UV Photolysis Study of Para-Aminobenzoic Acid Using Parahydrogen Matrix Isolated Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Matrix-Isolation Infrared Spectroscopy of PABA
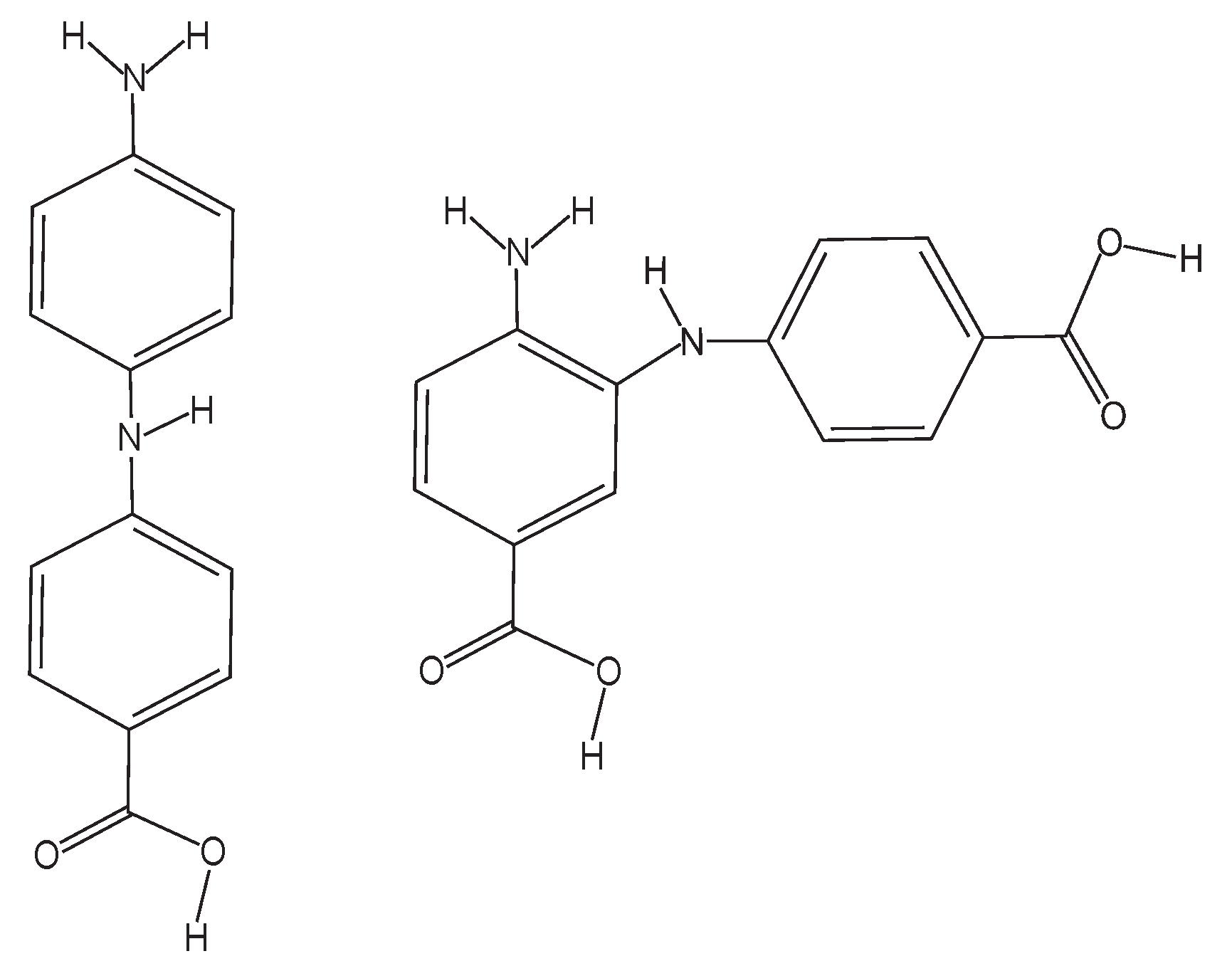
3.2. UVA: 355 nm Irradiation
3.3. UVB: >280 nm Irradiation
3.4. UVC: 266 nm Irradiation
3.5. UVC: 213 nm Irradiation
3.6. The PABA Radical Conformational Analysis
3.7. Overall Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abarca, J.F.; Casiccia, C.C. Skin Cancer and Ultraviolet-B Radiation Under the Antartic Ozone Hole: Southern Chile, 1987–2000. J. Am. Chem. Soc. 2002, 18, 294–302. [Google Scholar]
- Scott, H.W.; Dehority, B.A. Vitamin Requirements of Several Cellulolytic Rumen Bacteria. J. Bacteriol. 1964, 89, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Mahendiratta, S.; Sarma, P.; Kaur, H.; Kaur, S.; Kaur, H.; Bansal, S.; Prasad, D.; Prajapat, M.; Upadhay, S.; Kumar, S.; et al. Premature Graying of Hair: Risk Factors, Co-Morbid Conditions, Pharmacotherapy and Reversal—A Systematic Review and Meta-Analysis. Dermatol. Ther. 2020, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.S.; May, M. Ability of PABA to Protect Mammalian Skin from Ultraviolet Light-Induced Skin Tumors and Actinic Damage. J. Investig. Dermatol. 1975, 65, 543–546. [Google Scholar] [CrossRef][Green Version]
- Flindt-Hansen, H.; Thune, P.; Larsen, T.E. The Inhibiting Effect of PABA on Photocarcinogenesis. Arch. Dermatol. Res. 1990, 282, 38–41. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, Y.; Zeng, C.; Zhang, Y.; Wang, Z.; Yang, X. Aquatic Photodegradation of Sunscreen Agent p-Aminobenzoic Acid in the Presence of Dissolved Organic Matter. Water Res. 2013, 47, 153–162. [Google Scholar] [CrossRef]
- Wong, T. Sunscreen Allergy and its Investigation. Clin. Dermatol. 2011, 29, 306–310. [Google Scholar] [CrossRef]
- Chan, C.T.L.; Ma, C.; Chan, R.C.T.; Ou, H.M.; Xie, H.X.; Wong, A.K.W.; Wang, M.L.; Kwok, W.M. A Long Lasting Sunscreen Controversy of 4- Aminobenzoic Acid and 4-Dimethylaminobenzaldehyde Derivatives Resolved by Ultra-fast Spectroscopy Combined with Density Functional Theoretical Study. Phys. Chem. Chem. Phys. 2020, 22, 8006–8020. [Google Scholar] [CrossRef]
- Shaw, A.A.; Wainschel, L.A.; Shetlar, M.D. Photoaddition of p-Aminobenzoic acid to Thymine and Thymidine. Photochem. Photobiol. 1992, 55, 657–663. [Google Scholar] [CrossRef]
