Electrospun Carbon Nanofibers Decorated with Ag3PO4 Nanoparticles: Visible-Light-Driven Photocatalyst for the Photodegradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Carbon Nanofibers
2.3. Fabrication of Ag3PO4/CNFs Heterostructures
2.4. Characterization
2.5. Photocatalytic Test
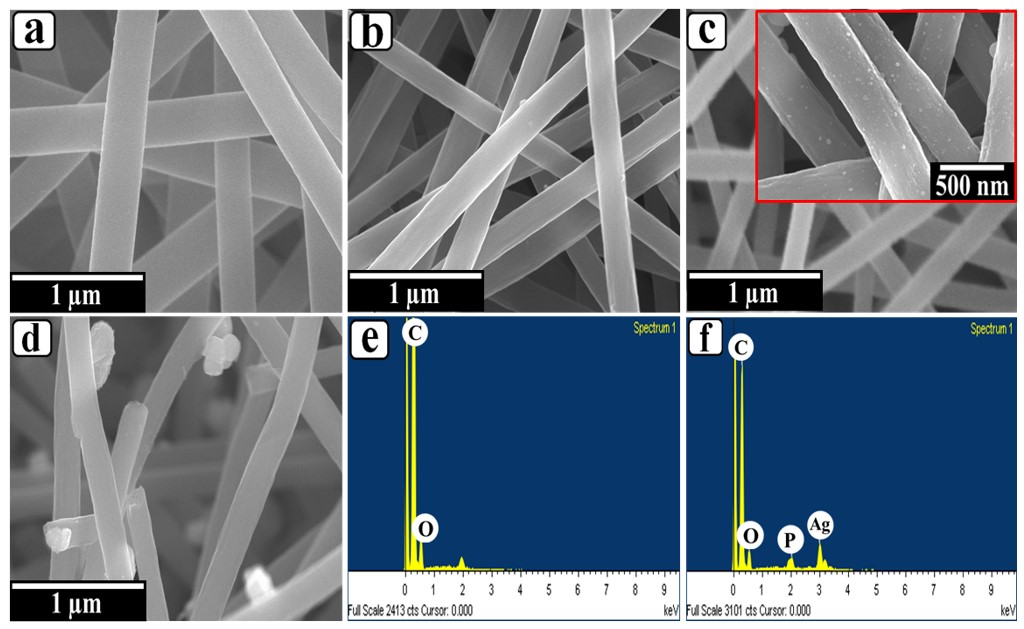
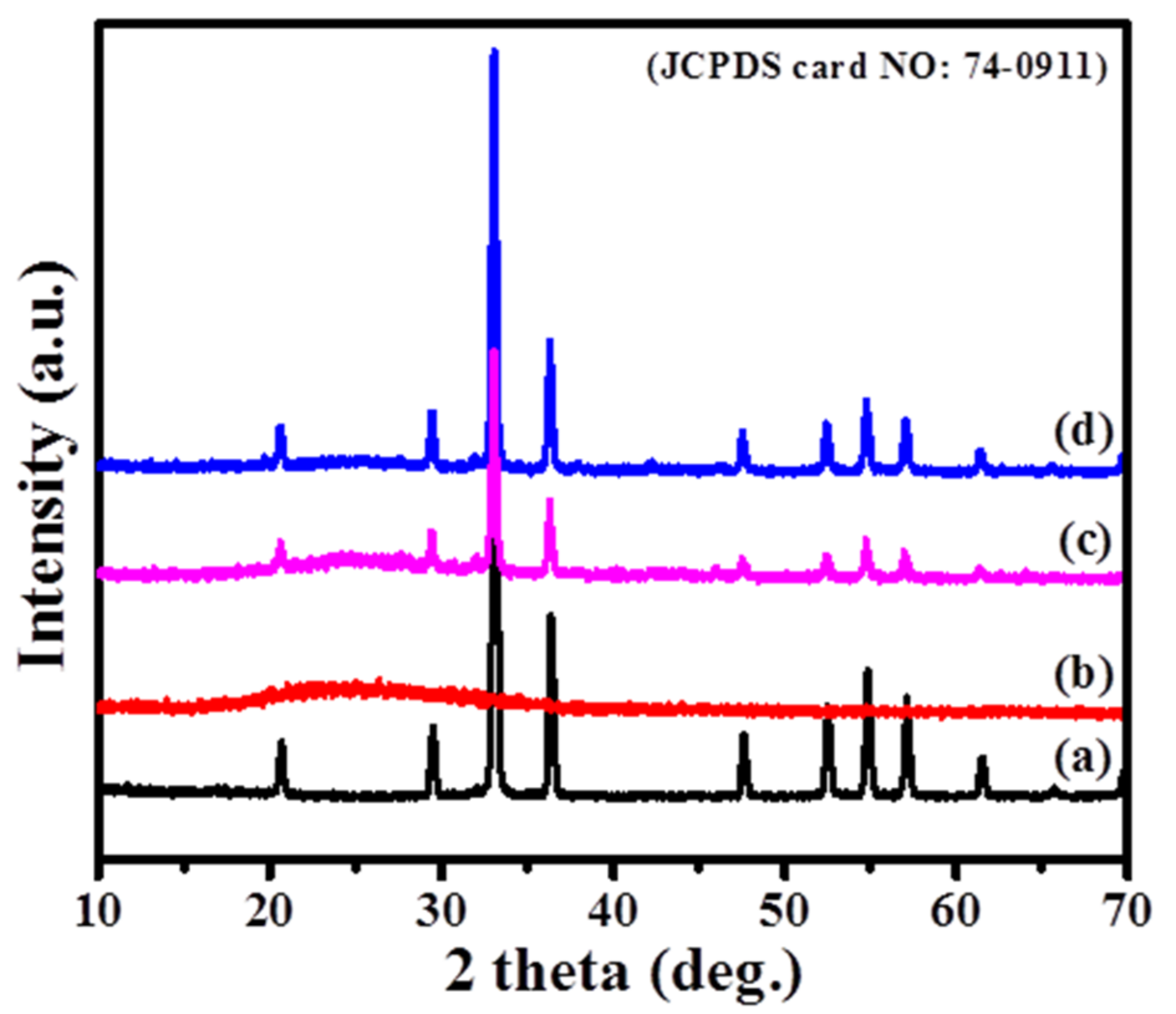
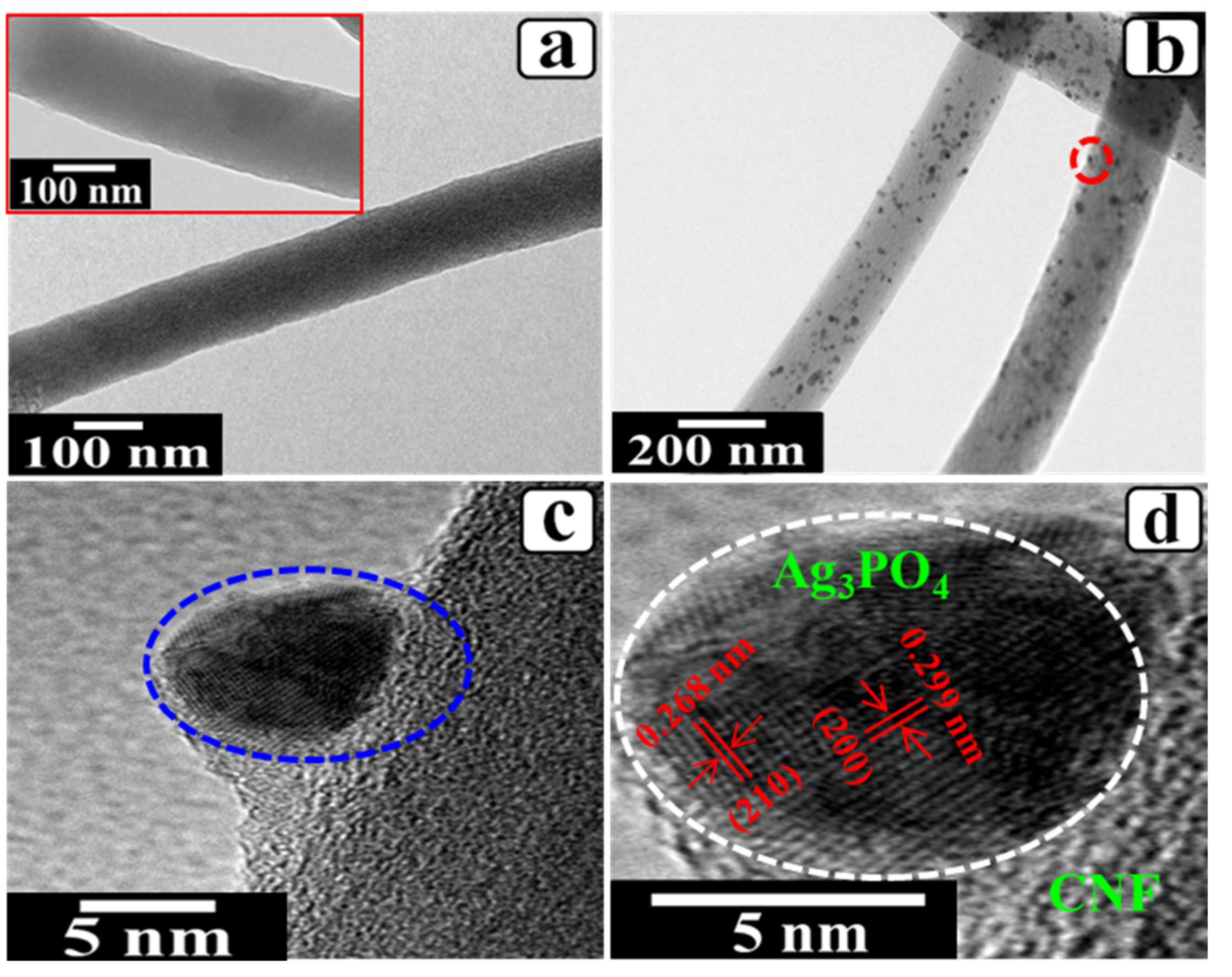
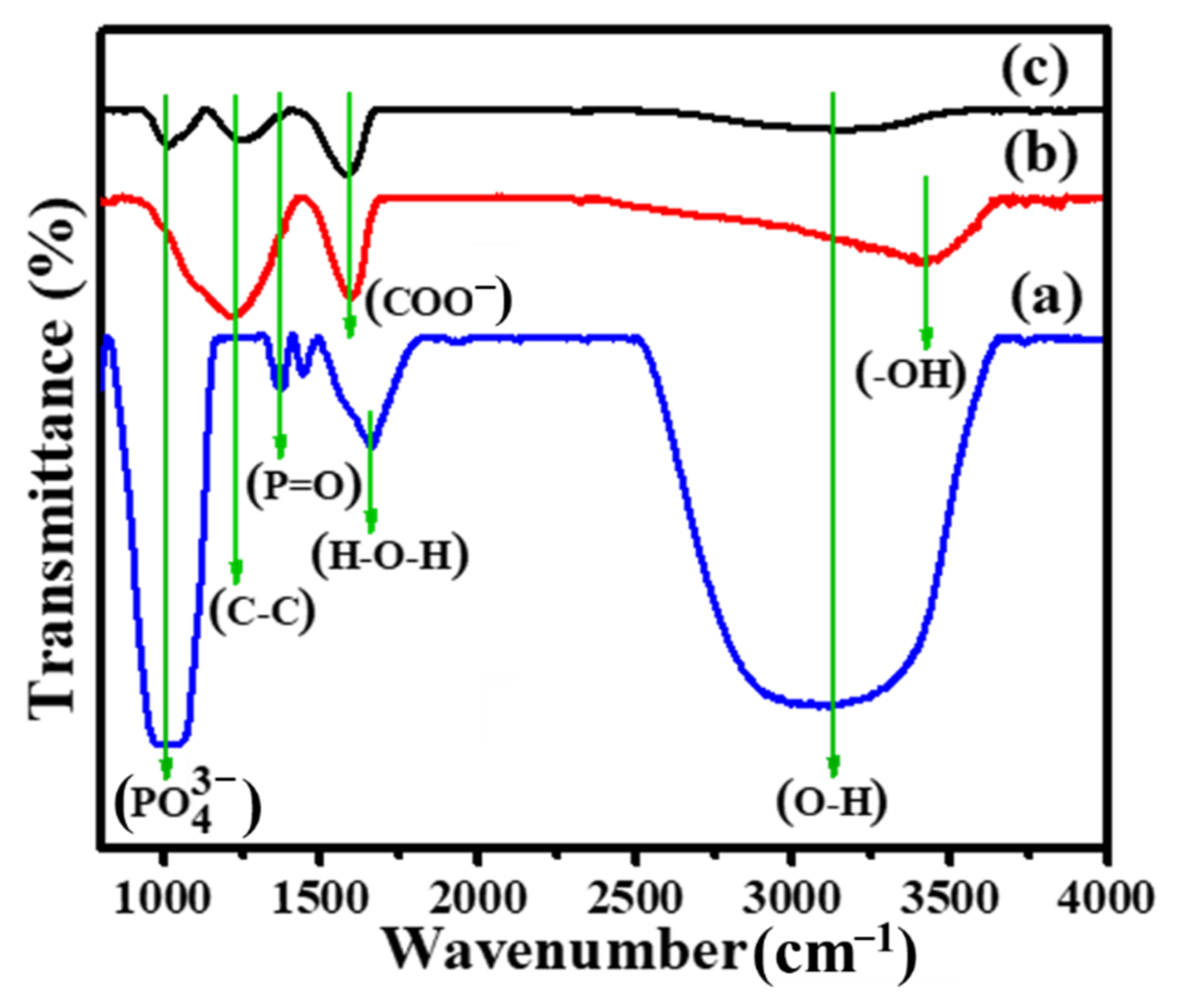
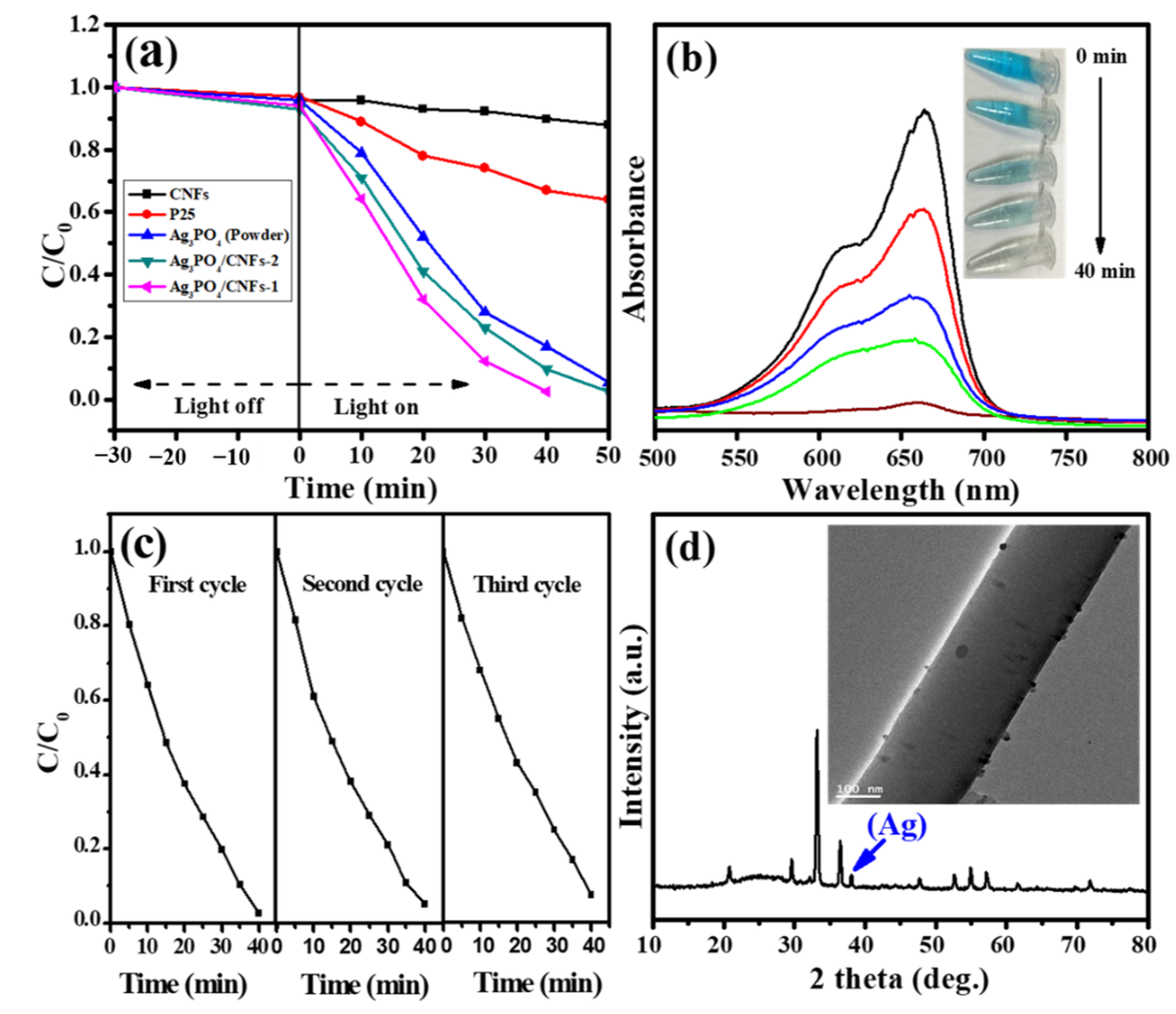
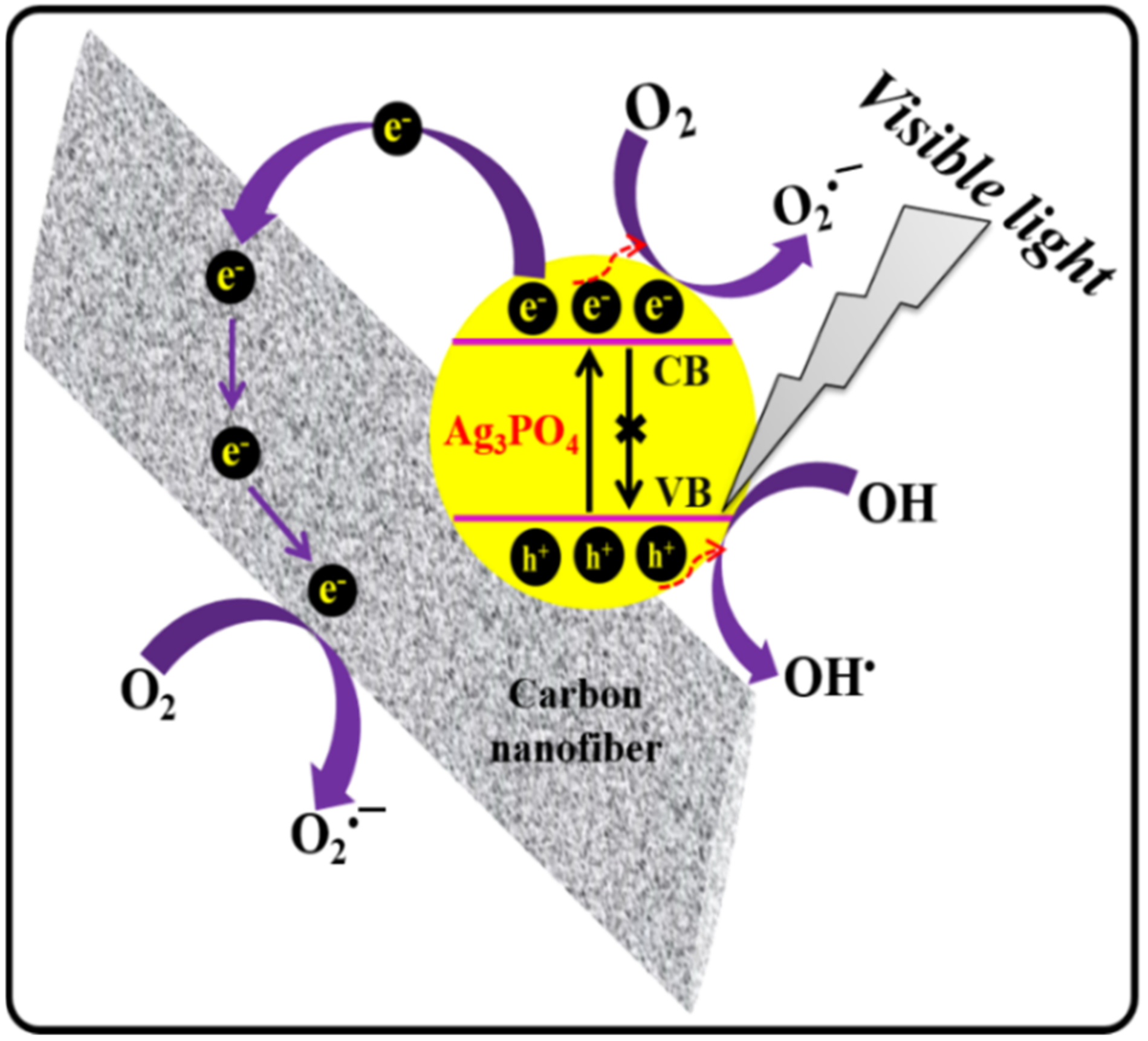
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Valentin, C.D.; Pacchioni, G. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, X.X.; Huang, B.B.; Zhang, X.Y.; Dai, Y. Hydrothermal synthesis and visible-light photocatalytic activity of novel cage-like ferric oxide hollow spheres. Cryst. Growth. Des. 2009, 9, 1474–1480. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. A. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Panthi, G.; Yousef, A.; Barakat, N.A.M.; Khalil, K.A.; Akhter, S.; Choi, Y.; Kim, H.Y. Mn2O3/TiO2 nanofibers with broad-spectrum antibiotics effect and photocatalytic activity for preliminary stage of water desalination. Ceram. Int. 2013, 39, 2239–2246. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.C.; Wang, Y.; Ma, W.H.; Zhao, J.C.; Rajh, T.; Zang, L. Enhanced photocatalytic degradation of dye pollutants under visible irradiation on Al (III)-modified TiO2: Structure, interaction, and interfacial electron transfer. Environ. Sci. Technol. 