Pharmaceutical Micropollutant Treatment with UV–LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Titanium Dioxide-Coated Membrane Synthesis
2.2.1. Nanomaterial Characterization
2.2.2. Experimental Setup
2.3. Sample Preparation and Analysis
3. Results
3.1. Nanomaterial Characterization
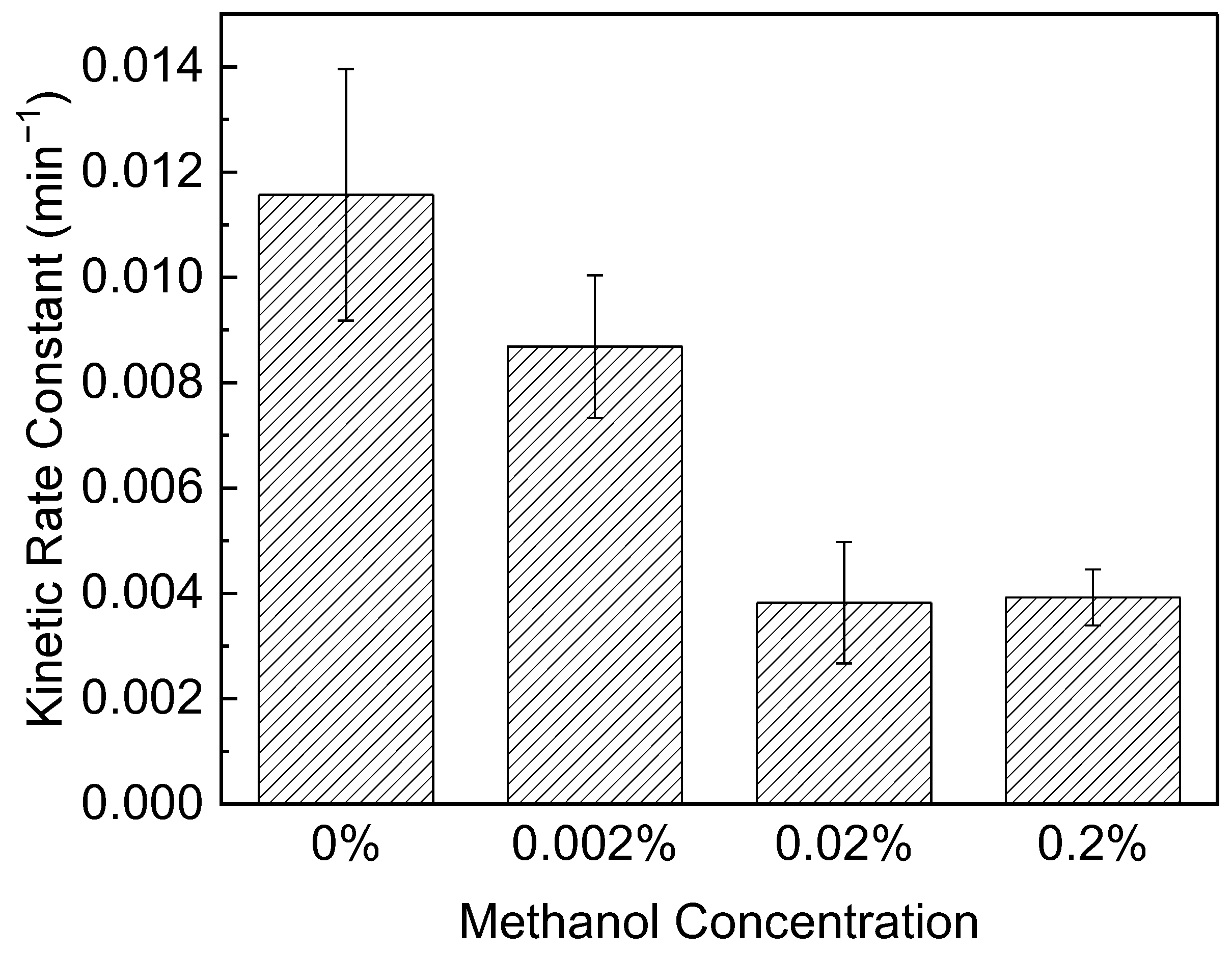
3.2. The Effect of Methanol on PPCP Photocatalysis
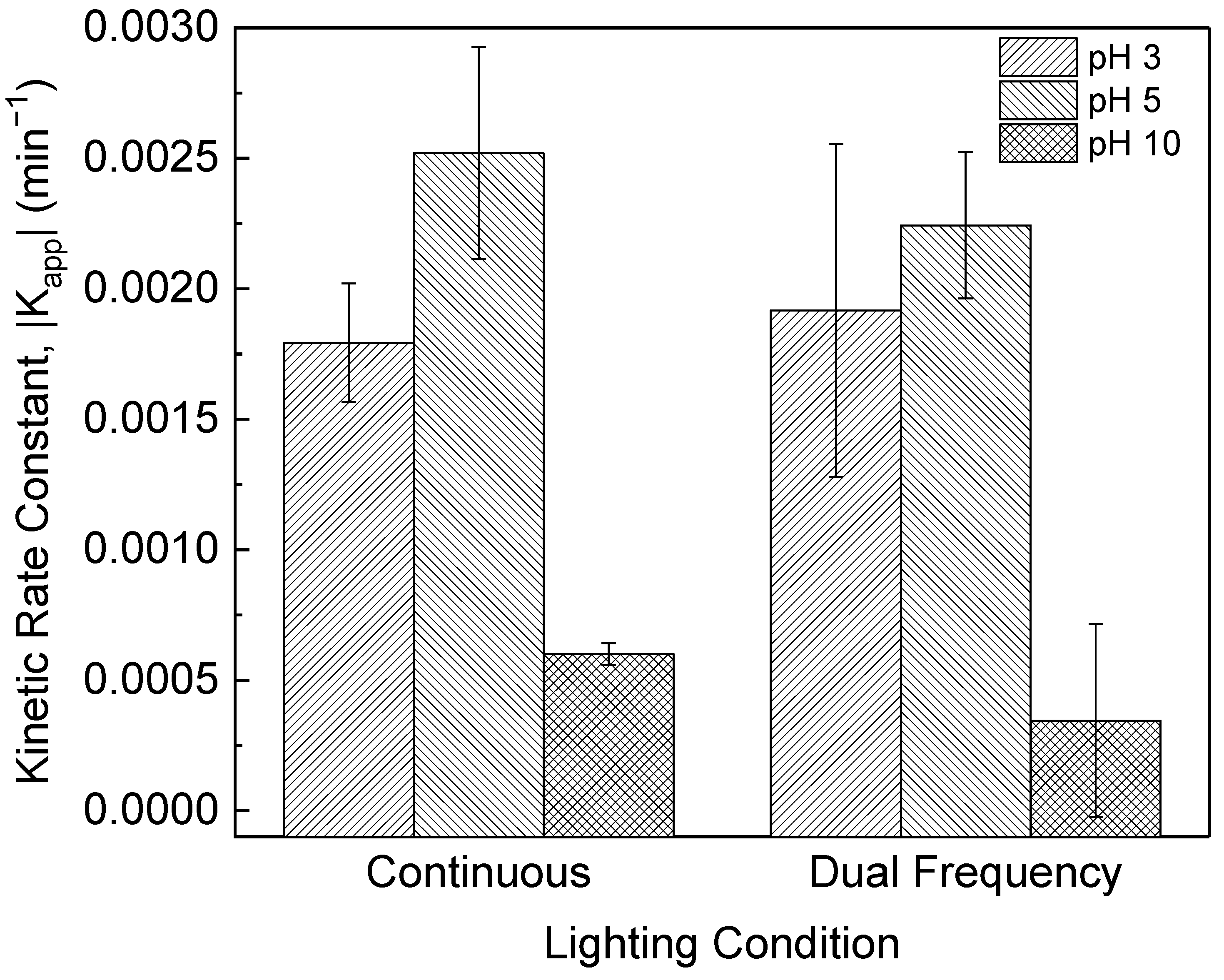
3.3. The Effect of Dual Pulse Frequencies on PPCP Photocatalysis
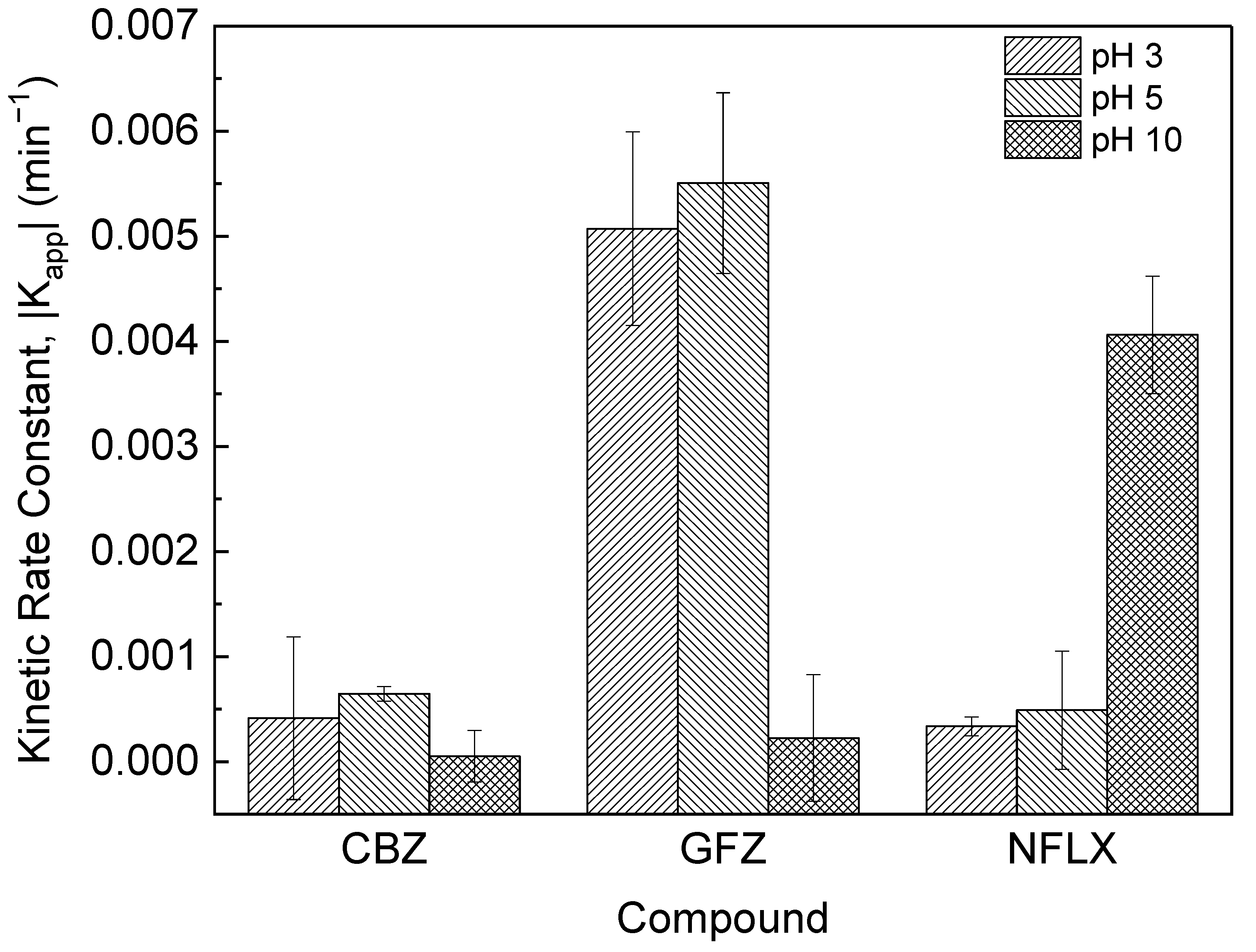
3.4. The Effect of pH on PPCP Photocatalysis
3.5. Literature Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Braund, R.; Warren, D.; Peake, B. TiO2-assisted photodegradation of pharmaceuticals—A review. Open Chem. 2012, 10, 989–1027. [Google Scholar] [CrossRef]
- Miranda-García, N.; Maldonado, M.I.; Coronado, J.; Malato, S. Degradation study of 15 emerging contaminants at low concentration by immobilized TiO2 in a pilot plant. Catal. Today 2010, 151, 107–113. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sanchez, B.; Coronado, J.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Guida, M.; Fatta-Kassinos, D.; Belgiorno, V. Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res. 2009, 43, 4070–4078. [Google Scholar] [CrossRef]
- Liang, R.; Hu, A.; Li, W.; Zhou, Y.N. Enhanced degradation of persistent pharmaceuticals found in wastewater treatment effluents using TiO2 nanobelt photocatalysts. J. Nanopart. Res. 2013, 15, 1990. [Google Scholar] [CrossRef]
- Martínez, C.; Fernández, M.; Santaballa, J.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Arlos, M.J.; Hatat-Fraile, M.M.; Liang, R.; Bragg, L.M.; Zhou, N.Y.; Andrews, S.A.; Servos, M.R. Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points. Water Res. 2016, 101, 351–361. [Google Scholar] [CrossRef]
