.; Bitton, P.P.; Wehrberger, F.; Griessler, T.; Meadows, M.G.; Champ, C.M.; Michiels, N.K. Do the Fluorescent Red Eyes of the Marine Fish Tripterygion Delaisi Stand out? In Situ and in Vivo Measurements at Two Depths. Ecol. Evol. 2018, 8, 4685–4694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.B.; Lam, Y.C.; Gaffney, J.P.; Weaver, J.C.; Krivoshik, S.R.; Hamchand, R.; Pieribone, V.; Gruber, D.F.; Crawford, J.M. Bright Green Biofluorescence in Sharks Derives from Bromo-Kynurenine Metabolism. iScience 2019, 19, 1291–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassoun, A.; Shumilina, E.; Di Donato, F.; Foschi, M.; Simal-Gandara, J.; Biancolillo, A. Emerging Techniques for Differentiation of Fresh and Frozen-Thawed Seafoods: Highlighting the Potential of Spectroscopic Techniques. Molecules 2020, 25, 4472. [Google Scholar] [CrossRef] [PubMed]
- Protzel, D.; Hes, M.; Scherz, M.D.; Schwager, M.; Padje, A.V.T.; Glaw, F. Widespread Bone-Based Fluorescence in Chameleons. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Prötzel, D.; Hes, M.; Schwager, M.; Glaw, F.; Scherz, M.D. Neon-Green Fluorescence in the Desert Gecko Pachydactylus Rangei Caused by Iridophores. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Goutte, S.; Mason, M.J.; Antoniazzi, M.M.; Jared, C.; Merle, D.; Cazes, L.; Toledo, L.F.; el-Hafci, H.; Pallu, S.; Portier, H.; et al. Intense Bone Fluorescence Reveals Hidden Patterns in Pumpkin Toadlets. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Taboada, C.; Brunetti, A.E.; Pedron, F.N.; Neto, F.C.; Estrin, D.A.; Bari, S.E.; Chemese, L.B.; Lopes, N.P.; Lagorio, M.G.; Faivovich, J. Naturally Occurring Fluorescence in Frogs. Proc. Natl. Acad. Sci. USA 2017, 114, 3672–3677. [Google Scholar] [CrossRef] [Green Version]
- Lamb, J.Y.; Davis, M.P. Salamanders and Other Amphibians Are Aglow with Biofluorescence. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Völker, O. Ueber Fluoreszierende, Gelbe Federpigmente Bei Papageien, Eine Neue Klasse von Federfarbstoffen. J. für Ornithol. 1937, 85, 136–146. [Google Scholar] [CrossRef]
- Stradi, R.; Pini, E.; Celentano, G. The Chemical Structure of the Pigments in Ara Macao Plumage. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 130, 57–63. [Google Scholar] [CrossRef]
- McGraw, K.J.; Nogare, M.C. Carotenoid Pigments and the Selectivity of Psittacofulvin-Based Coloration Systems in Parrots. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.B.; McGraw, K.J.; Butler, M.W.; Carrano, M.T.; Madden, O.; James, H.F. Ancient Origins and Multiple Appearances of Carotenoid-Pigmented Feathers in Birds. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [Green Version]
- Cooke, T.F.; Fischer, C.R.; Wu, P.; Jiang, T.X.; Xie, K.T.; Kuo, J.; Doctorov, E.; Zehnder, A.; Khosla, C.; Chuong, C.M.; et al. Genetic Mapping and Biochemical Basis of Yellow Feather Pigmentation in Budgerigars. Cell 2017, 171, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronelli, M.; Zerbi, G.; Stradi, R. In Situ Resonance Raman Spectra of Carotenoids in Bird’s Feathers. J. Raman Spectrosc. 1995, 26, 683–692. [Google Scholar] [CrossRef]
- Barnsley, J.E.; Tay, E.J.; Gordon, K.C.; Thomas, D.B. Frequency Dispersion Reveals Chromophore Diversity and Colour-Tuning Mechanism in Parrot Feathers. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.; Johnsen, S. Fluorescence as a Means of Colour Signal Enhancement. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [Green Version]
- Arnold, K.E.; Owens, I.P.F.; Marshall, N.J. Fluorescent Signaling in Parrots. Science 2002, 295, 92. [Google Scholar] [CrossRef]
- Camacho, C.; Negro, J.J.; Redondo, I.; Palacios, S.; Sáez-Gómez, P. Correlates of Individual Variation in the Porphyrin-Based Fluorescence of Red-Necked Nightjars (Caprimulgus Ruficollis). Sci. Rep. 2019, 9, 19115. [Google Scholar] [CrossRef]
- Pine, R.H.; Rice, J.E.; Bucher, J.E.; Tank, D.H.; Greenhall, A.M. Labile Pigments and Fluorescent Pelage in Didelphid Marsupials. Mammalia 1985, 49, 249–256. [Google Scholar] [CrossRef]
- Olson, E.R.; Carlson, M.R.; Ramanujam, V.M.S.; Sears, L.; Anthony, S.E.; Anich, P.S.; Ramon, L.; Hulstrand, A.; Jurewicz, M.; Gunnelson, A.S.; et al. Vivid Biofluorescence Discovered in the Nocturnal Springhare (Pedetidae). Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Hamchand, R.; Lafountain, A.M.; Büchel, R.; Maas, K.R.; Hird, S.M.; Warren, M.; Frank, H.A.; Brückner, C. Red Fluorescence of European Hedgehog (Erinaceus Europaeus) Spines Results from Free-Base Porphyrins of Potential Microbial Origin. J. Chem. Ecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Weagle, G.; Paterson, P.E.; Kennedy, J.; Pottier, R. The Nature of the Chromophore Responsible for Naturally Occurring Fluorescence in Mouse Skin. J. Photochem. Photobiol. B Biol. 1988, 2, 313–320. [Google Scholar] [CrossRef]
- Kohler, A.M.; Olson, E.R.; Martin, J.G.; Anich, P.S. Ultraviolet Fluorescence Discovered in New World Flying Squirrels (Glaucomys). J. Mammal. 2019, 100, 21–30. [Google Scholar] [CrossRef]
- Anich, P.S.; Anthony, S.; Carlson, M.; Gunnelson, A.; Kohler, A.M.; Martin, J.G.; Olson, E.R. Biofluorescence in the Platypus (Ornithorhynchus Anatinus). Mammalia 2021, 85, 179–181. [Google Scholar] [CrossRef]
- Widder, E.A. Bioluminescence in the Ocean: Origins of Biological, Chemical, and Ecological Diversity. Science 2010, 328, 704–708. [Google Scholar] [CrossRef] [Green Version]
- Brodl, E.; Winkler, A.; Macheroux, P. Molecular Mechanisms of Bacterial Bioluminescence. Comput. Struct. Biotechnol. J. 2018, 16, 551–564. [Google Scholar] [CrossRef]