2008, 42, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Panthi, G.; Kwon, O.H.; Kuk, Y.S.; Gyawali, K.R.; Park, Y.W.; Park, M. Ternary Composite of Co-Doped CdSe@electrospun Carbon Nanofibers: A Novel Reusable Visible LightDriven Photocatalyst with Enhanced Performance. Catalysts 2020, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ci, X.B.; Dai, H.J.; Yin, L.; Shi, H.X. One-step synthesis of highly active Ti-containing Cr-modified MCM-48 mesoporous material and the photocatalytic performance for decomposition of H2S under visible light. Appl. Surf. Sci. 2012, 258, 8258–8263. [Google Scholar] [CrossRef]
- Tang, J.T.; Gong, W.; Cai, T.J.; Xie, T.; Deng, C.; Peng, Z.H.; Deng, Q. Novel visible light responsive Ag@(Ag2S/Ag3PO4) photocatalysts: Synergistic effect between Ag and Ag2S for their enhanced photocatalytic activity. RSC. Adv. 2013, 3, 2543–2547. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Williams, H.S.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Wan, J.; Liu, E.; Fan, J.; Hu, X.; Sun, L.; Tang, C.; Yin, Y.; Li, H.; Hu, Y. In-situ synthesis of plasmonic Ag/Ag3PO4 tetrahedron with exposed {111} facets for high visible-light photocatalytic activity and stability. Ceram. Int. 2015, 41, 6933–6940. [Google Scholar] [CrossRef]
- Panthi, G.; Park, S.J.; Chae, S.H.; Kim, T.W.; Chung, H.J.; Hong, S.T.; Park, M.; Kim, H.Y. Immobilization of Ag3PO4 nanoparticles on electrospun PAN nanofibers via surface oximation: Bifunctional composite membrane with enhanced photocatalytic and antimicrobial activities. J. Ind. Eng. Chem. 2017, 45, 277–286. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Park, M.; Kim, H.K.; Park, S.J. Fabrication of PdS/ZnS NPs doped PVAc hybrid electrospun nanofibers: Effective and reusable catalyst for dye photodegradation. J. Ind. Eng. Chem. 2015, 21, 298–302. [Google Scholar] [CrossRef]
- Yang, X.F.; Cui, H.Y.; Li, Y.; Qin, J.L.; Zhang, R.X.; Tang, H. Fabrication of Ag3PO4-Graphene Composites with Highly Efficient and Stable Visible Light Photocatalytic Performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Tang, J.T.; Liu, Y.H.; Li, H.Z.; Tan, Z.; Li, D.T. A novel Ag3AsO4 visible-light-responsive photocatalyst: Facile synthesis and exceptional photocatalytic performance. Chem. Commun. 2013, 49, 5498–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panthi, G.; Gyawali, K.R.; Park, M. Towards the Enhancement in Photocatalytic Performance of Ag3PO4 Nanoparticles through Sulfate Doping and Anchoring on Electrospun Nanofibers. Nanomaterials 2020, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Gao, Z.D.; Han, K.; Liu, Y.; Song, Y.Y. Synthesis of Magnetically Separable Ag3PO4/TiO2/Fe3O4 Heterostructure with Enhanced Photocatalytic Performance under Visible Light for Photoinactivation of Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 15122–15131. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Dong, C.; Wu, K.L.; Li, M.R.; Liu, L.; Wei, X.W. Synthesis of Ag3PO4-ZnO nanorod composites with high visible-light photocatalytic activity. Catal. Commun. 2014, 46, 32–35. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Jiang, Y.; Li, R.; Li, Y.; Tang, H. Bifunctional TiO2/Ag3PO4/graphene composites with superior visible light photocatalytic performance and synergistic inactivation of bacteria. RSC Adv. 2014, 4, 18627–18636. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Sun, D.D. Graphene oxide enwrapped Ag3PO4 composite: Towards a highly efficient and stable visible-light-induced photocatalyst for water purification. Catal. Sci. Technol. 2012, 2, 2525–2532. [Google Scholar] [CrossRef]
- Liang, Q.; Shi, Y.; Ma, W.; Li, Z.; Yang, X. Enhanced photocatalytic activity and structural stability by hybridizing Ag3PO4 nanospheres with graphene oxide sheets. Phys. Chem. Chem. Phys. 2012, 14, 15657–15665. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, L.; Zhang, M.; Zhou, G.; Fei, H.; Shi, H.; Dai, H. Synthesis and characterization of Ag3PO4/multiwalled carbon nanotube composite photocatalyst with enhanced photocatalytic activity and stability under visible light. J Mater. Sci. 2014, 49, 1585–1593. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Song, Y.; Zhu, J.; Xu, Y.; Yan, J.; Song, Y.X.; Li, H. CNT/Ag3PO4 composites with highly enhanced visible light photocatalytic activity and stability. Chem. Eng. J. 2014, 241, 35–42. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Kong, Y.; Cui, B.; Zhu, R.; Li, D. Nanocomposite of attapulgite-Ag3PO4 for Orange II photodegradation. Appl. Catal. B Environ. 2014, 144, 36–40. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L.; Wu, Q.; Yao, W. Novel Synthesis of Ag3PO4/CNFs/Silica-fiber hybrid composite as an efficient photocatalyst. J. Taiwan Inst. Chem. Eng. 2016, 63, 506–511. [Google Scholar] [CrossRef]