- Arlos, M.J.; Liang, R.; Hatat-Fraile, M.M.; Bragg, L.M.; Zhou, N.Y.; Servos, M.R.; Andrews, S.A. Photocatalytic decomposition of selected estrogens and their estrogenic activity by UV-LED irradiated TiO2 immobilized on porous titanium sheets via thermal-chemical oxidation. J. Hazard. Mater. 2016, 318, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Shiu, S.-J.; Wu, H.-C. Decomposition of dimethyl phthalate in aqueous solution by UV–LED/TiO2 process under periodic illumination. J. Photochem. Photobiol. A Chem. 2017, 332, 299–305. [Google Scholar] [CrossRef]
- Buechler, K.J.; Nam, C.H.; Zawistowski, T.M.; Noble, R.D.; Koval, C.A. Design and Evaluation of a Novel-Controlled Periodic Illumination Reactor To Study Photocatalysis. Indust. Eng. Chem. Res. 1999, 38, 1258–1263. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Ma, D.; Liu, A.; Li, S.; Lu, C.; Chen, C. TiO2 photocatalysis for C–C bond formation. Catal. Sci. Technol. 2018, 8, 2030–2045. [Google Scholar] [CrossRef]
- Liu, A.; Ma, D.; Qian, Y.; Li, J.; Zhai, S.; Wang, Y.; Chen, C. A powerful azomethine ylide route mediated by TiO2 photocatalysis for the preparation of polysubstituted imidazolidines. Org. Biomol. Chem. 2021, 19, 2192–2197. [Google Scholar] [CrossRef]
- Ma, D.; Li, J.; Liu, A.; Chen, C. Carbon Gels-Modified TiO2: Promising Materials for Photocatalysis Applications. Materials 2020, 13, 1734. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.; Newkirk, J.; Liou, F. Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel. Materials 2021, 14, 560. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Maldonado, M.I.; Malato, S.; Sánchez, B. Regeneration approaches for TiO2 immobilized photocatalyst used in the elimination of emerging contaminants in water. Catal. Today 2014, 230, 27–34. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Mai, J.; Ni, J. Initial photocatalytic degradation intermediates/pathways of 17α-ethynylestradiol: Effect of pH and methanol. Chemosphere 2010, 81, 92–99. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Berk, D.; Yargeau, V. Photocatalytic removal of 17α-ethinylestradiol (EE2) and levonorgestrel (LNG) from contraceptive pill manufacturing plant wastewater under UVC radiation. Chem. Eng. J. 2012, 185–186, 52–60. [Google Scholar] [CrossRef]