- Oba, Y.; Stevani, C.V.; Oliveira, A.G.; Tsarkova, A.S.; Chepurnykh, T.; Yampolsky, I. Selected Least Studied but Not Forgotten Bioluminescent Systems. Photochem. Photobiol. 2017, 93, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Daubner, S.C.; Astorga, A.M.; Leisman, G.B.; Baldwin, T.O. Yellow Light Emission of Vibrio Fischeri Strain Y-1: Purification and Characterization of the Energy-Accepting Yellow Fluorescent Protein. Proc. Natl. Acad. Sci. USA 1987, 84, 8912–8916. [Google Scholar] [CrossRef] [Green Version]
- Vannier, T.; Hingamp, P.; Turrel, F.; Tanet, L.; Lescot, M.; Timsit, Y. Diversity and Evolution of Bacterial Bioluminescence Genes in the Global Ocean. NAR Genom. Bioinform. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.D.; Haddock, S.H.D.; Elvidge, C.D.; Lee, T.F. Detection of a Bioluminescent Milky Sea from Space. Proc. Natl. Acad. Sci. USA 2005, 102, 14181–14184. [Google Scholar] [CrossRef] [Green Version]
- Valiadi, M.; Iglesias-Rodriguez, D. Understanding Bioluminescence in Dinoflagellates—How Far Have We Come? Microorganisms 2013, 1, 3–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifaie-Graham, O.; Galensowske, N.F.B.; Dean, C.; Pollard, J.; Balog, S.; Gouveia, M.G.; Chami, M.; Vian, A.; Amstad, E.; Lattuada, M.; et al. Shear Stress-Responsive Polymersome Nanoreactors Inspired by the Marine Bioluminescence of Dinoflagellates. Angew. Chem. 2021, 133, 917–922. [Google Scholar] [CrossRef]
- Valiadi, M.; de Rond, T.; Amorim, A.; Gittins, J.R.; Gubili, C.; Moore, B.S.; Iglesias-Rodriguez, M.D.; Latz, M.I. Molecular and Biochemical Basis for the Loss of Bioluminescence in the Dinoflagellate Noctiluca Scintillans along the West Coast of the U.S.A. Limnol. Oceanogr. 2019, 64, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Cusick, K.D.; Widder, E.A. Bioluminescence and Toxicity as Driving Factors in Harmful Algal Blooms: Ecological Functions and Genetic Variability. Harmful Algae 2020, 98. [Google Scholar] [CrossRef]
- Cusick, K.D.; Widder, E.A. Intensity Differences in Bioluminescent Dinoflagellates Impact Foraging Efficiency in a Nocturnal Predator. Bull. Mar. Sci. 2014, 90, 797–811. [Google Scholar] [CrossRef]
- Duchatelet, L.; Delroisse, J.; Flammang, P.; Mahillon, J.; Mallefet, J. Etmopterus Spinax, the Velvet Belly Lanternshark, Does Not Use Bacterial Luminescence. Acta Histochem. 2019, 121, 516–521. [Google Scholar] [CrossRef]
- Dunlap, P.V. Bioluminescence, Microbial. In Encyclopedia of Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 45–61. ISBN 9780123739445. [Google Scholar]
- Gould, A.L.; Dunlap, P.V. Shedding Light on Specificity: Population Genomic Structure of a Symbiosis Between a Coral Reef Fish and Luminous Bacterium. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Bongrand, C.; Ruby, E.G. The Impact of Vibrio fischeri Strain Variation on Host Colonization. Curr. Opin. Microbiol. 2019, 50, 15–19. [Google Scholar] [CrossRef]
- Li, Y.D.; Kundrata, R.; Tihelka, E.; Liu, Z.; Huang, D.; Cai, C. Cretophengodidae, a New Cretaceous Beetle Family, Sheds Light on the Evolution of Bioluminescence. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202730. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.M.; Lee, H.H.; Chan-Yi, I.L.; Liu, Y.C.; Lu, M.R.; Hsieh, J.W.A.; Chang, C.C.; Wu, P.H.; Lu, M.J.; Li, J.Y.; et al. Mycena Genomes Resolve the Evolution of Fungal Bioluminescence. Proc. Natl. Acad. Sci. USA 2020, 117, 31267–31277. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, P.; Delean, S.; Wood, T.; Austin, A.D. Bioluminescence in the Ghost Fungus Omphalotus nidiformis Does Not Attract Potential Spore Dispersing Insects. IMA Fungus 2016, 7, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Morciano, G.; Sarti, A.C.; Marchi, S.; Missiroli, S.; Falzoni, S.; Raffaghello, L.; Pistoia, V.; Giorgi, C.; di Virgilio, F.; Pinton, P. Use of Luciferase Probes to Measure ATP in Living Cells and Animals. Nat. Protoc. 2017, 12, 1542–1562. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.M.; Esteves Da Silva, J.C.G. Firefly Bioluminescence: A Mechanistic Approach of Luciferase Catalyzed Reactions. IUBMB Life 2009, 61, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Molecular Origin of Color Variation in Firefly (Beetle) Bioluminescence: A Chemical Basis for Biological Imaging. Curr. Top. Med. Chem. 2016, 16, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Morise, H.; Shimomura, O.; Johnson, F.H.; Winant, J. Intermolecular Energy Transfer in the Bioluminescent System of Aequorea. Biochemistry 1974, 13, 2656–2662. [Google Scholar] [CrossRef]
- Chalfie, M. Green Fluorescent Protein. Photochem. Photobiol. 1995, 62, 651–656. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccolo, M.; Pozzan, T. Imaging Signal Transduction in Living Cells with GFP-Based Probes. IUBMB Life 2000, 49, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, A.; Katz, M.; Fleming, J.; Truty, M.; Thomas, R.; Moriwaki, H.; Bouvet, M.; Saji, S.; Hoffman, R.M. Multi-Color Palette of Fluorescent Proteins for Imaging the Tumor Microenvironment of Orthotopic Tumorgraft Mouse Models of Clinical Pancreatic Cancer Specimens. J. Cell. Biochem. 2012, 113, 2290–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambito, G.; Hall, M.P.; Wood, M.G.; Gaspar, N.; Ridwan, Y.; Stellari, F.F.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Lowik, C.; et al. Red-Shifted Click Beetle Luciferase Mutant Expands the Multicolor Bioluminescent Palette for Deep Tissue Imaging. iScience 2021, 24. [Google Scholar] [CrossRef] [PubMed]