- Meijer, G.; de Vries, M.S.; Hunziker, H.E.; Wendt, H.R. Laser Desorption Jet-Cooling Spectroscopy of Para-Amino Benzoic Acid Monomer, Dimer, and Clusters. J. Chem. Phys. 1990, 92, 7625–7635. [Google Scholar] [CrossRef]
- Magata, N. Solvent Effects on the Absorption and Fluorescence Spectra of Napthylamines and Isomeric Aminobenzoic Acids. Bull. Chem. Soc. Jpn. 1963, 36, 654–662. [Google Scholar] [CrossRef]
- Lynch, K.; Pergolizzi, R.G. In vitro Method to Quantify UV Mediated DNA Damage. J. Young Investig. 2010, 20, 1–16. [Google Scholar]
- Shaw, A.A.; Wainschel, L.A.; Shetlar, M.D. The Photochemistry of p-Aminobenzoic Acid. Photochem. Photobiol. 1992, 55, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Cismesia, A.P.; Nicholls, G.R.; Polfer, N.C. Amine vs. Carboxylic Acid Protonation in Ortho-, Meta-, and Para-Aminobenzoic Acid: An IRMPD Spectroscopy Study. J. Mol. Spectrosc. 2017, 332, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Momose, T.; Fushitani, M.; Hoshina, H. Chemical Reactions in Quantum Crystals. Int. Rev. Phys. Chem. 2005, 24, 533–552. [Google Scholar] [CrossRef]
- Momose, T.; Shida, T. Matrix-Isolation Spectroscopy Using Solid Parahydrogen as the Matrix: Application to High-Resolution Spectroscopy, Photochemistry, and Cryochemistry. J. Chem. Phys. 1998, 108, 4237–4241. [Google Scholar] [CrossRef]
- Momose, T.; Hoshina, H.; Fushitani, M.; Katsuki, H. High-Resolution Spectroscopy and its Analysis of Ro-Vibrational Transitions of Molecules in Solid Parahydrogen. Vib. Spectrosc. 2004, 34, 95–108. [Google Scholar] [CrossRef]
- Tom, B.A.; Bhasker, S.; Miyamoto, Y.; Momose, T.; McCall, B.J. Producing and Quantifying Enriched para-H2. Rev. Sci. Inst. 2009, 80, 16108–16111. [Google Scholar] [CrossRef]
- Wong, Y.T.A.; Toh, S.Y.; Djuricanin, P.; Momose, T. Conformational Composition and Population Analysis of β-alanine Isolated in Solid Parahydrogen and Argon Matrices. J. Mol. Spectrosc. 2015, 310, 23–31. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Momose, T.; Fajardo, M.E. Matrix Isolation Spectroscopy and Spectral Simulations of Isotopically Substituted C60 Molecules. J. Chem. Phys. 2019, 151, 234301. [Google Scholar] [CrossRef]
- Schmidt, W.; Polik, J.R. WebMO Enterprise; Version 17.0.012e; WebMO LLC: Holland, MI, USA, 2016; Available online: https://www.webmo.net (accessed on 13 July 2021).