- Kralchevska, R.; Milanova, M.; Bistan, M.; Pintar, A.; Todorovsky, D. The photocatalytic degradation of 17α-ethynylestradiol by pure and carbon nanotubes modified TiO2 under UVC illumination. Open Chem. 2012, 10, 1137–1148. [Google Scholar] [CrossRef]
- Marinho, B.A.; de Liz, M.V.; Lopes Tiburtius, E.R.; Nagata, N.; Peralta-Zamora, P. TiO2 and ZnO mediated photocatalytic degradation of E2 and EE2 estrogens. Photochem. Photobiol. Sci. 2013, 12, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.L.; McDonald, J.; Khan, S.; Le-Clech, P. Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (MPR). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, L.; Xiao, M.; Li, H.; Pan, X.; Jiang, F. One-step hydrothermal synthesis of surface fluorinated TiO2/reduced graphene oxide nanocomposites for photocatalytic degradation of estrogens. Mater. Sci. Semicond. Process. 2015, 40, 183–193. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Identification and Roles of the Active Species Generated on Various Photocatalysts. In Photocatalysis and Water Purification; Pichat, P., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013; pp. 3–24. [Google Scholar] [CrossRef]
- Paul, T.; Miller, P.L.; Strathmann, T.J. Visible-Light-Mediated TiO2 Photocatalysis of Fluoroquinolone Antibacterial Agents. Environ. Sci. Technol. 2007, 41, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Arlos, M.J.; Liang, R.; Fong, L.C.L.C.; Zhou, N.Y.; Ptacek, C.J.; Andrews, S.A.; Servos, M.R. Influence of methanol when used as a water-miscible carrier of pharmaceuticals in TiO2 photocatalytic degradation experiments. J. Environ. Chem. Eng. 2017, 5, 4497–4504. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca-Martínez, F.; Li Puma, G. Modeling the photocatalytic mineralization in water of commercial formulation of estrogens 17-β estradiol (E2) and nomegestrol acetate in contraceptive pills in a solar powered compound arabolic collector. Molecules 2015, 20, 13354–13373. [Google Scholar] [CrossRef] [Green Version]