- Unalan, H.; Wei, D.; Suzuki, K.; Dalal, S.; Hiralal, P.; Matsumoto, H.; Imaizumi, S.; Minagawa, M.; Tanioka, A.; Flewitt, A.; et al. Photoelectrochemical cell using dye sensitized zinc oxide nanowires grown on carbon fibers. Appl. Phys. Lett. 2008, 93, 133116–133118. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO-Carbon Nanofiber Heteroarchitectures. ACS Appl. Mater Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, M.; Zhang, Z.; Zhang, P.; Chen, B.; Liu, Y.N. In2O3 nanocubes/carbon nanofibers heterostructures with high visible light photocatalytic activity. J. Mater. Chem. 2012, 22, 1786–1793. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, O.S.; Park, S.J.; Park, E.Y.; You, S.A.; Yoon, H.; Jang, J. Fabrication of Ultrafine Metal-Oxide-Decorated Carbon Nanofibers for DMMP Sensor Application. ACS Nano 2011, 5, 7992–8001. [Google Scholar] [CrossRef]
- Lakshminarayanan, P.V.; Toghiani, H.; Pittman, C.U., Jr. Nitric acid oxidation of vapor grown carbon nanofibers. Carbon 2004, 42, 2433–2442. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, S.J. Influence of air-oxidation on electric double layer capacitances of multi-walled carbon nanotube electrodes. Curr. Appl. Phys. 2010, 10, 241–244. [Google Scholar] [CrossRef]
- Okajima, K.; Ohta, K.; Sudoh, M. Capacitance behavior of activated carbon fibers with oxygen-plasma treatment. Electrochim. Acta 2005, 50, 2227–2231. [Google Scholar] [CrossRef]