- Zambito, G.; Gaspar, N.; Ridwan, Y.; Hall, M.P.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Lowik, C.; Mezzanotte, L. Evaluating Brightness and Spectral Properties of Click Beetle and Firefly Luciferases Using Luciferin Analogues: Identification of Preferred Pairings of Luciferase and Substrate for In Vivo Bioluminescence Imaging. Mol. Imaging Biol. 2020, 22, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, P. Biochemistry and Genetics of Bacterial Bioluminescence. Adv. Biochem. Eng. Biotechnol. 2014, 144, 37–64. [Google Scholar] [CrossRef]
- Wizenty, J.; Schumann, T.; Theil, D.; Stockmann, M.; Pratschke, J.; Tacke, F.; Aigner, F.; Wuensch, T. Recent Advances and the Potential for Clinical Use of Autofluorescence Detection of Extra-Ophthalmic Tissues. Molecules 2020, 25, 2095. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus Autofluorescence Imaging. Prog. Retin. Eye Res. 2021, 81. [Google Scholar] [CrossRef] [PubMed]
- Langhout, G.C.; Spliethoff, J.W.; Schmitz, S.J.; Aalbers, A.G.J.; van Velthuysen, M.L.F.; Hendriks, B.H.W.; Ruers, T.J.M.; Kuhlmann, K.F.D. Differentiation of Healthy and Malignant Tissue in Colon Cancer Patients Using Optical Spectroscopy: A Tool for Image-Guided Surgery. Lasers Surg. Med. 2015, 47, 559–565. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; Galema, H.A.; Hardillo, J.A.U.; Sewnaik, A.; Monserez, D.; van Driel, P.B.A.A.; Verhoef, C.; Baatenburg de Jong, R.J.; Hilling, D.E.; Keereweer, S. Current Intraoperative Imaging Techniques to Improve Surgical Resection of Laryngeal Cancer: A Systematic Review. Cancers 2021, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Glover, B.; Teare, J.; Patel, N. A Review of New and Emerging Techniques for Optical Diagnosis of Colonic Polyps. J. Clin. Gastroenterol. 2019, 53, 495–506. [Google Scholar] [CrossRef]
- Osman, H.; Georges, J.; Elsahy, D.; Hattab, E.M.; Yocom, S.; Cohen-Gadol, A.A. In Vivo Microscopy in Neurosurgical Oncology. World Neurosurg. 2018, 115, 110–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, S.; Halicek, M.; Fabelo, H.; Callico, G.M.; Fei, B. Hyperspectral and Multispectral Imaging in Digital and Computational Pathology: A Systematic Review [Invited]. Biomed. Opt. Express 2020, 11, 3195. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Yamanaka, M.; Fujihara, J.; Matsuoka, Y.; Gohto, Y.; Obana, A.; Tanito, M. Evaluation of Relevance between Advanced Glycation End Products and Diabetic Retinopathy Stages Using Skin Autofluorescence. Antioxidants 2020, 9, 1100. [Google Scholar] [CrossRef]
- Wang, M.; Long, F.; Tang, F.; Jing, Y.; Wang, X.; Yao, L.; Ma, J.; Fei, Y.; Chen, L.; Wang, G.; et al. Autofluorescence Imaging and Spectroscopy of Human Lung Cancer. Appl. Sci. 2017, 7, 32. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Y.; Chen, M.; Wang, L.; Pan, H.; Zhang, X.; Wagnieres, G.; Mohammad, Y.; Barreiro, E.; Pirozzolo, G.; et al. Comparison of Autofluorescence and White-Light Bronchoscopies Performed with the Evis Lucera Spectrum for the Detection of Bronchial Cancers: A Meta-Analysis. Transl. Lung Cancer Res. 2020, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Borst, M.K.H. Untersuchungen Über Porphyrie, mit Besonderer Berücksichtigung der Porphyria Congenita; Hirzel: Leipzig, Germany, 1929. [Google Scholar]
- Dhéré, C. La Fluorescence En Biochimie; La Presse Universitaire de France: Paris, France, 1937. [Google Scholar] [CrossRef]
- Policard, A. Etude Sur Les Aspects Offerts Par Des Tumeurs Experimentales Examinees a La Lumiere de Wood. Comptes Rendus Des Séances Société Biol. 1924, 91, 1423–1424. [Google Scholar]
- Popper, H.; Gyorgy, P.; Goldblatt, H. Fluorescent Material (Ceroid) in Experimental Nutritional Cirrhosis. Arch. Path. 1944, 37, 161–168. [Google Scholar]
- Spikes, J.D. Photodynamic Action: From Paramecium to Photochemotherapy*. Photochem. Photobiol. 1997, 65, S142–S147. [Google Scholar] [CrossRef]
- Spikes, J.D. Porphyrins and Related Compounds as Photodynamic Sensitizers. Ann. N. Y. Acad. Sci. 1975, 244, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Popper, H. Distribution of Vitamin A in Tissue as Visualized by Fluorescence Microscopy. Physiol. Rev. 1944, 24, 205–224. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Quinn, K.P. Optical Imaging Using Endogenous Contrast to Assess Metabolic State. Annu. Rev. Biomed. Eng. 2012, 14, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Rost, B.F.W.D. Fluorescence Microscopy, Volume II by F.W.D. Rost Cambridge University Press, Cambridge and New York (1995) ISBN 0-521-41088-6; 457 Pages; $175.00. Scanning 2006, 18, 593. [Google Scholar] [CrossRef]
- Hamperl, H. Die Fluoreszenzmikroskopie Menschlicher Gewebe Itle. Virchows Arch. Pathol. Anat. Physiol. 1934, 292, 1–51. [Google Scholar] [CrossRef]
- Chance, B.; Legallais, V. Differential Microfluorimeter for the Localization of Reduced Pyridine Nucleotide in Living Cells. Rev. Sci. Instrum. 1959, 30, 732. [Google Scholar] [CrossRef]
- Kunz, W.S. Spectral Properties of Fluorescent Flavoproteins of Isolated Rat Liver Mitochondria. FEBS Lett. 1986, 195, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Kosowski, H.; Schild, L.; Kunz, D.; Halangk, W. Energy Metabolism in Rat Pancreatic Acinar Cells during Anoxia and Reoxygenation. Biochim. Biophys. Acta 1998, 1367, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating NADH and NADPH Fluorescence in Live Cells and Tissues Using FLIM. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, J.M.; Kohen, E.; Viallet, P.; Hirschberg, J.G.; Wouters, A.W.; Kohen, C.; Thorell, B. Microspectrofluorometric Approach to the Study of Free/Bound NAD(P)H Ratio as Metabolic Indicator in Various Cell Types. Photochem. Photobiol. 1982, 36, 585–593. [Google Scholar] [CrossRef]
