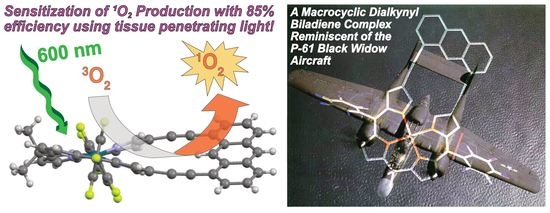
A P-61 Black Widow Inspired Palladium Biladiene Complex for Efficient Sensitization of Singlet Oxygen Using Visible Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Compound Characterization
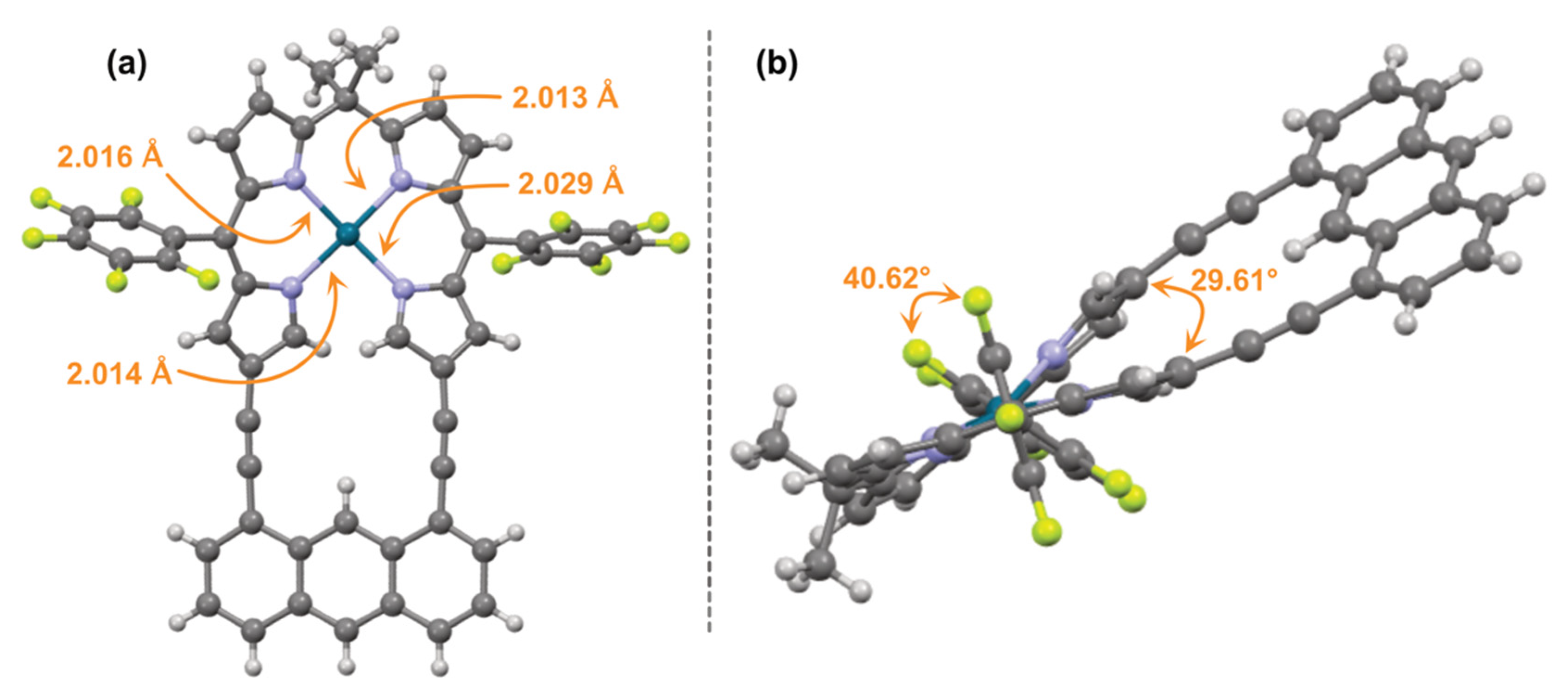
2.2. X-ray Crystallography
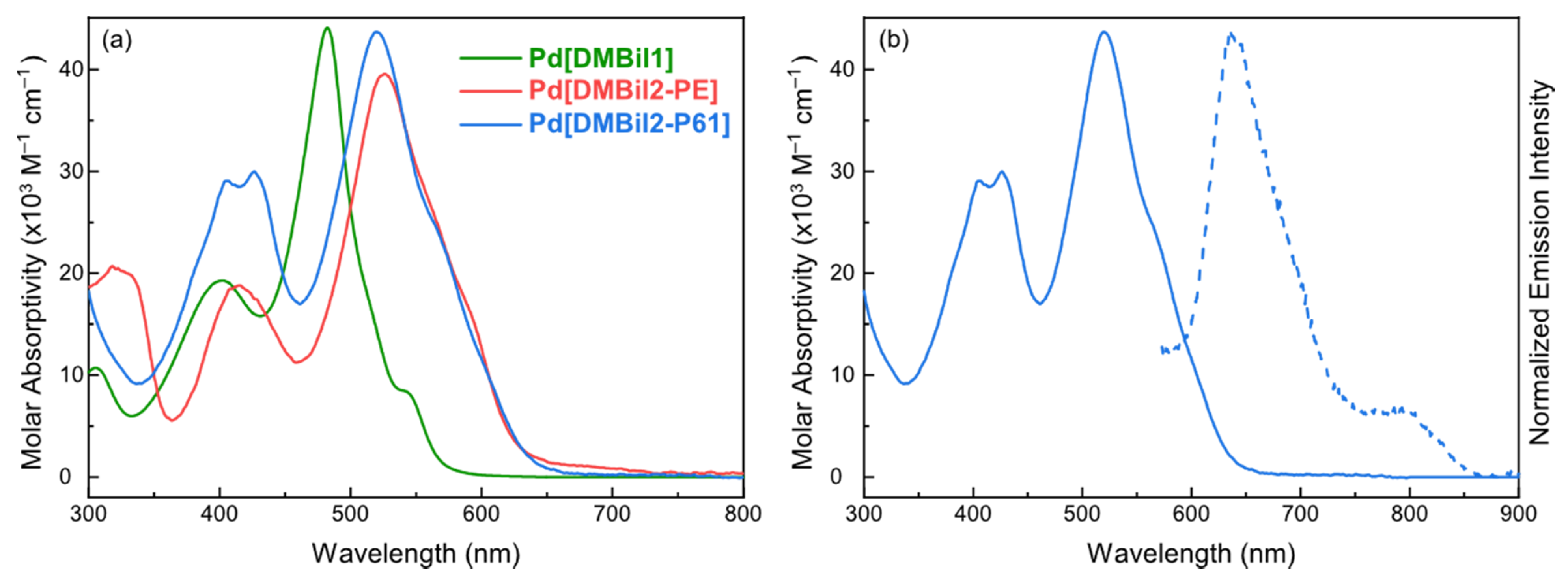
2.3. UV-Vis Absorption Experiments
2.4. Emission Experiments
2.5. Singlet Oxygen Experiments
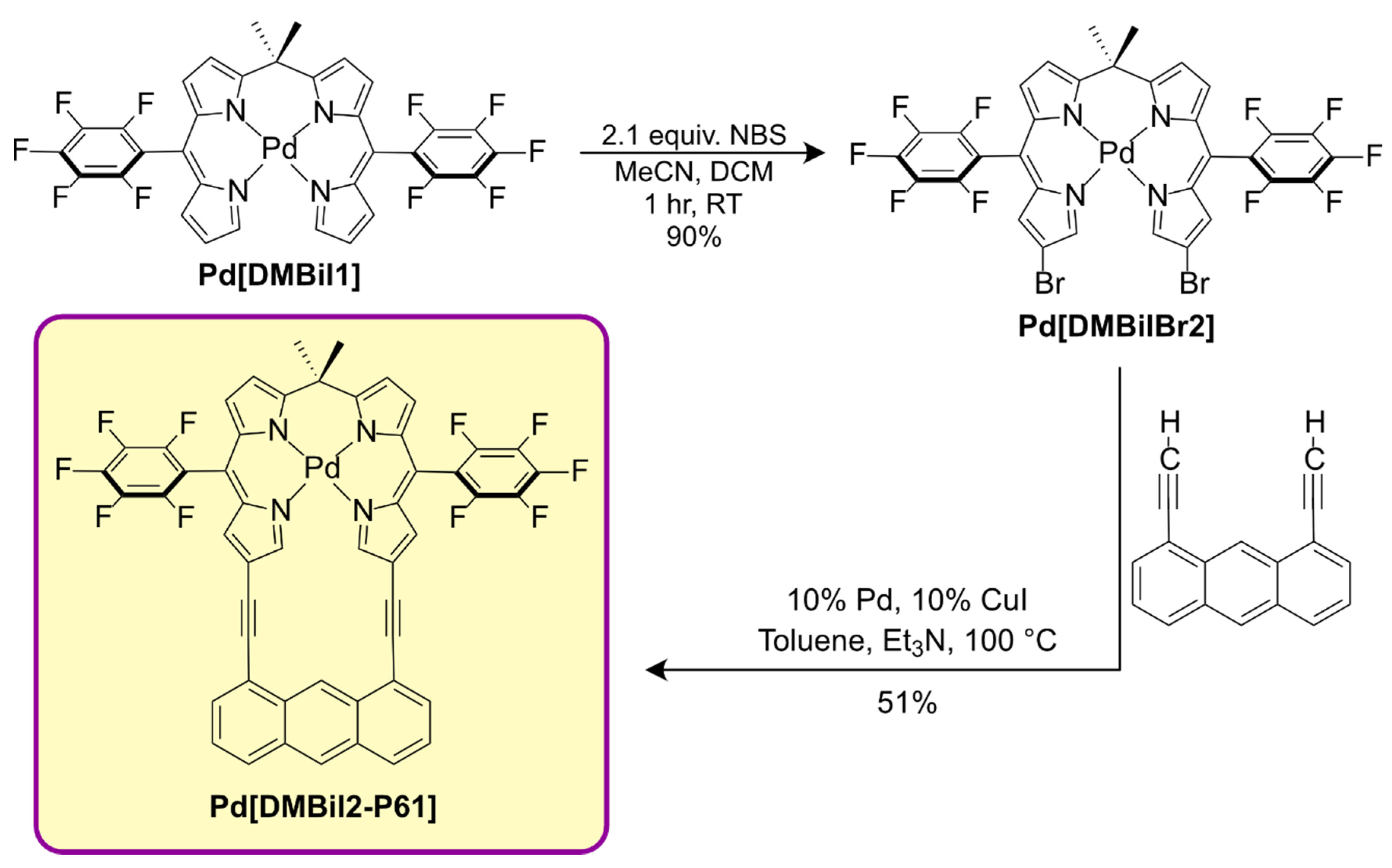
3. Results and Discussion
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferreira dos Dantos, A.; Queiroz de Almeida, D.R.; Ferriera Terra, L.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar]
- Dolmans, D.; Fukumura, D.; Jain, R. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28, 1734–1742. [Google Scholar] [CrossRef]
- Miller, J. Photodynamic Therapy: The Sensitization of Cancer Cells to Light. J. Chem. Educ. 1999, 76, 592–594. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Robertson, C.A.; Hawkins Evans, D.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photo-dynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Abdelkarim-Elafifi, H.; Parada-Avendaño, I.; Arnabat-Dominguez, J. Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review. Antibiotics 2021, 10, 1106. [Google Scholar] [CrossRef]
- Lotufo, M.A.; Tempestini Horliana, A.C.R.; Santana, T.; de Queiroz, A.C.; Gomes, A.O.; Motta, L.J.; Ferrari, R.A.M.; dos Santos Fernandes, K.P.; Bussadori, S.K. Efficacy of Photodynamic Therapy on the Treatment of Herpes Labialis: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 29, 101536. [Google Scholar] [CrossRef]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Gonçalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Bonfim-Mendonça, P.S.; Kioshima, E.S. A Systematic Review of Photodynamic Therapy as an Antiviral Treatment: Potential Guidance for Dealing with SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2021, 34, 102221. [Google Scholar] [CrossRef] [PubMed]