- Oh, H.S.; Kim, K.; Ko, Y.J.; Kim, H. Effect of chemical oxidation of CNFs on the electrochemical carbon corrosion in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2010, 35, 701–708. [Google Scholar] [CrossRef]
- Yu, H.; Jin, Y.; Peng, F. Kinetically Controlled Side-Wall Functionalization of Carbon Nanotubes by Nitric Acid Oxidation. J. Phys. Chem. C 2008, 112, 6758–6763. [Google Scholar] [CrossRef]
- Khan, A.; Qamar, M.; Muneer, M. Synthesis of highly active visible-light-driven colloidal silver orthophosphate. Chem. Phys. Lett. 2012, 519–520, 54–58. [Google Scholar] [CrossRef]
- Lafdi, K.; Wright, M.A. Carbon Fibers. In Handbook of Composites; Peters, S.T., Ed.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Zhou, Z.; Lai, C.; Zhang, L.; Qian, Y.; Hou, H.; Reneker, D.H. Development of carbon nanofibers from aligned electrospun polyacrylonitrile nanofiber bundles and characterization of their microstructural, electrical, and mechanical properties. Polymer 2009, 50, 2999–3006. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z.; Xu, S.; Han, D.; Lu, D. Enhanced visible-light photocatalytic activities of Ag3PO4/MWCNT nanocomposites fabricated by facile in situ precipitation method. J. Alloys Compd. 2014, 596, 19–24. [Google Scholar] [CrossRef]
- Miller, L.M.; Vairavamurthy, V.; Chance, M.R.; Mendelsohn, R.; Paschalis, E.P.; Betts, F.; Boskey, A.L. In situ analysis of mineral content and crystallinity in bone using infrared micro spectroscopy of the v4 PO43− vibration. Biochim. Biophys. Acta Gen. Subj. 2001, 1527, 11–19. [Google Scholar] [CrossRef]