- Wolfbeiss, O. The Fluorescence of Organic Natural Products. In Molecular Fluorescence Spectroscopy. Methods and Applications; Schulman, S.G., Ed.; John Wiley & Sons: New York, NY, USA, 1985; pp. 167–360. [Google Scholar]
- Chance, B.; Schoener, B.; Oshino, R.; Itshak, F.; Nakase, Y. Oxidation-Reduction Ratio Studies of Mitochondria in Freeze-Trapped Samples. NADH and Flavoprotein Fluorescence Signals. J. Biol. Chem. 1979, 254, 4764–4771. [Google Scholar] [CrossRef]
- Sato, B.; Tanaka, A.; Mori, S.; Yanabu, N.; Kitai, T.; Tokuka, A.; Inomoto, T.; Iwata, S.; Yamaoka, Y.; Chance, B. Quantitative Analysis of Redox Gradient within the Rat Liver Acini by Fluorescence Images: Effects of Glucagon Perfusion. Biochim. Biophys. Acta 1995, 1268, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Heaster, T.M.; Walsh, A.J.; Zhao, Y.; Hiebert, S.W.; Skala, M.C. Autofluorescence Imaging Identifies Tumor Cell-Cycle Status on a Single-Cell Level. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Santin, G.; Paulis, M.; Vezzoni, P.; Pacchiana, G.; Bottiroli, G.; Croce, A.C. Autofluorescence Properties of Murine Embryonic Stem Cells during Spontaneous Differentiation Phases. Lasers Surg. Med. 2013, 45, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.L.; Kaplan, D.L.; Georgakoudi, I. Two-Photon Microscopy for Non-Invasive, Quantitative Monitoring of Stem Cell Differentiation. PLoS ONE 2010, 5, e10075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, K.P.; Sridharan, G.V.; Hayden, R.S.; Kaplan, D.L.; Lee, K.; Georgakoudi, I. Quantitative Metabolic Imaging Using Endogenous Fluorescence to Detect Stem Cell Differentiation. Sci. Rep. 2013, 3, 3432. [Google Scholar] [CrossRef] [Green Version]
- Uchugonova, A.; König, K. Two-Photon Autofluorescence and Second-Harmonic Imaging of Adult Stem Cells. J. Biomed. Opt. 2008, 13, 054068. [Google Scholar] [CrossRef]
- Wright, B.K.; Andrews, L.M.; Markham, J.; Jones, M.R.; Stringari, C.; Digman, M.A.; Gratton, E. NADH Distribution in Live Progenitor Stem Cells by Phasor-Fluorescence Lifetime Image Microscopy. Biophys. J. 2012, 103, L7–L9. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.-W.; Yu, J.-S.; Hsu, S.-H.; Wei, Y.-H.; Lee, O.K.; Dong, C.-Y.; Wang, H.-W. Correlation of NADH Fluorescence Lifetime and Oxidative Phosphorylation Metabolism in the Osteogenic Differentiation of Human Mesenchymal Stem Cell. J. Biomed. Opt. 2015, 20, 017004. [Google Scholar] [CrossRef] [Green Version]
- Stringari, C.; Cinquin, A.; Cinquin, O.; Digman, M.A.; Donovan, P.J.; Gratton, E. Phasor Approach to Fluorescence Lifetime Microscopy Distinguishes Different Metabolic States of Germ Cells in a Live Tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 13582–13587. [Google Scholar] [CrossRef] [Green Version]
- Croce, A.C.; Spano, A.; Locatelli, D.; Barni, S.; Sciola, L.; Bottiroli, G. Dependence of Fibroblast Autofluorescence Properties on Normal and Transformed Conditions. Role of the Metabolic Activity. Photochem. Photobiol. 1999, 69, 364–374. [Google Scholar] [CrossRef]
- König, J.; Ott, C.; Hugo, M.; Jung, T.; Bulteau, A.L.; Grune, T.; Höhn, A. Mitochondrial Contribution to Lipofuscin Formation. Redox Biol. 2017, 11, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Ferrigno, A.; Vairetti, M.; Bertone, R.; Freitas, I.; Bottiroli, G. Autofluorescence Spectroscopy of Rat Liver during Experimental Transplantation Procedure. An Approach for Hepatic Metabolism Assessment. Photochem. Photobiol. Sci. 2005, 4, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; de Simone, U.; Vairetti, M.; Ferrigno, A.; Bottiroli, G. Autofluorescence Properties of Rat Liver under Hypermetabolic Conditions. Photochem. Photobiol. Sci. 2007, 6, 1202–1209. [Google Scholar] [CrossRef]
- Tirapelli, L.F.; Trazzi, B.F.M.; Bagnato, V.S.; Tirapelli, D.P.C.; Kurachi, C.; da Costa, M.M.; Tucci, S.; Cologna, A.J.; Martins, A.C.P. Histopathology and Laser Autofluorescence of Ischemic Kidneys of Rats. Lasers Med Sci. 2009, 24, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Kuzmiak-Glancy, S.; Jaimes, R.; Kay, M.W. Enzyme-Dependent Fluorescence Recovery of NADH after Photobleaching to Assess Dehydrogenase Activity of Isolated Perfused Hearts. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Chorvat, D.; Chorvatova, A. Spectrally Resolved Time-Correlated Single Photon Counting: A Novel Approach for Characterization of Endogenous Fluorescence in Isolated Cardiac Myocytes. Eur. Biophys. J. 2006, 36, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Hartl, B.A. Fluorescence Lifetime Spectroscopy and Imaging in Neurosurgery. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1465–1477. [Google Scholar] [CrossRef]
- Fatakdawala, H.; Griffiths, L.G.; Humphrey, S.; Marcu, L. Time-Resolved Fluorescence Spectroscopy and Ultrasound Backscatter Microscopy for Nondestructive Evaluation of Vascular Grafts. J. Biomed. Opt. 2014, 19, 080503. [Google Scholar] [CrossRef] [Green Version]
- Marsden, M.; Weaver, S.S.; Marcu, L.; Campbell, M.J. Intraoperative Mapping of Parathyroid Glands Using Fluorescence Lifetime Imaging. J. Surg. Res. 2021, 265, 42–48. [Google Scholar] [CrossRef]
- Knorr, F.; Yankelevich, D.R.; Liu, J.; Wachsmann-Hogiu, S.; Marcu, L. Two-Photon Excited Fluorescence Lifetime Measurements through a Double-Clad Photonic Crystal Fiber for Tissue Micro-Endoscopy. J. Biophotonics 2012, 5, 14–19. [Google Scholar] [CrossRef]
- Alfonso-Garcia, A.; Bec, J.; Weyers, B.; Marsden, M.; Zhou, X.; Li, C.; Marcu, L. Mesoscopic Fluorescence Lifetime Imaging: Fundamental Principles, Clinical Applications and Future Directions. J. Biophotonics 2021. [Google Scholar] [CrossRef] [PubMed]