- Tosa, M.; Ogawa, R. Photodynamic Therapy for Keloids and Hypertrophic Scars: A Review. Scars Burn. Heal. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2004, 73, 1–28. [Google Scholar] [CrossRef]
- Diwu, Z.; Lown, J.W. Phototherapeutic potential of alternative photosensitizers to porphyrins. Pharmacol. Ther. 1994, 63, 1–35. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyziol, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Pistner, A.J.; Yap, G.P.; Rosenthal, J. A Tetrapyrrole Macrocycle Displaying a Multielectron Redox Chemistry and Tunable Absorbance Profile. J. Phys. Chem. C 2012, 32, 16918–16924. [Google Scholar] [CrossRef]
- Pistner, A.J.; Lutterman, D.A.; Ghidiu, M.J.; Ma, Y.Z.; Rosenthal, J. Synthesis, electrochemistry, and photophysics of a family of phlorin macrocycles that display cooperative fluoride binding. J. Am. Chem. Soc. 2013, 17, 6601–6607. [Google Scholar] [CrossRef][Green Version]
- O’Brien, A.Y.; McGann, J.P.; Geier, G.R., III. Dipyrromethane + dipyrromethanedicarbinol routes to an electron deficient meso-substituted phlorin with enhanced stability. J. Org. Chem. 2007, 11, 4084–4092. [Google Scholar] [CrossRef]
- Pistner, A.J.; Lutterman, D.A.; Ghidiu, M.J.; Walker, E.; Yap, G.P.A.; Rosenthal, J. Factors Controlling the Spectroscopic Properties and Supramolecular Chemistry of an Electron Deficient 5,5-Dimethylphlorin Architecture. J. Phys. Chem. C 2014, 118, 14124–14132. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chun, H.-J.; Donnelly, C.C.; Geier, G.R., III. Two-Step, One-Flask Synthesis of a Me-so-Substituted Phlorin. J. Org. Chem. 2016, 81, 5021–5031. [Google Scholar] [CrossRef]