- Li Puma, G.; Puddu, V.; Tsang, H.K.; Gora, A.; Toepfer, B. Photocatalytic oxidation of multicomponent mixtures of estrogens (estrone (E1), 17β-estradiol (E2), 17α- ethynylestradiol (EE2) and estriol (E3)) under UVA and UVC radiation: Photonabsorption, quantum yields and rate constants independent of photon absorption. Appl. Catal. B Environ. 2010, 99, 388–397. [Google Scholar]
- Tada, H.; Suzuki, F.; Ito, S.; Akita, T.; Tanaka, K.; Kawahara, T.; Kobayashi, H. Au-Core/Pt-Shell Bimetallic Cluster-Loaded TiO2. 1. Adsorption of Organosulfur Compound. J. Phys. Chem. B 2002, 106, 8714–8720. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, J. Decomposition of acetaminophen (Ace) using TiO2/UVA/LED system. Catal. Today 2017, 282, 48–56. [Google Scholar] [CrossRef]
- Korovin, E.; Selishchev, D.; Besov, A.; Kozlov, D. UV-LED TiO2 photocatalytic oxidation of acetone vapor: Effect of high frequency controlled periodic illumination. Appl. Catal. B Environ. 2015, 163, 143–149. [Google Scholar] [CrossRef]
- Sczechowski, J.G.; Koval, C.A.; Noble, R.D. Evidence of critical illumination and dark recovery times for increasing the photoefficiency of aqueous heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 1993, 74, 273–278. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.; Robertson, P. Controlled periodic illumination in semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 2016, 319–320, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Helsel, D.R.; Hirsch, R.M. Statistical Methods in Water Resources; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Liang, R.; Van Leeuwen, J.C.; Bragg, L.M.; Arlos, M.J.; Fong, L.C.L.C.; Schneider, O.M.; Jaciw-Zurakowsky, I.; Fattahi, A.; Rathod, S.; Peng, P.; et al. Utilizing UV-LED pulse width modulation on TiO2 advanced oxidation processes to enhance the decomposition efficiency of pharmaceutical micropollutants. Chem. Eng. J. 2019, 361, 439–449. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Adsorption. In MWH’s Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1117–1262. [Google Scholar]