- Moustafa, Y.M.; El-Egili, K. Infrared studies on the structure of sodium phosphate glasses. J. Non-Cryst. Solids 1998, 240, 144–153. [Google Scholar] [CrossRef]
- Tang, T.; Shi, Z.; Yin, J. Poly(benzimidazole) functionalized multi-walled carbon nanotubes/100% acidified poly(hydroxyaminoether) composites: Synthesis, characterization and properties. Mater. Chem. Phys. 2011, 129, 356–364. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yang, H.H.; Zhang, X.S.; Li, P.; Zhou, X.G.; Niu, L.; Yuan, W.K. Electrophoretic deposition of network like carbon nanofibers as a palladium catalyst support for ethanol ixidation in alkaline media. Carbon 2010, 48, 48–3323. [Google Scholar] [CrossRef]
- Mawhinney, D.; Naumenko, V.; Kuznetsova, A.; Yates, J.; Liu, J.R. Smalley, Infrared Spectral Evidence for the Etching of Carbon Nanotubes: Ozone Oxidation at 298 K. J. Am. Chem. Soc. 2000, 122, 2383–2384. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Wang, P.; Xing, L.; Fang, Y.; Zhai, Y.; Dong, S. One-pot, water-phase approach to high-quality graphene/TiO2 composite nanosheets. Chem. Commun. 2010, 46, 7148–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panthi, G.; Ranjit, R.; Kim, H.Y.; Mulmi, D.D. Size dependent optical and antibacterial properties of Ag3PO4 synthesized by facile precipitation and colloidal approach in aqueous solution. Optik 2018, 156, 60–68. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, K.; Zhang, W.; Yang, B.; Chi, F.; Ran, S.; Liu, X. One step synthesis of Ag/Ag3PO4/BiPO4 double-heterostructured nanocomposites with enhanced visible-light photocatalytic activity and stability. Ceram. Int. 2015, 40, 8087–8092. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M.; Park, S.J.; Kim, H.K. PAN electrospun nanofibers reinforced with Ag2CO3 nanoparticles: Highly efficient visible light photocatalyst for photodegradation of Organic Contaminants in waste water. Macromol. Res. 2015, 23, 149–155. [Google Scholar] [CrossRef]
- Dong, P.Y.; Wang, Y.H.; Cao, B.C.; Xin, S.Y.; Guo, L.N.; Zhang, J.; Li, F.H. Ag3PO4/reduced graphite oxide sheets nanocomposites with highly enhanced visible light photocatalytic activity and stability. Appl. Catal. B Environ. 2013, 132–133, 45–53. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panthi, G.; Park, M. Electrospun Carbon Nanofibers Decorated with Ag3PO4 Nanoparticles: Visible-Light-Driven Photocatalyst for the Photodegradation of Methylene Blue. Photochem 2021, 1, 345-357. https://doi.org/10.3390/photochem1030022
Panthi G, Park M. Electrospun Carbon Nanofibers Decorated with Ag3PO4 Nanoparticles: Visible-Light-Driven Photocatalyst for the Photodegradation of Methylene Blue. Photochem. 2021; 1(3):345-357. https://doi.org/10.3390/photochem1030022
Chicago/Turabian StylePanthi, Gopal, and Mira Park. 2021. "Electrospun Carbon Nanofibers Decorated with Ag3PO4 Nanoparticles: Visible-Light-Driven Photocatalyst for the Photodegradation of Methylene Blue" Photochem 1, no. 3: 345-357. https://doi.org/10.3390/photochem1030022