- Bruce, A.M.; Weyburne, E.S.; Engle, J.T.; Ziegler, C.J.; Geier, G.R., III. Phlorins Bearing Different Substituents at the sp3-Hybridized Meso-Position. J. Org. Chem. 2014, 79, 5664–5672. [Google Scholar] [CrossRef] [PubMed]
- LeSaulnier, T.D.; Graham, B.W.; Geier, G.R., III. Enhancement of Phlorin Stability by the In-corporation of meso-Mesityl Substituents. Tetrahedron Lett. 2005, 46, 5633–5637. [Google Scholar] [CrossRef]
- Nieto-Pescador, J.; Abraham, B.; Pistner, A.J.; Rosenthal, J.; Gundlach, L. Electronic State Dependence of Heterogeneous Electron Transfer: Injection from the S 1 and S 2 State of Phlorin into TiO2. Phys. Chem. Chem. Phys. 2015, 17, 7914–7923. [Google Scholar] [CrossRef]
- Pistner, A.J.; Martin, M.I.; Yap, G.P.A.; Rosenthal, J. Synthesis, structure, electronic characterization and halogenation of gold(III) phlorin complexes. J. Porphyr. Phthalocyanines 2021, 25, 683–695. [Google Scholar] [CrossRef]
- Flint, D.L.; Fowler, R.L.; LeSaulnier, T.D.; Long, A.C.; O’Brien, A.Y.; Geier, G.R., III. Investigation of Complementary Reactions of a Dipyrromethane with a Dipyrromethanemonocarbinol Leading to a 5-Isocorrole. J. Org. Chem. 2010, 75, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Geier, G.R., III; Ziegler, C.J. Structure and Spectroscopic Characterization of Free Base and Metal Complexes of 5,5-Dimethyl-10,15-Bis(Pentafluorophenyl)Isocorrole. Dalton Trans. 2011, 40, 4384–4386. [Google Scholar] [CrossRef]
- Hoffmann, M.; Cordes, B.; Kleeberg, C.; Schweyen, P.; Wolfram, B.; Broring, M. Template Synthesis of Alkyl-Substituted Metal Isocorroles. Eur. J. Inorg. Chem. 2016, 19, 3076–3085. [Google Scholar] [CrossRef]