- Laera, G.; Jin, B.; Zhu, H.; Lopez, A. Photocatalytic activity of TiO2 nanofibers in simulated and real municipal effluents. Catal. Today 2011, 161, 147–152. [Google Scholar] [CrossRef]

| Compound | Abbreviation | Use | Deuterated Standard |
|---|---|---|---|
| Atenolol | ATEN | Beta-blocker | Atenolol-d7 a |
| Atorvastatin | ATOR | Lipid lowering | Atorvastatin-d5 b |
| Atrazine | ATRZ | Herbicide | Atrazine-d5 a |
| Carbamazepine | CBZ | Anti-epileptic | Carbamazepine-d5 a |
| Carbamazepine-10,11-epoxide | e-CBZ | Carbamazepine | Carbamazepine-10,11-epoxide-d10 a |
| Diclofenac | DCF | Anti-inflammatory | Diclofenac-d4 a |
| Fluoxetine | FLX | Antidepressant | Fluoxetine-d5 a |
| Gemfibrozil | GFZ | Lipid lowering | Gemfibrozil-d6 a |
| Ibuprofen | IBU | Anti-inflammatory | Ibuprofen-d3 a |
| Naproxen | NPX | Anti-inflammatory | Naproxen-d3 a |
| Norfluoxetine | NFLX | Fluoxetine | Norfluoxetine-d5 a |
| o-Hydroxy atorvastatin | o-ATOR | Atorvastatin | o-Hydroxyatorvastatin-d5 b |
| p-Hydroxy atorvastatin | p-ATOR | Atorvastatin | p-Hydroxyatorvastatin-d5 b |
| Sulfamethoxazole | SULF | Antibiotic | Sulfamethoxazole-d4 a |
| Triclosan | TCS | Antimicrobial | Triclosan-d3 a |
| Triclocarban | TCCB | Antimicrobial | Triclocarban-d4 a |
| Trimethoprim | TRIM | Antibiotic | Trimethoprim-d3 a |
| Venlafaxine | VEN | Antidepressant | Venlafaxine-d6 a |
| Compound | Molecular Weight (g/mol) | Charge at pH 3 a | Charge at pH 5 a | Charge at pH 10 a | logS at pH 3 a | logS at pH 5 a | logS at pH 10 a |
|---|---|---|---|---|---|---|---|
| ATEN | 266.34 | 1.00 | 1.00 | 0.32 | 0.43 | 0.43 | −1.41 |
| ATOR | 558.64 | −0.05 | −0.83 | −1.00 | −7.03 | −6.28 | −1.36 |
| ATRZ | 215.68 | 0.51 | 0.01 | 0.00 | −3.39 | −3.8 | −3.8 |
| CBZ | 236.27 | 0.00 | 0.00 | 0.00 | −3.79 | −3.79 | −3.79 |
| e-CBZ | 252.7 | 0.99 | 0.46 | 0.00 | −2.62 | −3.11 | 0 |
| DCF | 296.15 | −0.09 | −0.91 | −1.00 | −4.26 | −3.25 | 0 |