- Borah, B.; Gomti Devi, T. The Vibrational Study on the Molecular interaction of L- Proline and Para-Aminobenzoic Acid. J. Mol. Struct. 2020, 1203, 1–15. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD. 69 Online. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C150130&Units=SI&Type=IR-SPEC&Index=0#IR-SPEC (accessed on 20 June 2021).
- Fehrensen, B.; Luckhaus, D.; Quack, M. Inversion Tunneling in Aniline from High Resolution Infrared Spectroscopy and an Adiabatic Reaction Path Hamiltonian Approach. Z. Phys. Chem. 1999, 209, 1–19. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Morgan, P.J.; Pratt, D.W. Push-Pull Molecules in the Gas Phase: Stark Effect Measurements of the Permanent Dipole Moments of p-Aminobenzoic Acid in Its Ground and Electronically Excited States. J. Phys. Chem. A 2008, 112, 12597–12601. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.J.; Morgan, P.J.; Pratt, D.W. High-Resolution Electronic Spectroscopy Studies of meta-Aminobenzoic Acid in the Gas Phase Reveal the Origins of its Solvatochromic Behaviour. J. Mol. Struct. 2020, 55, 657–663. [Google Scholar]
- Moore, B.; Toh, C.S.Y.; Wong, Y.T.A.; Bashiri, T.; McKinnon, A.; Wai, Y.; Lee, A.; Ovchinikov, P.; Chiang, C.; Djuricanin, P.; et al. Hydrocarboxyl Radical as a Product of α-Alanine Ultraviolet Photolysis. J. Chem. Phys. Lett. 2021, 12, 11992–11997. [Google Scholar] [CrossRef]
- Ruzi, M.; Anderson, D.T. Matrix Isolation Spectroscopy and Nuclear Spin Conversion of NH3 and ND3 in Solid Parahydrogen. J. Phys. Chem. A 2013, 117, 9712–9724. [Google Scholar] [CrossRef]
- Ruzi, M.; Anderson, D.T. Fourier Transform Infrared Studies of Ammonia Photochemistry in Solid Parahydrogen. J. Phys. Chem. A 2013, 117, 13832–13842. [Google Scholar] [CrossRef]
- Gée, C.; Crépin, S.D.C.; Bréchignac, P. Infrared Spectroscopy of Aniline (C6H5NH2) and its Cation in a Cryogenic Argon Matrix. Chem. Phys. Lett. 2001, 338, 130–136. [Google Scholar]
- Tsuge, M.; Chen, Y.H.; Lee, Y.P. Infrared Spectra of Isomers of Protonated Aniline in Solid para-Hydrogen. J. Phys. Chem. A 2020, 124, 2253–2263. [Google Scholar] [CrossRef]
- Stephanian, S.; Reva, I.D.; Radchenko, E.D.; Sheina, G.G. Infrared Spectra of Benzoic Acid Monomers and Dimers in Argon Matrix. Vib. Spectrosc. 1996, 11, 123–133. [Google Scholar] [CrossRef]
- Winther, F.; Meyer, S.; Nicolaisen, F.M. The Infrared Spectrum of Methylketene. J. Mol. Struct. 2002, 611, 9–22. [Google Scholar] [CrossRef]
- Ryazantsev, S.V.; Feldman, V.I. Matrix-Isolation Studies on the Radiation-Induced Chemistry in H2O/CO2 Systems: Reactions of Oxygen Atoms and Formation of HOCO Radical. J. Phys. Chem. A 2014, 119, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, M.E.; Lindsay, C.M.; Momose, T. Crystal Field Theory Analysis of Rovibrational Spectra of Carbon Monoxide Monomers Isolated in Solid Parahydrogen. J. Chem. Phys. 2009, 130, 244508–244517. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Fajardo, M.E. Observation of the High-Resolution Infrared Absorption Spectrum of CO2 Molecules Isolated in Solid Parahydrogen. Low. Temp. Phys. 2000, 26, 889–898. [Google Scholar] [CrossRef]