- Marsden, M.; Fukazawa, T.; Deng, Y.-C.; Weyers, B.W.; Bec, J.; Gregory Farwell, D.; Marcu, L. FLImBrush: Dynamic Visualization of Intraoperative Free-Hand Fiber-Based Fluorescence Lifetime Imaging. Biomed. Opt. Express 2020, 11, 5166. [Google Scholar] [CrossRef] [PubMed]
- Thorling, C.A.; Crawford, D.; Burczynski, F.J.; Liu, X.; Liau, I.; Roberts, M.S. Multiphoton Microscopy in Defining Liver Function. J. Biomed. Opt. 2014, 19, 90901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liang, X.; Gravot, G.; Thorling, C.A.; Crawford, D.H.G.; Xu, Z.P.; Liu, X.; Roberts, M.S. Visualizing Liver Anatomy, Physiology and Pharmacology Using Multiphoton Microscopy. J. Biophotonics 2017, 10, 46–60. [Google Scholar] [CrossRef]
- Gratton, E.; Breusegem, S.; Sutin, J.; Ruan, Q.; Barry, N. Fluorescence Lifetime Imaging for the Two-Photon Microscope: Time-Domain and Frequency-Domain Methods. J. Biomed. Opt. 2003, 8, 381–390. [Google Scholar] [CrossRef]
- Digman, M.A.; Caiolfa, V.R.; Zamai, M.; Gratton, E. The Phasor Approach to Fluorescence Lifetime Imaging Analysis. Biophys. J. 2008, 94, L14–L16. [Google Scholar] [CrossRef] [Green Version]
- Ranjit, S.; Dvornikov, A.; Stakic, M.; Hong, S.H.; Levi, M.; Evans, R.M.; Gratton, E. Imaging Fibrosis and Separating Collagens Using Second Harmonic Generation and Phasor Approach to Fluorescence Lifetime Imaging. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Gillette, A.A.; Babiarz, C.P.; VanDommelen, A.R.; Pasch, C.A.; Clipson, L.; Matkowskyj, K.A.; Deming, D.A.; Skala, M.C. Autofluorescence Imaging of Treatment Response in Neuroendocrine Tumor Organoids. Cancers 2021, 13, 1873. [Google Scholar] [CrossRef]
- Leupold, D.; Pfeifer, L.; Hofmann, M.; Forschner, A.; Wessler, G.; Haenssle, H. From Melanocytes to Melanoma Cells: Characterization of the Malignant Transformation by Four Distinctly Different Melanin Fluorescence Spectra (Review). Int. J. Mol. Sci. 2021, 22, 5265. [Google Scholar] [CrossRef]
- Ranjit, S.; Dvornikov, A.; Dobrinskikh, E.; Wang, X.; Luo, Y.; Levi, M.; Gratton, E. Measuring the Effect of a Western Diet on Liver Tissue Architecture by FLIM Autofluorescence and Harmonic Generation Microscopy. Biomed. Opt. Express 2017, 8, 3143. [Google Scholar] [CrossRef] [Green Version]
- Bottiroli, G.; Croce, A.C.; Locatelli, D.; Marchesini, R.; Pignoli, E.; Tomatis, S.; Cuzzoni, C.; DiPalma, S.; Dalfante, M.; Spinellu, P. Natural Fluorescence of Normal and Neoplastic Human Colon: A Comprehensive “Ex Vivo” Study. Lasers Surg. Med. 1995, 16, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, R.; Pignoli, E.; Tomatis, S.; Fumagalli, S.; Sichirollo, A.E.; Dipalma, S.; Dalfante, M.; Spinelli, P.; Croce, A.C.; Bottiroli, G. Ex-Vivo Optical-Properties of Human Colon Tissue. Lasers Surg. Med. 1994, 15, 351–357. [Google Scholar] [CrossRef]
- Krupka, M.; Bartusik-Aebisher, D.; Strzelczyk, N.; Latos, M.; Sieroń, A.; Cieślar, G.; Aebisher, D.; Czarnecka, M.; Kawczyk-Krupka, A.; Latos, W. The Role of Autofluorescence, Photodynamic Diagnosis and Photodynamic Therapy in Malignant Tumors of the Duodenum. Photodiagnosis Photodyn. Ther. 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Moriichi, K.; Fujiya, M.; Okumura, T. The Efficacy of Autofluorescence Imaging in the Diagnosis of Colorectal Diseases. Clin. J. Gastroenterol. 2016, 9, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korneva, Y.S.; Dorosevich, A.E.; Maryakhina, V.S. Fluorescent Diagnostics of Epithelial Neoplasms of Different Colon Parts. Lasers Surg. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Moriichi, K.; Fujiya, M.; Kobayashi, Y.; Murakami, Y.; Iwama, T.; Kunogi, T.; Sasaki, T.; Ijiri, M.; Takahashi, K.; Tanaka, K.; et al. Autofluorescence Imaging Reflects the Nuclear Enlargement of Tumor Cells as Well as the Cell Proliferation Ability and Aberrant Status of the P53, Ki-67, and P16 Genes in Colon Neoplasms. Molecules 2019, 24, 1106. [Google Scholar] [CrossRef] [Green Version]
- Semenov, A.N.; Yakimov, B.P.; Rubekina, A.A.; Gorin, D.A.; Drachev, V.P.; Zarubin, M.P.; Velikanov, A.N.; Lademann, J.; Fadeev, V.V.; Priezzhev, A.V.; et al. The Oxidation-Induced Autofluorescence Hypothesis: Red Edge Excitation and Implications for Metabolic Imaging. Molecules 2020, 25, 1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladurner, R.; Lerchenberger, M.; al Arabi, N.; Gallwas, J.K.S.; Stepp, H.; Hallfeldt, K.K.J. Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules 2019, 24, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lualdi, M.; Cavalleri, A.; Battaglia, L.; Colombo, A.; Garrone, G.; Morelli, D.; Pignoli, E.; Sottotetti, E.; Leo, E. Early Detection of Colorectal Adenocarcinoma: A Clinical Decision Support Tool Based on Plasma Porphyrin Accumulation and Risk Factors. BMC Cancer 2018, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courrol, L.C.; de Oliveira Silva, F.R.; Coutinho, E.L.; Piccoli, M.F.; Mansano, R.D.; Vieira, N.D., Jr.; Schor, N.; Bellini, M.H. Study of Blood Porphyrin Spectral Profile for Diagnosis of Tumor Progression. J. Fluoresc. 2007, 17, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Tristão, V.R.; de Carvalho, F.F.; Gomes, C.Z.; Miranda, A.R.; Vequi-Suplicy, C.C.; Lamy, M.T.; Schor, N.; Bellini, M.H. Study of Blood Porphyrin Spectral Profile for Diagnosis of Chronic Renal Failure. J. Fluoresc. 2010, 20, 665–669. [Google Scholar] [CrossRef]
- Alsalhi, M.; Masilamani, V.; Trinka, V.; Elangovan, M.; Kochupillai, V.; Shah, N. Detection of Cancer by Optical Analysis of Body Fluids—A Single Blind Study. Technol. Cancer Res. Treat. 2011, 10, 145–152. [Google Scholar] [CrossRef]