- Pomarico, G.; Xiao, X.; Nardis, S.; Paolesse, R.; Fronczek, F.R.; Smith, K.M.; Fang, Y.; Ou, Z.; Kadish, K.M. Synthesis and characterization of free-base, copper, and nickel isocorroles. Inorg. Chem. 2010, 12, 5766–5774. [Google Scholar] [CrossRef]
- Thomas, K.E.; Beavers, C.M.; Gagnon, K.J.; Ghosh, A. beta-Octabromo- and beta-Octakis(trifluoromethyl)isocorroles: New Sterically Constrained Macrocyclic Ligands. ChemistryOpen 2017, 3, 402–409. [Google Scholar] [CrossRef]
- Martin, M.I.; Cai, Q.; Yap, G.P.A.; Rosenthal, J. Synthesis, Redox, and Spectroscopic Proper-ties of Pd(II) 10,10-Dimethylisocorrole Complexes Prepared via Bromination of Dimethylbila-diene Oligotetrapyrroles. Inorg. Chem. 2020, 59, 18241–18252. [Google Scholar] [CrossRef] [PubMed]
- Pistner, A.J.; Pupillo, R.C.; Yap, G.P.A.; Lutterman, D.A.; Ma, Y.Z.; Rosenthal, J. Electro-chemical, Spectroscopic and Singlet Oxygen Sensitization Characteristics of 10,10-Dimethylbiladiene Complexes of Zinc and Copper. J. Phys. Chem A 2014, 118, 10639–10648. [Google Scholar] [CrossRef]
- Potocny, A.; Pistner, A.; Yap, G.; Rosenthal, J. Electrochemical, Spectroscopic, and 1O2 Sensi-tization Characteristics of Synthetically Accessible Linear Tetrapyrrole Complexes of Palladium and Platinum. Inorg. Chem. 2017, 56, 12703–12711. [Google Scholar] [CrossRef] [PubMed]
- Clara De Simone, B.; Mazzone, G.; Russo, N.; Sicilia, E.; Toscano, M. Metal Atom Effect on the Photophysical Properties of Mg(II), Zn(II), Cd(II), and Pd(II) Tetraphenylporphyrin Complexes Proposed as Possible Drugs in Photodynamic Therapy. Molecules 2016, 21, 1093. [Google Scholar]
- Cai, Q.; Rice, A.T.; Pointer, C.A.; Martin, M.I.; Davies, B.; Yu, A.; Ward, K.; Hertler, P.R.; Warndorf, M.C.; Yap, G.P.A.; et al. Synthesis, Electrochemistry and Photophysics of Pd(II) Biladiene Complexes Bearing Varied Substituents at the sp3-Hybridized 10-Position. Inorg. Chem. 2021, 60, 15797–15807. [Google Scholar] [CrossRef]
