Structural, Morphological and Optical Properties of MoS2-Based Materials for Photocatalytic Degradation of Organic Dye
Abstract
:1. Introduction
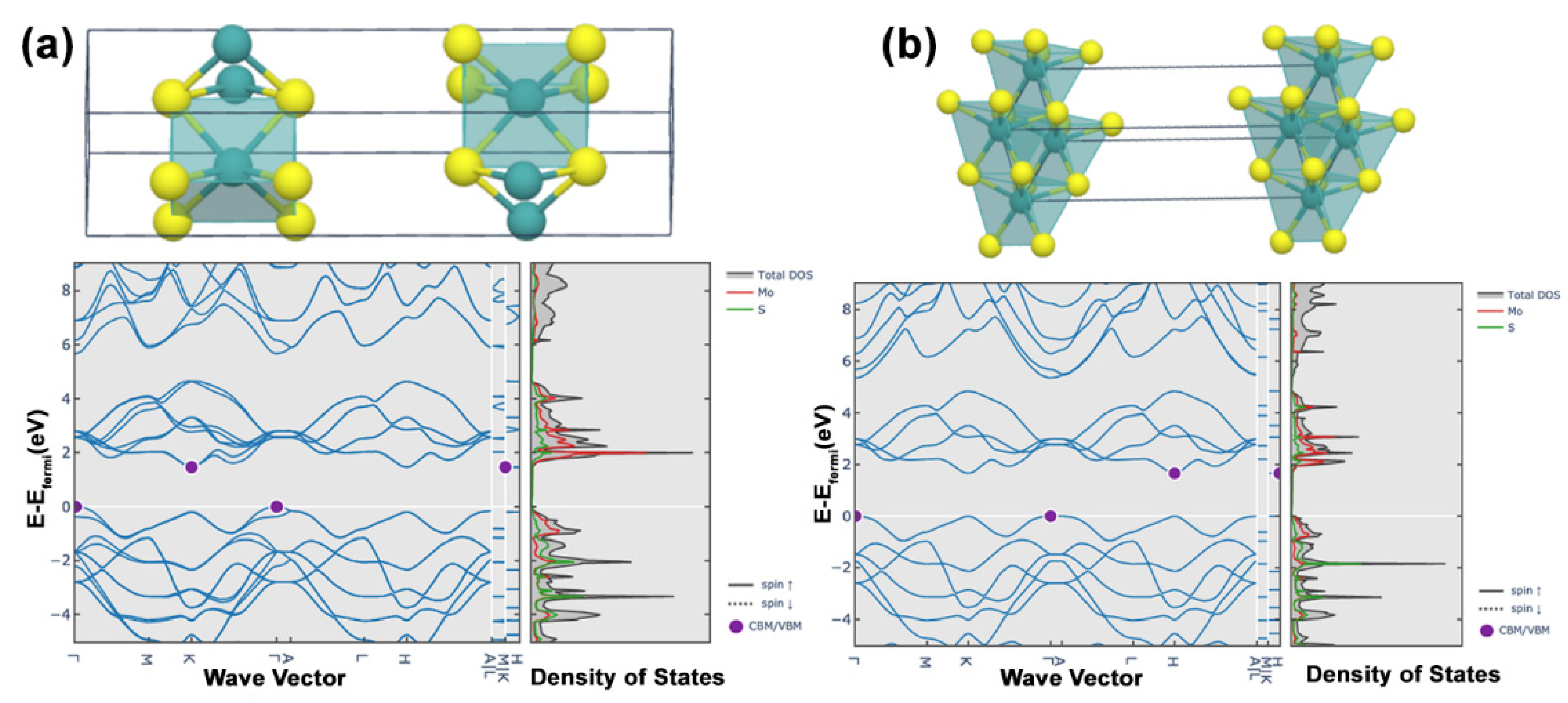