- Atif, M.; AlSalhi, M.S.; Devanesan, S.; Masilamani, V.; Farhat, K.; Rabah, D. A Study for the Detection of Kidney Cancer Using Fluorescence Emission Spectra and Synchronous Fluorescence Excitation Spectra of Blood and Urine. Photodiagnosis Photodyn. Ther. 2018, 23, 40–44. [Google Scholar] [CrossRef]
- AlSalhi, M.; al Mehmadi, A.M.; Abdo, A.A.; Prasad, S.; Masilamani, V. Diagnosis of Liver Cancer and Cirrhosis by the Fluorescence Spectra of Blood and Urine. Technol. Cancer Res. Treat. 2012, 11, 345–351. [Google Scholar] [CrossRef]
- Al-Salhi, M.; Masilamani, V.; Vijmasi, T.; Al-Nachawati, H.; VijayaRaghavan, A.P. Lung Cancer Detection by Native Fluorescence Spectra of Body Fluids—A Preliminary Study. J. Fluoresc. 2011, 21, 637–645. [Google Scholar] [CrossRef]
- Masilamani, V.; Vijmasi, T.; al Salhi, M.; Govindaraj, K.; Vijaya-Raghavan, A.P.; Antonisamy, B. Cancer Detection by Native Fluorescence of Urine. J. Biomed. Opt. 2010, 15, 057003. [Google Scholar] [CrossRef]
- Kalaivani, R.; Masilamani, V.; AlSalhi, M.S.; Devanesan, S.; Ramamurthy, P.; Palled, S.R.; Ganesh, K.M. Cervical Cancer Detection by Time-Resolved Spectra of Blood Components. J. Biomed. Opt. 2014, 19, 057011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masilamani, V.; Devanesan, S.; Ravikumar, M.; Perinbam, K.; Al Salhi, M.S.A.; Prasad, S.; Palled, S.; Ganesh, K.M.A.; Alsaeed, A.H. Fluorescence Spectral Diagnosis of Malaria: A Preliminary Study. Diagn. Pathol. 2014, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, A.; Bottiroli, G.; di Pasqua, L.; Berardo, C.; Siciliano, V.; Rizzo, V.; Vairetti, M.; Ferrigno, A. Serum and Hepatic Autofluorescence as a Real-Time Diagnostic Tool for Early Cholestasis Assessment. Int. J. Mol. Sci. 2018, 19, 2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, A.C.; Ferrigno, A.; Santin, G.; Vairetti, M.; Bottiroli, G. Bilirubin: An Autofluorescence Bile Biomarker for Liver Functionality Monitoring. J. Biophotonics 2014, 7, 810–817. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mostovnikov, V.A.; Mostovnikova, G.R.; Tret’yakova, A.I. Spectral Fluorescence and Polarization Characteristics of Z,Z-Bilirubin IXα. J. Appl. Spectrosc. 2007, 74, 120–132. [Google Scholar] [CrossRef]
- Mazzoni, M.; Agati, G.; Troup, G.J.; Pratesi, R. Analysis of Wavelength-Dependent Photoisomerization Quantum Yields in Bilirubins by Fitting Two Exciton Absorption Bands. J. Opt. A Pure Appl. Opt. 2003, 5, S374. [Google Scholar] [CrossRef]
- Granucci, G.; Mazzoni, M.; Persico, M.; Toniolo, A. A Computational Study of the Excited States of Bilirubin IX. Phys. Chem. Chem. Phys. 2005, 7, 2594–2598. [Google Scholar] [CrossRef]
- Vreman, H.J.; Kourula, S.; Jašprová, J.; Ludvíková, L.; Klán, P.; Muchová, L.; Vítek, L.; Cline, B.K.; Wong, R.J.; Stevenson, D.K. The Effect of Light Wavelength on in Vitro Bilirubin Photodegradation and Photoisomer Production. Pediatric Res. 2019, 85, 865–873. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Berardo, C.; Bottiroli, G.; Vairetti, M.; di Pasqua, L.G. Spectrofluorometric Analysis of Autofluorescing Components of Crude Serum from a Rat Liver Model of Ischemia and Reperfusion. Molecules 2020, 25, 1327. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.M.; Hong, S.H.; Kipp, Z.A.; Hinds, T.D. Identification of Binding Regions of Bilirubin in the Ligand-Binding Pocket of the Peroxisome Proliferator-Activated Receptor-A (PPARalpha). Molecules 2021, 26, 2975. [Google Scholar] [CrossRef] [PubMed]
- Monici, M.; Agati, G.; Fusi, F.; Pratesi, R.; Paglierani, M.; Santini, V.; Bernabei, P.A. Dependence of Leukemic Cell Autofluorescence Patterns on the Degree of Differentiation. Photochem. Photobiol. Sci. 2003, 2, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; De Simone, U.; Vairetti, M.; Ferrigno, A.; Boncompagni, E.; Freitas, I.; Bottiroli, G. Liver Autofluorescence Properties in Animal Model under Altered Nutritional Conditions. Photochem. Photobiol. Sci. 2008, 7, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Tsin, A.T.C.; Pedrozo-Fernandez, H.A.; Gallas, J.M.; Chambers, J.P. The Fluorescence Quantum Yield of Vitamin A2. Life Sci. 1988, 43, 1379–1384. [Google Scholar] [CrossRef]
- Crespi, F.; Croce, A.C.; Fiorani, S.; Masala, B.; Heidbreder, C.; Bottiroli, G. Autofluorescence Spectrofluorometry of Central Nervous System (CNS) Neuromediators. Lasers Surg. Med. 2004, 34, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L. Characterization of Type I, II, III, IV, and V Collagens by Time-Resolved Laser-Induced Fluorescence Spectroscopy. In Proceedings of the SPIE BiOS 2000 The International Symposium on Biomedical Optics 2000, San Jose, CA, USA, 22 January 2000; pp. 93–101. [Google Scholar] [CrossRef]
- Thornhill, D.P. Separation of a Series of Chromophores and Fluorophores Present in Elastin. Biochem. J. 1975, 147, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.D.; Rogers, M.A.; et al. New Consensus Nomenclature for Mammalian Keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Zatloukal, K.; Stumptner, C.; Fuchsbichler, A.; Fickert, P.; Lackner, C.; Trauner, M.; Denk, H. The Keratin Cytoskeleton in Liver Diseases. J. Pathol. 2004, 204, 367–376. [Google Scholar] [CrossRef]
- Wu, Y.; Qu, J.Y. Autofluorescence Spectroscopy of Epithelial Tissues. J. Biomed. Opt. 2006, 11, 054023. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence Spectroscopy for Monitoring Metabolism in Animal Cells and Tissues. In Methods in Molecular Biology; Springer: Clifton, NJ, USA, 2017; Volume 1560, pp. 15–43. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. New Light in Flavin Autofluorescence. Eur. J. Histochem. 2015, 59, 2576. [Google Scholar] [CrossRef] [Green Version]