- Land, E.J.; Porter, G. Primary Photochemical Processes in Aromatic Molecules. Trans. Faraday Soc. 1963, 59, 2027–2037. [Google Scholar] [CrossRef]
- Roscher, N.M.; Lindemann, M.K.; Kong, S.B.; Cho, C.G.; Jiang, P. Photodecomposition of Several Compounds Commonly Used as Sunscreen Agents. J. Photochem. Photobiol. A Chem. 1994, 80, 417–421. [Google Scholar] [CrossRef]
- Fisher, M.S.; Menter, J.M.; Willis, I. Ultraviolet Radiation-Induced Suppression of Contact Hypersensitivity in Relation to Padimate O and Oxybenzone. Soc. Investig. Dermatol. 1989, 92, 337–341. [Google Scholar] [CrossRef]
- Knowland, J.; McKenzie, E.A.; McHugh, P.J.; Cridland, N.A. Sunlight-Induced Mutagenicity of a Common Sunscreen Ingredient. FEBS Lett. 1993, 324, 309–313. [Google Scholar] [CrossRef]










| Vibrational Mode | Theoretical | Solid | Gas | pH |
|---|---|---|---|---|
| Conformer (I) | State [22] | Phase [23] | Matrix | |
| C=C-C Wag | 697.92 (23.09) | 694 | 698.0 (20.62) | |
| O-C-O Scissor | 725.94 (7.59) | 734 | 732.3 (4.13) | |
| C-C-C Bend | 770.40 (27.35) | 773.43 | 770 | 771.6 (10.92) |
| C-C-C Bend | 836.13 (14.97) | 832.14 | 840 | 840 * |
| C=C-C Scissor | 841.92 (8.41) | 843 * | ||
| C-H Bend | 947.20 (0.74) | 954.6 (0.18) | ||
| H-C-C Scissor | 964.63 (0.21) | 967.4 (0.12) | ||
| H-C-C Rock | 1006.49 (26.28) | 1012.6 (0.68) | ||
| NH Rock | 1046.07 (17.85) | 1048.5 (6.52) | ||
| NH Rock | 1087.64 (123.18) | 1065.26 | 1080 | 1086.6 (54.61) |
| C-O Stretch | 1124.00 (3.82) | 1127.9 (1.74) | ||
| H-C-C Bend | 1154.98 (178.25) | 1169.17 | 1166 | 1164.8 (81.01) |
| C-O-H Scissor | 1187.34 (38.55) | 1198 | 1194.7 (19.26) | |
| C-H Bend | 1295.27 (7.09) | 1260 | 1274.6 (1.23) | |
| C-O Stretch | 1305.85 (63.39) | 1288.92 | 1286 | 1300.6 (32.48) |
| C-O Stretch | 1347.04 (16.10) | 1325 | 1335 * | |
| H-C-O Bend | 1360.55 (121.57) | 1367.66 | 1360 | 1364.2, 1367.8 (41.33) |
| C=C Stretch | 1441.77 (14.14) | 1421.41 | 1434 | 1440.9 (6.18) |
| C=C Asym. Str. | 1513.15 (17.55) | 1518 | 1523.8 (13.82) | |
| C=C Asym. Str. | 1578.09 (5.01) | 1583.3 (2.96) | ||
| NH Scissor | 1596.06 (8.61) | 1606.7 (12.26) | ||
| NH Scissor | 1623.35 (271.97) | 1620.18 | 1622 | 1624.2 (100.00) |
| C=O Stretch | 1767.10 (287.04) | 1690.90 | 1754 | 1743.9, 1746.3 (109.75) |
| C-H Asym. Str. | 3010.33 (17.31) | 3010 | 3016.2 (0.70) | |
| C-H Asym. Str. | 3016.28 (14.37) | 3028.9 (2.57) | ||
| C-H Sym. Str. | 3061.17 (1.94) | 3044 | 3052.3 (3.25) | |
| C-H Sym. Str. | 3070.83 (11.15) | 3074 | 3079.3 (3.01) | |
| NH Asym. Stetch | 3399.32 (26.90) | 3460.70 | 3422 | 3436.2, 3437.3 (19.59) |