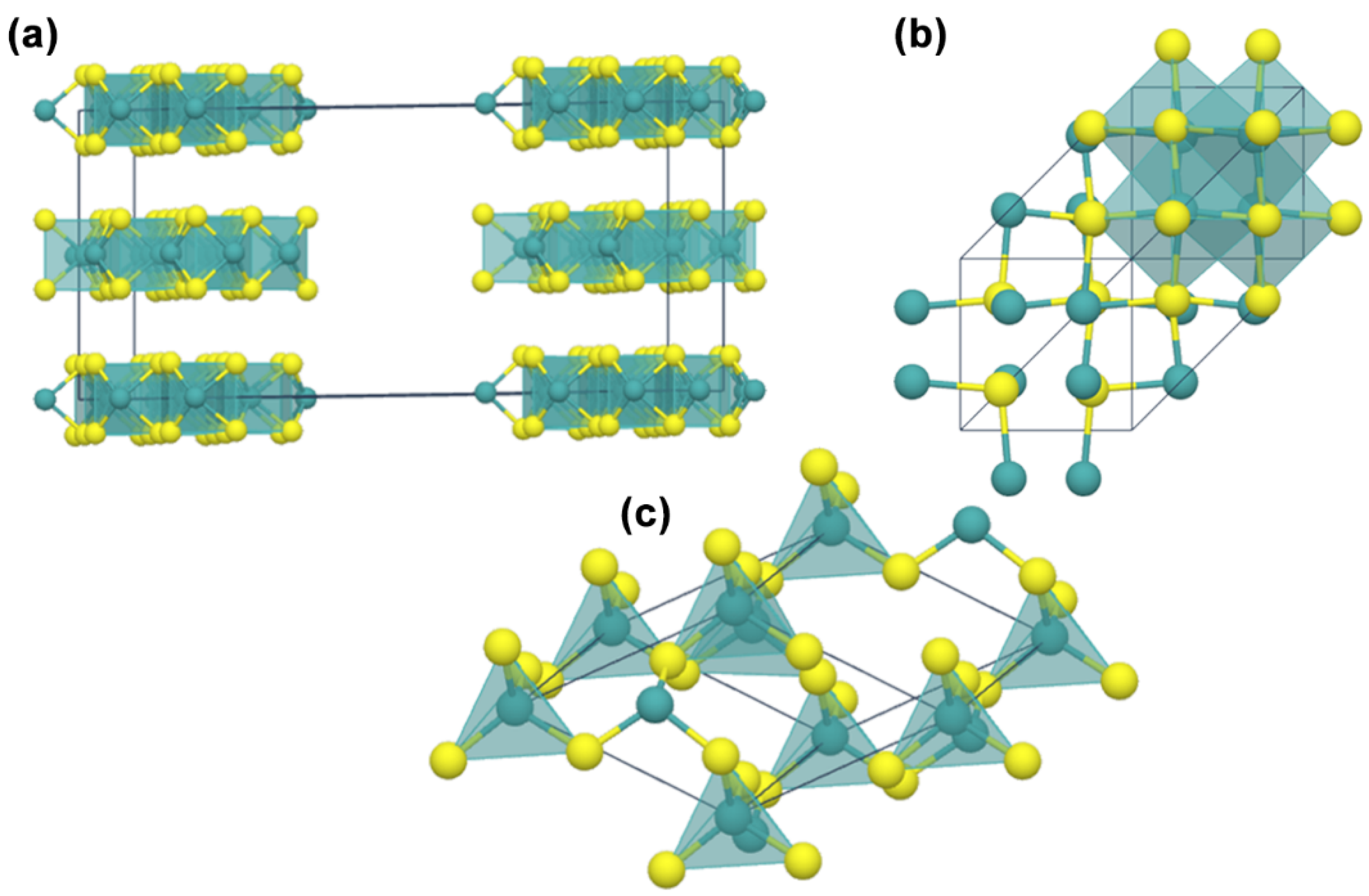
2. Structure and Geometries of MoS2
3. Different Methods of Preparation of MoS2
3.1. Physical Methods
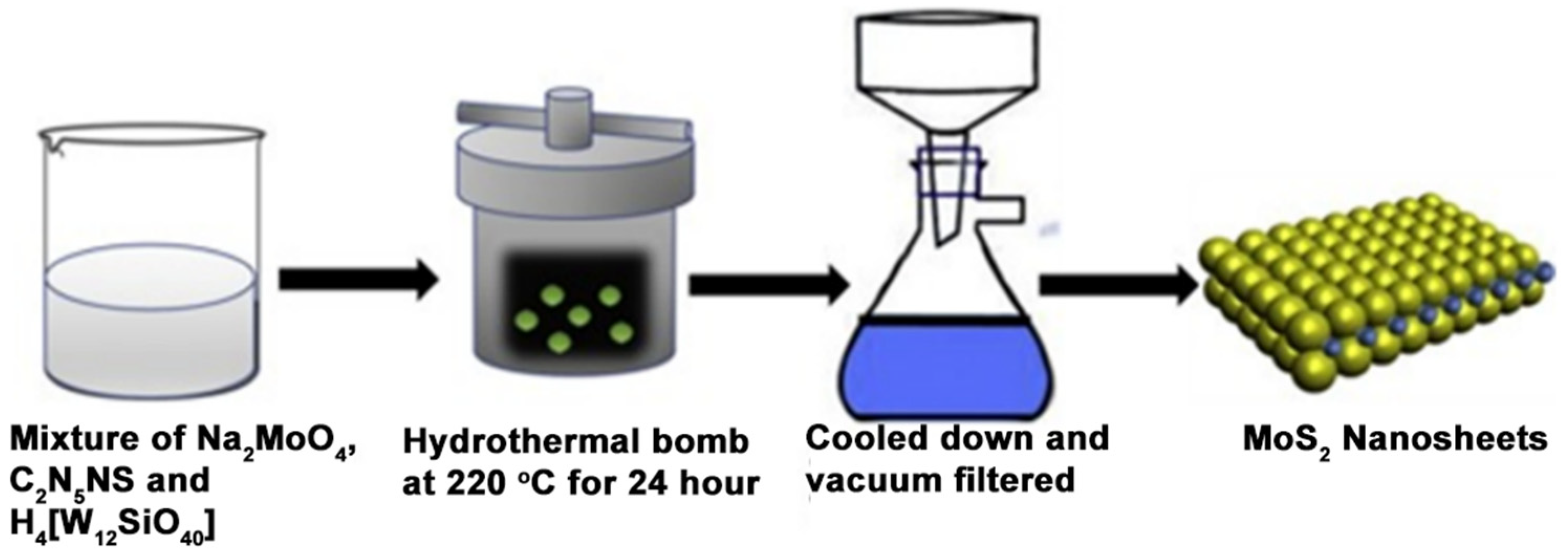