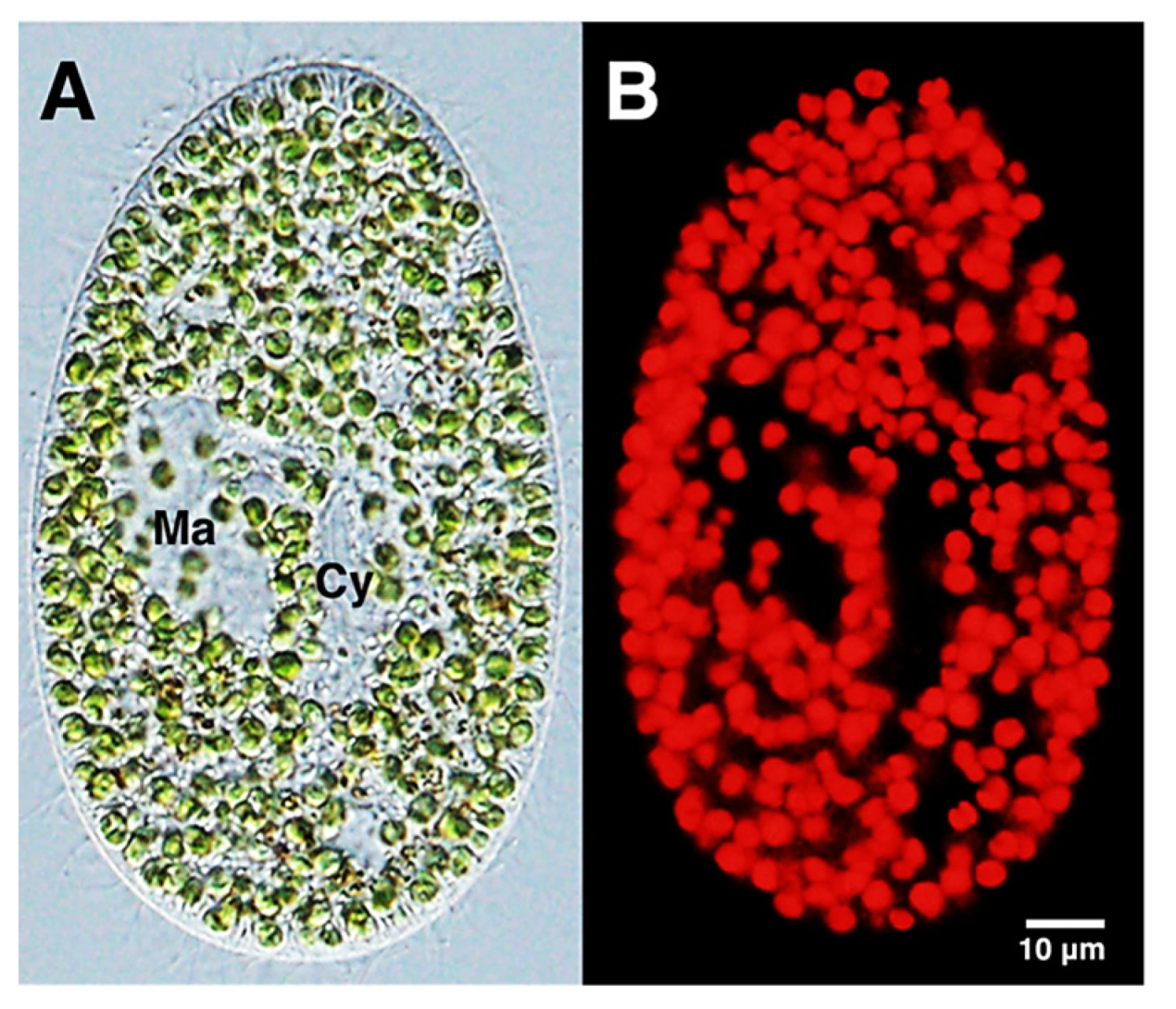
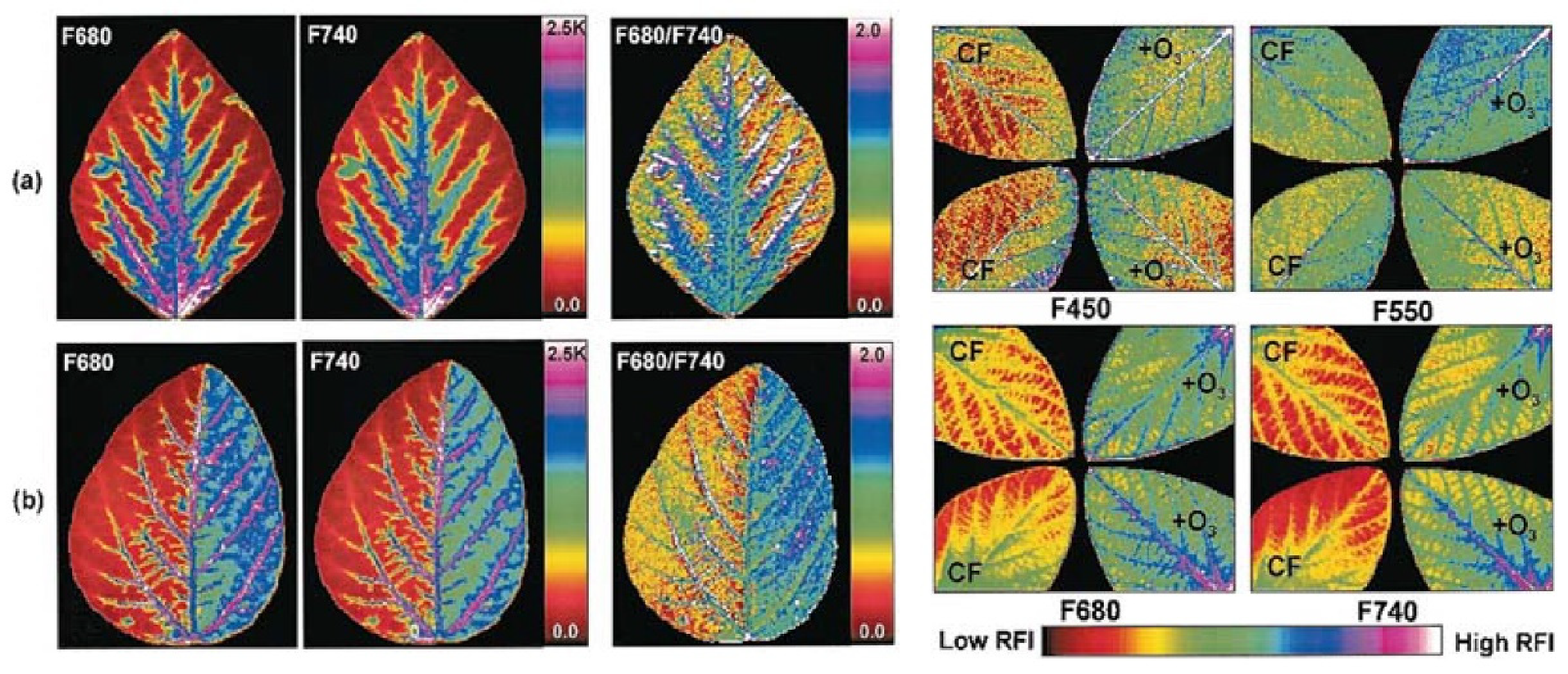


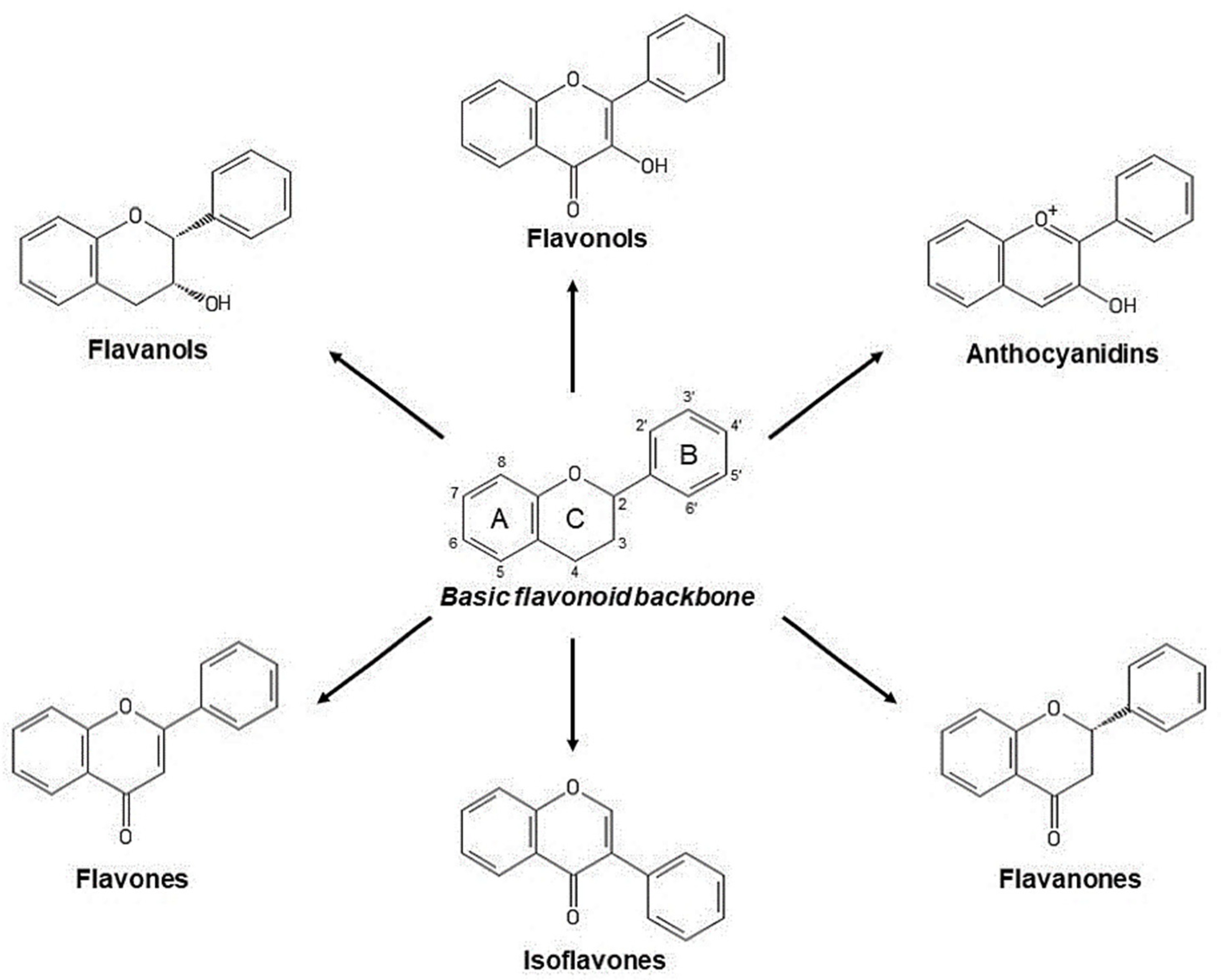

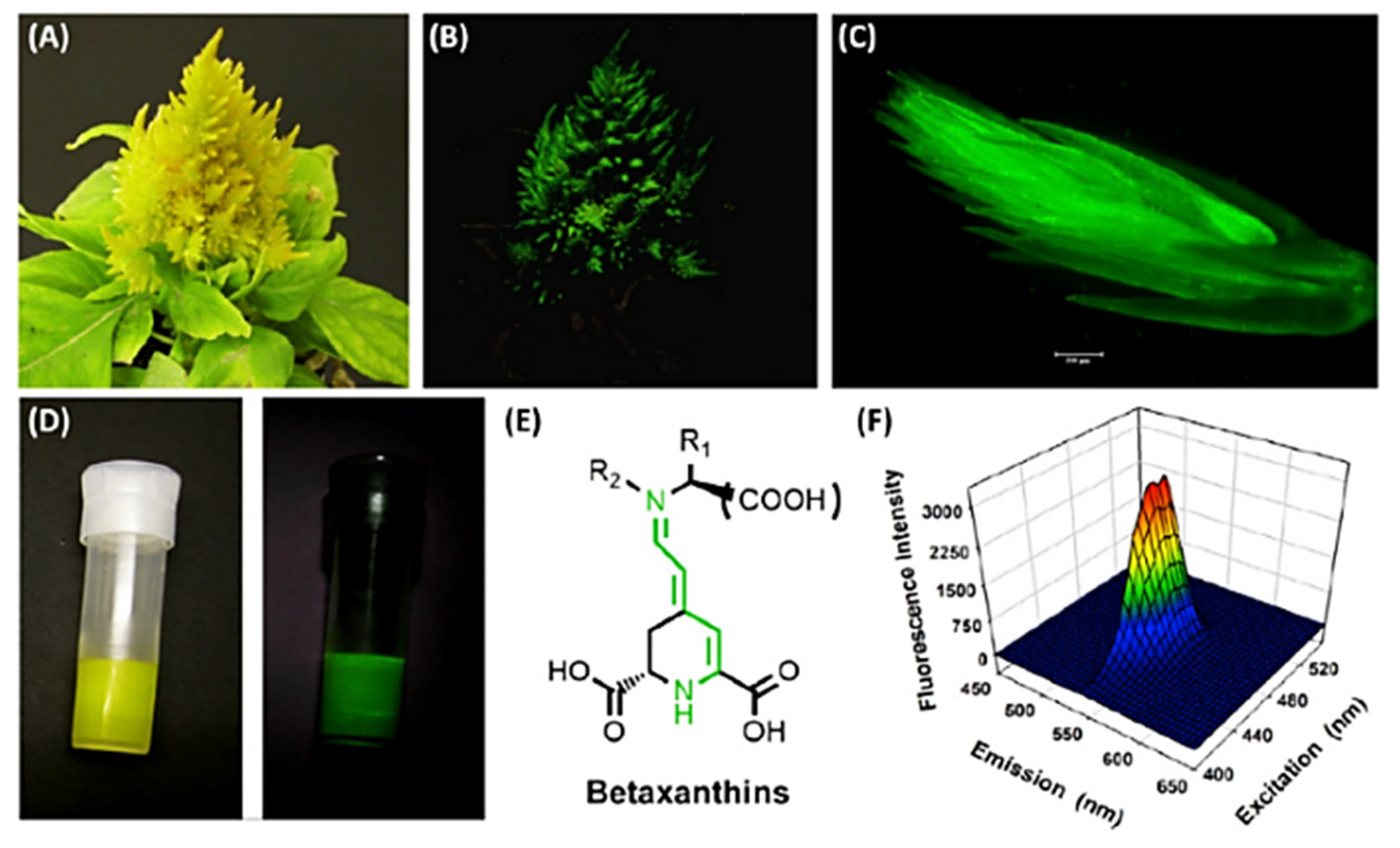



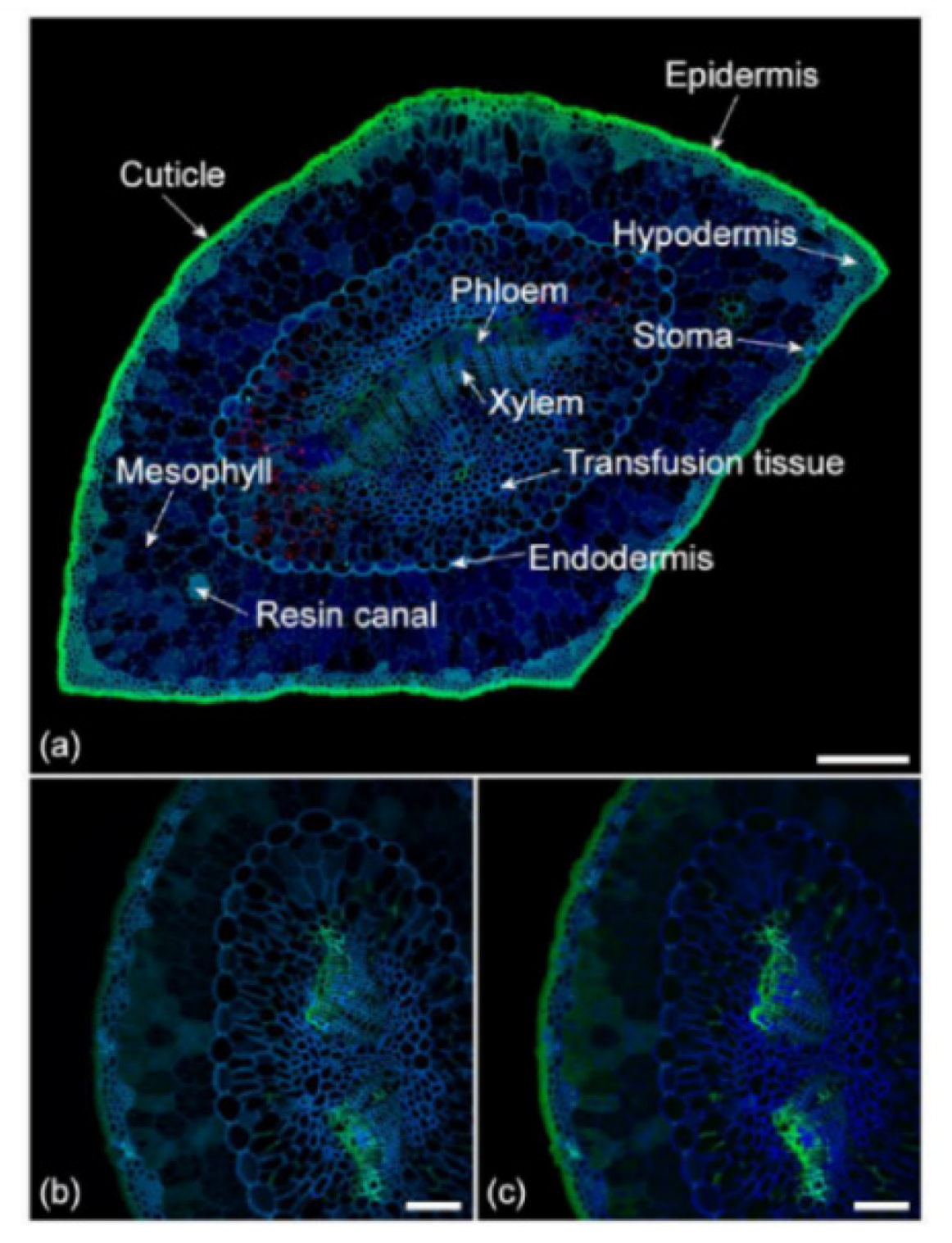



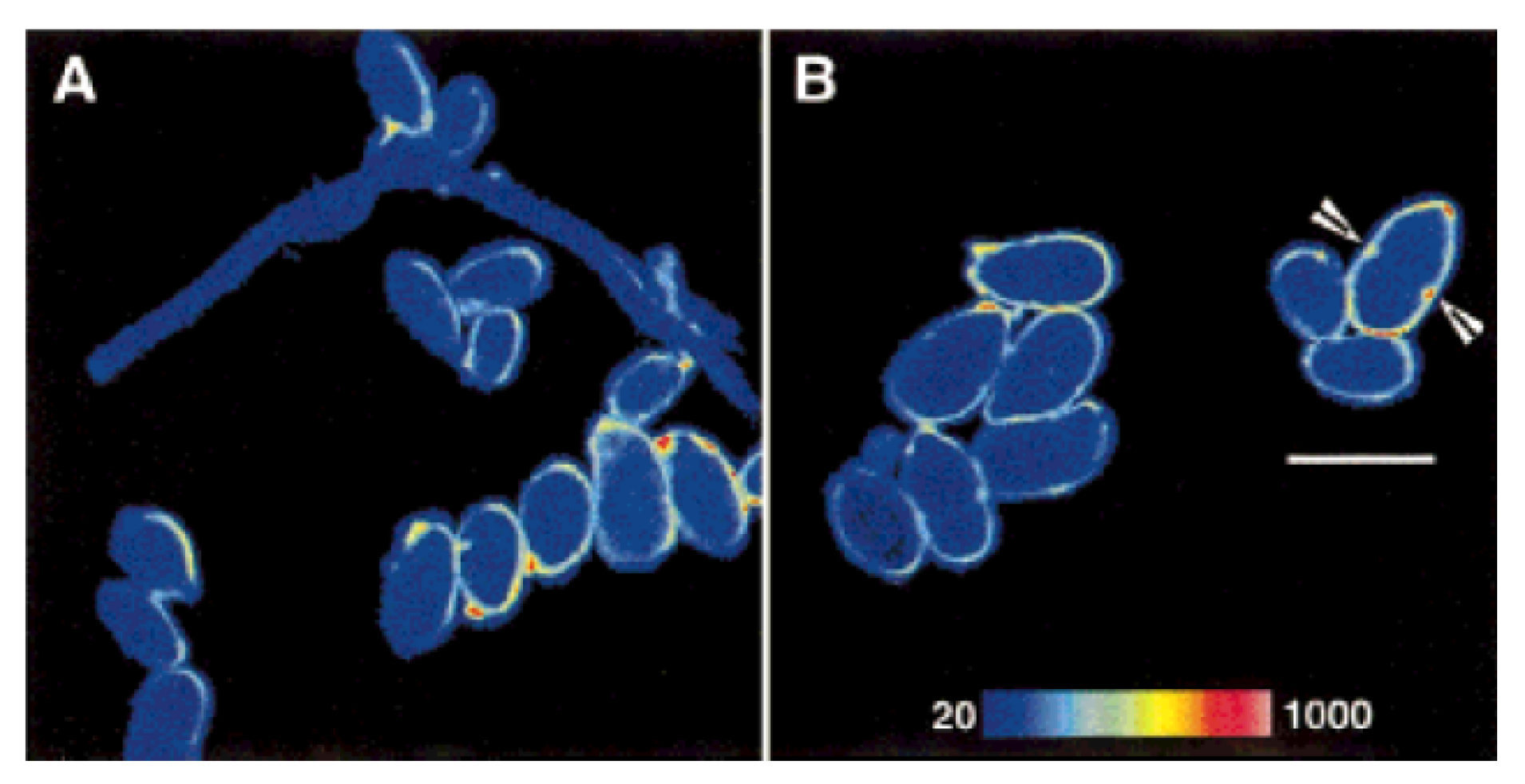











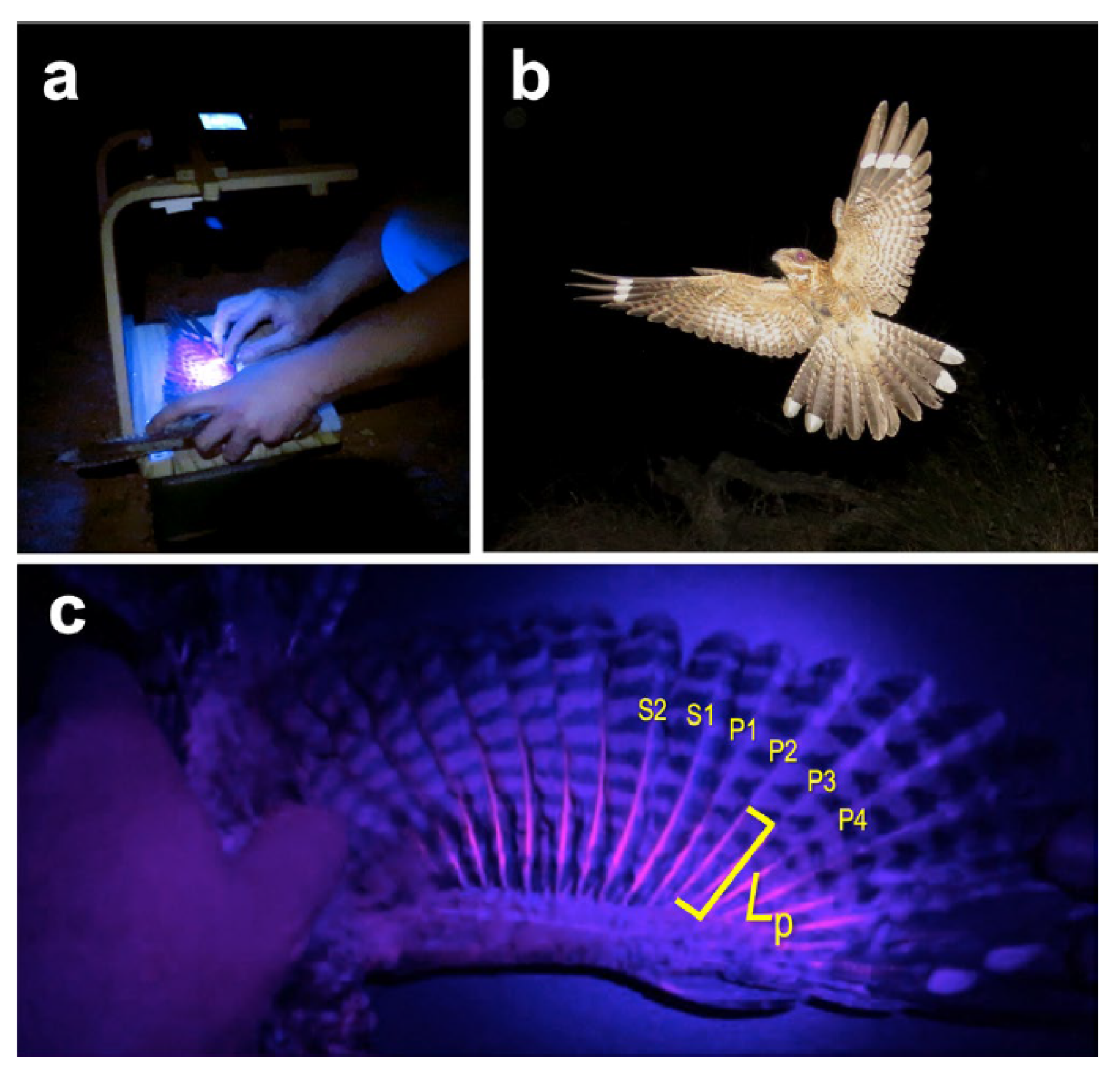
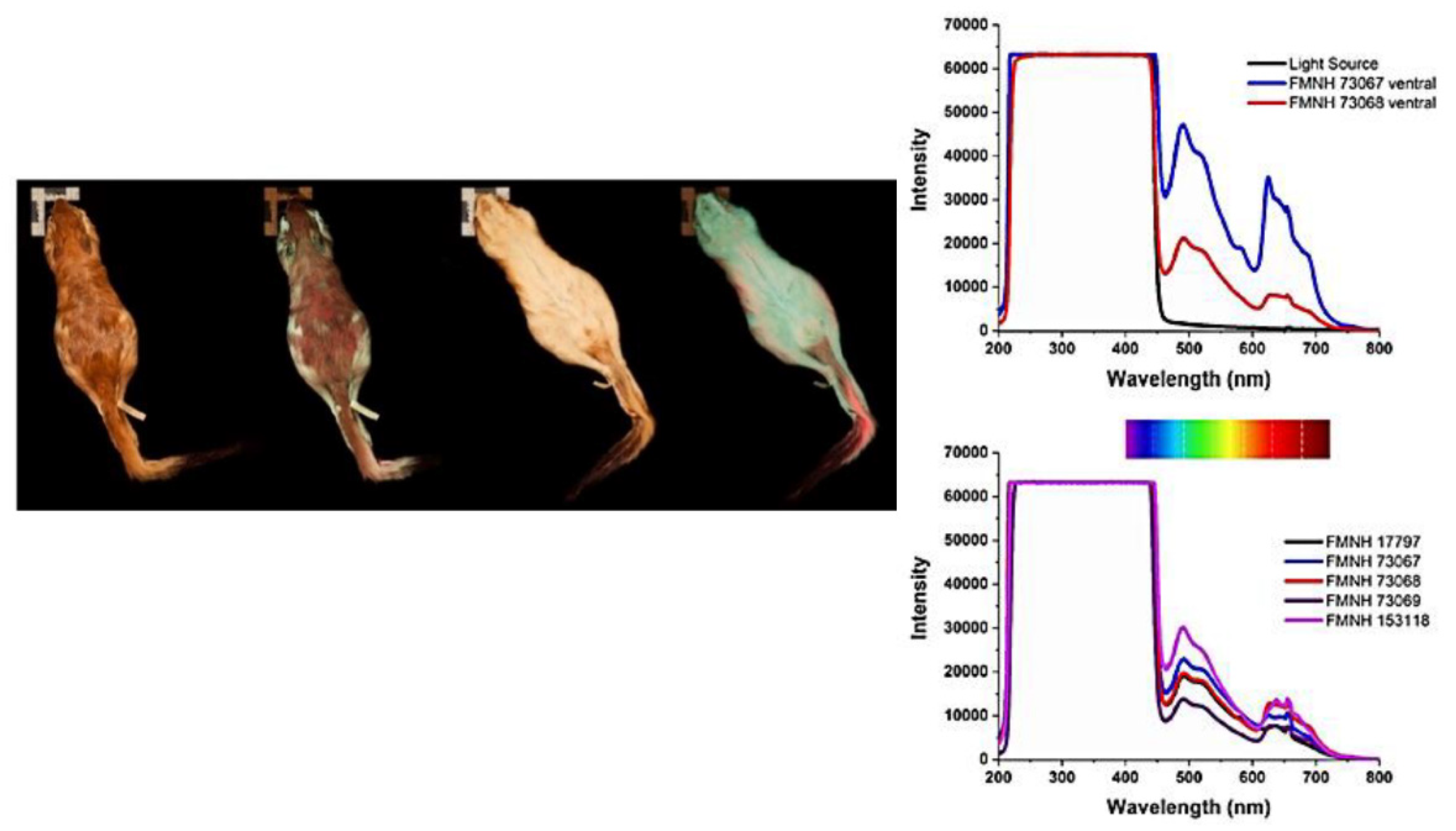





| Fluorophores | Absorption/Excitation (nm) | Emission (nm) | References |
|---|---|---|---|
| Coumarins | 400–450 nm | 450–480 nm | [8] |
| Quinine | 300–400 nm | 400–600 nm | [9] |
| Ferulate | 355 nm | 450–650 nm | [7] |
| Lignin | 355 nm/488 nm | 400–600 nm/500–700 nm | |
| Suberin | 355 nm | 400–600 nm | |
| Flavonoids | 355 nm/488 nm | 400–600 nm/500–700 nm | |
| Kaempherol | 355 nm | 500 nm–800 nm | |
| Dihydrokampherol2 | 355 nm | 500–700 nm | |
| Tannins2 | 488 nm | 490–700 nm | |
| Stilbenes | UV | 400 nm/490 nm–700 nm | |
| Anthocyanins | 310–410 nm 860 nm multiphoton | 360–530 nm 680 nm | |
| Chlorophyll | 350–475 nm/625–690 nm | 640–850 nm | [10,11] |
| Porphyrins | 405 nm | 600–630 nm/670–750 m | [12,13,14] |
| Phycocyanine | 550–670 nm | 650–700 nm | [15] |
| Phycoerythrin | 500–590 nm | 550–650 nm | [16] |
| Riboflavin/flavins | 360/445 nm | 480/540 nm | [17] |
| Nicotinamides | 330–380 nm | 440/480 nm, free/bound | [17] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Chlorophylls | 350–475 nm/ 625–690 nm | 640–850 nm | Light harvesting | [10,11] |
| 400–450 nm/ 650–690 nm | 640–850 nm | Spontaneous fluorescence: energy dispersion Induced fluorescence, biomarker of: alga in endosymbiotic studies; of functionality photosystem II; abiotic stress in higher plants | [19] [11,25,26,27,28,31,34,37] | |
| Chlorophyll a | 400–450 nm/ 650–690 nm | 640–850 nm | ||
| Abiotic stress in algae | [62,67] | |||
| Photophysical biomarker to indirectly estimate flavonoids in fruit ripening; biotic stress | [40,41] | |||
| Carotenoid | 400–520 nm | 520–640 nm | Participation to light harvesting; regulation of energy flow in photosynthesis; excessive energy dissipation | [10,22,23] |
| Abiotic stress in higher plants | [39] | |||
| Phycocyanin Allo-phycocyanin | 550–670 nm | 650–700 nm | Light harvesting, efficient energy conversion, photoprotection in cyanobacteria | [58] |
| Food and pharmaceutic additives | [15,50,51,54,55,56,57,58,59] | |||
| Models to improve photovoltaic applications | [15] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Phenolic compounds | ||||
| 400–520 nm, >550 nm | 600–700 nm/700–800 nm | Fruit phenolic maturity | [93,94,96,128] | |
| Monitoring of own metabolism, trafficking and cell vacuole sequestration | [80,83] | |||
| Anthocyanins | Abiotic/biotic resistance, biomarker of oxidative stress | [85,93] | ||
| Estimated indirectly from chlorophyll AF as biomarkers in fruit ripening; biotic stress; nutritional advance of herbal food | [40,41,97] | |||
| P-Coumaric, Caffeic, Ferulic Acids | 366 nm. | 430–480 nm | Attract and guide insect preys to the pitcher trap in some carnivorous plants | [113] |
| Catechin | 280/450 nm | 300–340, 400–490 nm/ 550–600 nm | Tea quality and origin | [114,117] |
| Ferulate | 355 nm | 450–650 nm | Diagnosis of plant disorders | [119] |
| Coumarins | 400–500 nm | 500–600 nm | Photosensitizers. Chemical derivatives: pharmacology (e.g., anticoagulants); laser and labelling dyes. Spectral properties vary depending on substituents | [121,122,129] |
| Lignin | 355/488 nm | 400–600 nm/500–700 nm | Wood quality, lignocellulosic biomass processing | [124,127] |
| Porphyrins | 405 nm | 600–630 nm/670–750 m | Intermediates in chlorophylls biosynthesis; biomarkers of leaf photooxidation | [6,55] |
| Quercetin Kaempherol, | 440 nm | 520–600 nm | Spice components, e.g., paprika between flavonoids with a role in auxin transport, gravitropism, and phototropism | [109] |
| 355 nm | 500–800 nm | [7,109] | ||
| Betalains | ||||
| Betaxanthins | 430–500 nm | 500–600 nm | Flower industry; studies on flowers vs. pollinator signaling; biomarker in protein tagging, diagnosis, betalamic production | [105,108,130,131] |
| Betacyanins | 480–540 nm | --- | ||
| Alkaloids | ||||
| Quinine | 300–400 nm | 400–600 nm | Traditional medicine; fluorescence reference standard; pharmacology; soft drinks | [9,132,133,134] |
| Campothecin | 300–400 nm | 450–600 nm | Anticancer drug development | [135] |
| Berberine | 420 nm | 500–600 nm | Traditional medicine, anticancer drug development | [136] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Porphyrins | 405 nm | 600–630 nm/670–750 m | Intermediates/catabolites in heme biosynthesis; Biomarkers of bacteria/photodynamic treatment of acne | [143,144,147] |
| Pyoverdine | 360–410 nm 450–480 nm | 450–480 nm | Biomarkers of Pseudomonas aureaginosa infection | [138,141,153] |
| Riboflavin/ flavins | 360/445 nm | 480/540 nm | Intra/extracellular product biomarkers of performance in biowaste processing | [17] |
| Nicotinamides | 330–380 nm | 440/480 nm, free/bound | [17,151] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Ergosterol | 360–370 nm | 400–600 nm | Diet, precursors of vitamin D; marker of fungal infestation. | [160]. |
| Flavins | 360/445 nm | 480/540 nm | Markers of fungal infestation, energy metabolism | [157,169] |
| NADH | 330–380 nm | 440/480 nm, free/bound | [167,168,177] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Pteridines | 320–325 nm | 425–450 nm | Butterfly classifying tool | [181] |
| Papiliochrome II | ~400 nm | ~470 nm | ||
| Resiline | 320–380 nm | 420–470 nm | Functional morphology and biomechanism in insects | [195] |