- Potocny, A.; Riley, R.; O’Sullivan, R.; Day, E.; Rosenthal, J. Photochemotherapeutic Properties of a Linear Tetrapyrrole Palladium (II) Complex displaying an Exceptionally High Phototoxicity Index. Inorg. Chem. 2018, 57, 10608–10615. [Google Scholar] [CrossRef]
- Riley, R.S.; O’Sullivan, R.K.; Potocny, A.M.; Rosenthal, J.; Day, E.S. Evaluating Nanoshells and a Potent Biladiene Photosensitizer for Dual Photothermal and Photodynamic Therapy of Triple Negative Breast Cancer Cells. Nanomaterials 2018, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Potocny, A.M.; Rosenthal, J.; Day, E.S. Upconverting Gold Nanoshell- Linear Tetrapyrrole Conjugates for Near Infrared-Activated Dual Photodynamic and Photothermal Therapies. ACS Omega 2020, 5, 926–940. [Google Scholar] [CrossRef]
- Rice, A.T.; Martin, M.I.; Warndorf, M.C.; Yap, G.P.A.; Rosenthal, J. Synthesis, Spectroscopic and 1O2 Sensitization Characteristics of Extended Pd(II) 10,10-Dimethylbiladiene Complexes Bearing Alkynyl-Aryl Appendages. Inorg. Chem. 2021, 60, 11154–11163. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, F.; Rey, D.; Fatayer, S.; Schulz, F.; Perez, D.; Pena, D.; Gross, L. Intramolecular Coupling of Terminal Alkynes by Atom Manipulation. Angew. Chem. Int. Ed. 2020, 59, 22989–22993. [Google Scholar] [CrossRef] [PubMed]
- Apex3 [Computer Software]; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Chemistry and structure in Acta Crystallographica Section C. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Brouwer, A. Standards for Photoluminescence Quantum Yield Measurements in Solution. Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Singh, A.; McIntyre, N.; Koroll, G. Photochemical Formation of Metastable Species from 1,3-Diphenylisobenzofuran. Photochem. Photobiol. 1978, 28, 595–601. [Google Scholar] [CrossRef]