| NH Sym. Stretch | 3491.38 (10.41) | 3510 | 3531.0, 3532.3 (8.59) | |
| O-H Stretch | 3525.95 (64.46) | 3607.25 | 3586 | 3580.4, 3583.0 (19.32) |
| Mode | Sym | Assignment | Theoretical | Theoretical | pH | Conformer |
|---|---|---|---|---|---|---|
| Conformer (I) | Conformer (II) | Matrix | Assignment | |||
| 9 | A’ | C-C-C Bend | 778.48 (38.0) | 778.74 (39.9) | 774.3 (16.5) | (I) |
| 774.6 (19.0) | (II) | |||||
| 11 | C-C-C Bend | 851.22 (17.0) | 854.31 (14.0) | 849.1 (13.0) | (I) | |
| 850.5 (11.7) | (II) | |||||
| 26 | O-H Bend | 1117.83 (8.3) | 1124.85 (6.9) | 1119.5 (22.5) | (I)/(II) | |
| 20 | A” | O-C-O Scissor | 721.73 (19.7) | 721.77 (38.0) | 737.6 (36.4) | (I)/(II) |
| 22 | H-C-C Wag | 984.84 (2.0) | 984.84 (1.4) | 997.3 (8.3) | (I)/(II) | |
| 23 | H-C-C Rock | 1083.84 (70.4) | 1078.48 (49.8) | 1077.2 (35.3) | (II) | |
| 1079.5 (49.0) | (I) | |||||
| 24 | O-C-C Stretch | 1091.25 (0.8) | 1093.57 (21.0) | 1095.0 (25.6) | (II) | |
| 25 | H-C-C Bend | 1134.02 (132.2) | 1134.69 (183.4) | 1137.4 (92.3) | (I)/(II) | |
| 27 | H-O-C Scissor | 1175.83 (103.5) | 1176.46 (109.9) | 1180.8 (24.0) | (I) | |
| 1184.2 (100.0) | (II) | |||||
| 30 | H-N-C Stretch | 1348.41 (13.9) | 1336.48 (82.3) | 1329.3 * | (I)/(II) | |
| 1332.0 * | (I)/(II) | |||||
| 31 | C-C-O Stretch | 1362.56 (147.4) | 1369.37 (20.8) | 1374.6 (13.5) | (I)/(II) | |
| 32 | C=C Asym. Str. | 1420.91 (15.3) | 1420.76 (14.7) | 1423.3 (5.8) | (I)/(II) | |
| 1429.5 (5.9) | (I)/(II) | |||||
| 33 | C=C Sym. Str. | 1455.42 (19.6) | 1450.59 (6.3) | 1460.0 (1.1) | (II) | |
| 1461.1 (3.3) | (I) | |||||
| 34 | C=C-C Asym. Str. | 1514.64 (0.7) | 1515.47(20.5) | 1518.5 (4.5) | (II) | |
| 36 | C=O Stretch | 1764.16 (197.0) | 1765.90 (93.0) | 1744–1746 * | (I)/(II) | |
| 42 | O-H Stretch | 3531.75 (81.5) | 3535.61 (81.7) | 3570.9 (2.3) | (I)/(II) | |
| 3572.7 (15.5) | (I)/(II) | |||||
| 3574.3 (29.0) | (I)/(II) | |||||
| 3575.5 (41.3) | (I)/(II) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKinnon, A.; Moore, B.; Djuricanin, P.; Momose, T. UV Photolysis Study of Para-Aminobenzoic Acid Using Parahydrogen Matrix Isolated Spectroscopy. Photochem 2022, 2, 88-101. https://doi.org/10.3390/photochem2010008
McKinnon A, Moore B, Djuricanin P, Momose T. UV Photolysis Study of Para-Aminobenzoic Acid Using Parahydrogen Matrix Isolated Spectroscopy. Photochem. 2022; 2(1):88-101. https://doi.org/10.3390/photochem2010008
Chicago/Turabian StyleMcKinnon, Alexandra, Brendan Moore, Pavle Djuricanin, and Takamasa Momose. 2022. "UV Photolysis Study of Para-Aminobenzoic Acid Using Parahydrogen Matrix Isolated Spectroscopy" Photochem 2, no. 1: 88-101. https://doi.org/10.3390/photochem2010008
APA StyleMcKinnon, A., Moore, B., Djuricanin, P., & Momose, T. (2022). UV Photolysis Study of Para-Aminobenzoic Acid Using Parahydrogen Matrix Isolated Spectroscopy. Photochem, 2(1), 88-101. https://doi.org/10.3390/photochem2010008