| Other exoskeleton components (e.g., chitin) | 450–490 nm/530–560 nm | >510 nm/ 570–640 nm | ||
| Lipofuscin-like lipopigment | Near UV, 400–500 nm | >460 nm | Membrane lipid peroxidation in Apis Mellifera | [200,201] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Bilirubin-fatty acid a fatty acid binding protein complex | 450–500 nm | 500–580 nm | Advantage in communication and antioxidant protection in cryptic eels | [207,208,209] |
| Guanine crystals | 450–600 nm | 600–700 nm | Visual contrast and signaling functions in cryptid fishes | [210] |
| Bromo-tryptophan-kynurenine derivative | 300–350 nm/380–420 nm | 430–550 nm | Antibacterial role in some shark species | [212] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Hyloin (dedihydroisoquinolinone) | 400 nm | 420–570 nm | Visual perception in terrestrial environments in Hypsiboas punctatus, a South America frog | [217] |
| Likely a fluorescent-iridophore-specific protein. | 330–390 nm/400–500 nm | 400–500 nm/500–600 nm | Night communication and signaling, proposed in desert gecko Pachydactylus rangei | [215] |
| Undefined, from skull bone tubercles | 353 nm | 360–500 nm | Communication and signaling, proposed in Chameleons | [214] |
| Undefined, from bone | 365 nm | 400–600 nm | Alternate communication vs. loss of high-frequency hearing, proposed in South America pumpkin toadlets | [216] |
| Fluorophores | Absorption /Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Psittacofulvins | 300–400 nm | 450–600 nm | Typical in parrots, proved to drive sexual choice, but un-influent on the social life of the Australian budgerigars Melopsittacus undulatus | [226,227] |
| Porphyrins | 405 nm | 600–630 nm/670–750 m | Typical in crepuscular and nocturnal birds, e.g., Caprimulgus ruficollis, still undefined | [228] |
| Fluorophore | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Porphyrins in furs in: Didelphid marsupials; Pedetidae family rodents, likely endogenous from hair cuticle; Erinaceus europaeus, exogenous from spine microbiome; North American Glaucomys flying squirrels, from the diet | 405 nm | 600–630 nm/670–750 m | Undefined/crepuscular signaling functions | [229,230,231,233] |
| Fluorescing System: Luciferase Enzyme-luminescent Luciferine | Energy Source/Absorption | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| Luciferase-flavine based luciferin | biochemical reactions by redox enzymatic system | ~490 nm | Poorly defined biological meaning, apart reciprocal advantage from symbiosis with fishes; taxonomic and luminescence mechanisms studies. Sensing by biomechanical stimuli in Dinoflagellates, a model for artificial shear stress activatable polymersomes nanoreactors | [235,236,239,241,242,243,245,248,263] |
| Luciferase-flavine based luciferine/yellow fluorescing protein | ~490/~534 nm | Vibrio fischeri, taxonomic, luminescence mechanism studies; association with the squid Euprymna scolopes: host interaction mechanisms studies | [238,249] | |
| Not better-defined luciferin/luciferase couple | ~470 nm | Regulation mechanism by hormonal control in deep sea shark studies | [246] | |
| Luciferase-3-hydroxyhispidin based luciferin | Genome plasticity in evolution, mechanism and possible functional roles of bioluminescence in the ecology of fungi (Agaricales, Armillaria and Mycene species). | [251,252] | ||
| Non enzymatic, calcium dependent photoprotein (aequorin)/energy transfer to natural GFP | Absorption: 200 nm/400 nm/480 nm | 470 nm (aequorin)/509 (GFP) | Expansive production of fluorescing proteins for multipurpose labelling in biomedicine (FP palette generation) | [257,258,259,260,261,262] |
| Fluorophores | Absorption/ Excitation (nm) | Emission (nm) | Main Functions | References |
|---|---|---|---|---|
| NAD(P)H | 330–380 nm | 440/480 nm, free/bound | Energy/redox metabolism, reductive biosynthesis; phlogosis; carcinogenesis | [17,292,351] |
| Flavins | 360/445 nm | 480/540 nm | Energy/redox metabolism; phlogosis; carcinogenesis | [17,289] |
| Vitamin A | near UV/366 nm | <460 nm | Regulation of systemic body metabolism, cell proliferation-differentiation, immunity, development, reproduction; vision | [349,352,353] |
| Fluorescing Free Fatty acids | 330–350 nm | 470–480 nm | Signaling in metabolic syndrome/liver steatosis | [349] |
| Bilirubin | 366–450 nm | 540–600 nm | Heme/iron catabolism, liver functionality | [344] |
| Porphyrins | 405 nm | 600–630 nm/670–750 m | Heme/iron metabolism/PDD/PDT | [12,278,279,280,281,282,283] |
| 5-hydroxytriptamine (5-HT) | near UV/366 nm | <400 nm | Uptake disorder at synapsis | [354] |
| Collagen | 280–325nm) | 400–410 nm | Connective tissue/fibrosis | [17,355] |
| Elastin | 300–400 nm | 400–500 nm | Connective tissue | [356] |
| Cytokeratins | 355 nm/405 nm | 350–500 nm/450–600 nm | Intermediate filaments in epithelia, tumor markers | [357,358,359] |
| Lipofuscin-like lipopigment | Near UV, 400–500 nm | >460 nm | Oxidative stress | [360,361] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.C. Light and Autofluorescence, Multitasking Features in Living Organisms. Photochem 2021, 1, 67-124. https://doi.org/10.3390/photochem1020007
Croce AC. Light and Autofluorescence, Multitasking Features in Living Organisms. Photochem. 2021; 1(2):67-124. https://doi.org/10.3390/photochem1020007
Chicago/Turabian StyleCroce, Anna C. 2021. "Light and Autofluorescence, Multitasking Features in Living Organisms" Photochem 1, no. 2: 67-124. https://doi.org/10.3390/photochem1020007
APA StyleCroce, A. C. (2021). Light and Autofluorescence, Multitasking Features in Living Organisms. Photochem, 1(2), 67-124. https://doi.org/10.3390/photochem1020007