- Tanielian, C.; Wolff, C. Determination of the parameters controlling singlet oxygen production via oxygen heavy-atom enhancement of triplet yields. J. Phys. Chem. 1995, 99, 9831–9837. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination Polymers and Molecular Structures Among Complexes of Mercury(II) Halides with Selected 1-Benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 3817–3840. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Photosensitizers of the Porphyrin and Phthalocyanine Series for Photodynamic Therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rice, A.T.; Yap, G.P.A.; Rosenthal, J. A P-61 Black Widow Inspired Palladium Biladiene Complex for Efficient Sensitization of Singlet Oxygen Using Visible Light. Photochem 2022, 2, 58-68. https://doi.org/10.3390/photochem2010005
Rice AT, Yap GPA, Rosenthal J. A P-61 Black Widow Inspired Palladium Biladiene Complex for Efficient Sensitization of Singlet Oxygen Using Visible Light. Photochem. 2022; 2(1):58-68. https://doi.org/10.3390/photochem2010005
Chicago/Turabian StyleRice, Anthony T., Glenn P. A. Yap, and Joel Rosenthal. 2022. "A P-61 Black Widow Inspired Palladium Biladiene Complex for Efficient Sensitization of Singlet Oxygen Using Visible Light" Photochem 2, no. 1: 58-68. https://doi.org/10.3390/photochem2010005
APA StyleRice, A. T., Yap, G. P. A., & Rosenthal, J. (2022). A P-61 Black Widow Inspired Palladium Biladiene Complex for Efficient Sensitization of Singlet Oxygen Using Visible Light. Photochem, 2(1), 58-68. https://doi.org/10.3390/photochem2010005