| FLX | 309.33 | 1.00 | 1.00 | 0.39 | 0 | 0 | −3.86 |
| GFZ | 250.33 | −0.04 | −0.79 | −1.00 | −3.29 | −2.63 | 0 |
| IBU | 206.28 | −0.01 | −0.58 | −1.00 | −3.54 | −3.16 | 0 |
| NPX | 230.60 | −0.06 | −0.87 | −1.00 | −3.42 | −2.58 | 0 |
| NFLX | 295.30 | 1.00 | 1.00 | 0.37 | 0 | 0 | −4.05 |
| o-ATOR | 573.65 | −0.05 | −0.83 | −1.95 | −7.43 | −6.68 | −1.09 |
| p-ATOR | 573.65 | −0.05 | −0.83 | −1.79 | −7.43 | −6.68 | −1.09 |
| SULF | 253.28 | 0.08 | −0.06 | −1 | −2.16 | −2.17 | 0 |
| TCS | 289.54 | 0.00 | 0.00 | −1.00 | −5.28 | −5.27 | −2.95 |
| TCCB | 315.58 | 0.00 | 0.00 | −0.04 | −5.67 | −5.67 | −5.66 |
| TRIM | 290.32 | 1.00 | 0.99 | 0.00 | 0 | −0.64 | −2.8 |
| VEN | 277.40 | 1.00 | 1.00 | 0.07 | 0 | 0 | −2.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snowdon, M.; Liang, R.; Van Leeuwen, J.C.; Schneider, O.; Khan, A.; Li Chun Fong, L.C.M.; Zhou, N.Y.; Servos, M.R. Pharmaceutical Micropollutant Treatment with UV–LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions. Photochem 2022, 2, 503-514. https://doi.org/10.3390/photochem2030035
Snowdon M, Liang R, Van Leeuwen JC, Schneider O, Khan A, Li Chun Fong LCM, Zhou NY, Servos MR. Pharmaceutical Micropollutant Treatment with UV–LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions. Photochem. 2022; 2(3):503-514. https://doi.org/10.3390/photochem2030035
Chicago/Turabian StyleSnowdon, Monika, Robert Liang, Jocelyn C. Van Leeuwen, Olivia Schneider, Abrar Khan, Lena C. M. Li Chun Fong, Norman Y. Zhou, and Mark R. Servos. 2022. "Pharmaceutical Micropollutant Treatment with UV–LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions" Photochem 2, no. 3: 503-514. https://doi.org/10.3390/photochem2030035
APA StyleSnowdon, M., Liang, R., Van Leeuwen, J. C., Schneider, O., Khan, A., Li Chun Fong, L. C. M., Zhou, N. Y., & Servos, M. R. (2022). Pharmaceutical Micropollutant Treatment with UV–LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions. Photochem, 2(3), 503-514. https://doi.org/10.3390/photochem2030035