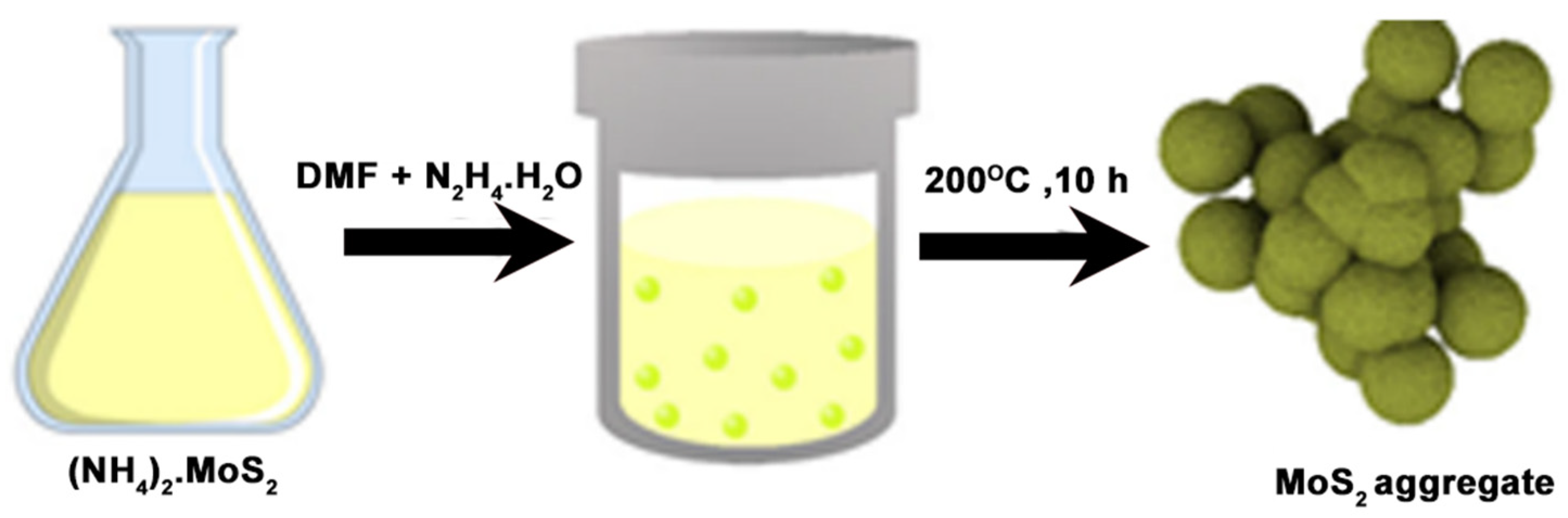
3.2. Chemical Methods
4. Characterization of MoS2-Based Materials
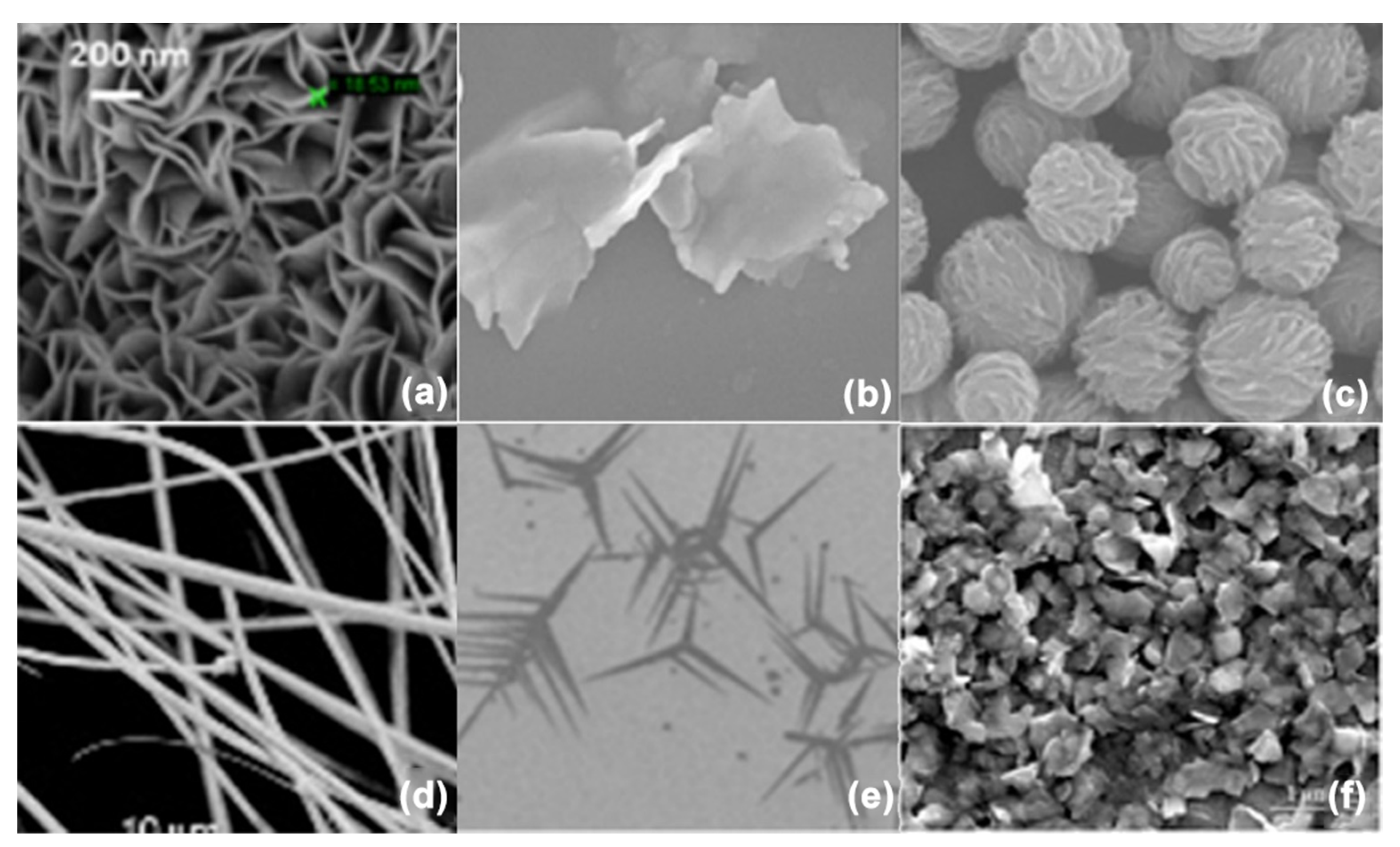
4.1. Morphological Properties
4.2. Structural Properties
4.3. Optical Properties
5. MoS2-Based System for Degradation of Organic Dyes
5.1. Binary Systems of MoS2
5.2. Ternary Systems of MoS2
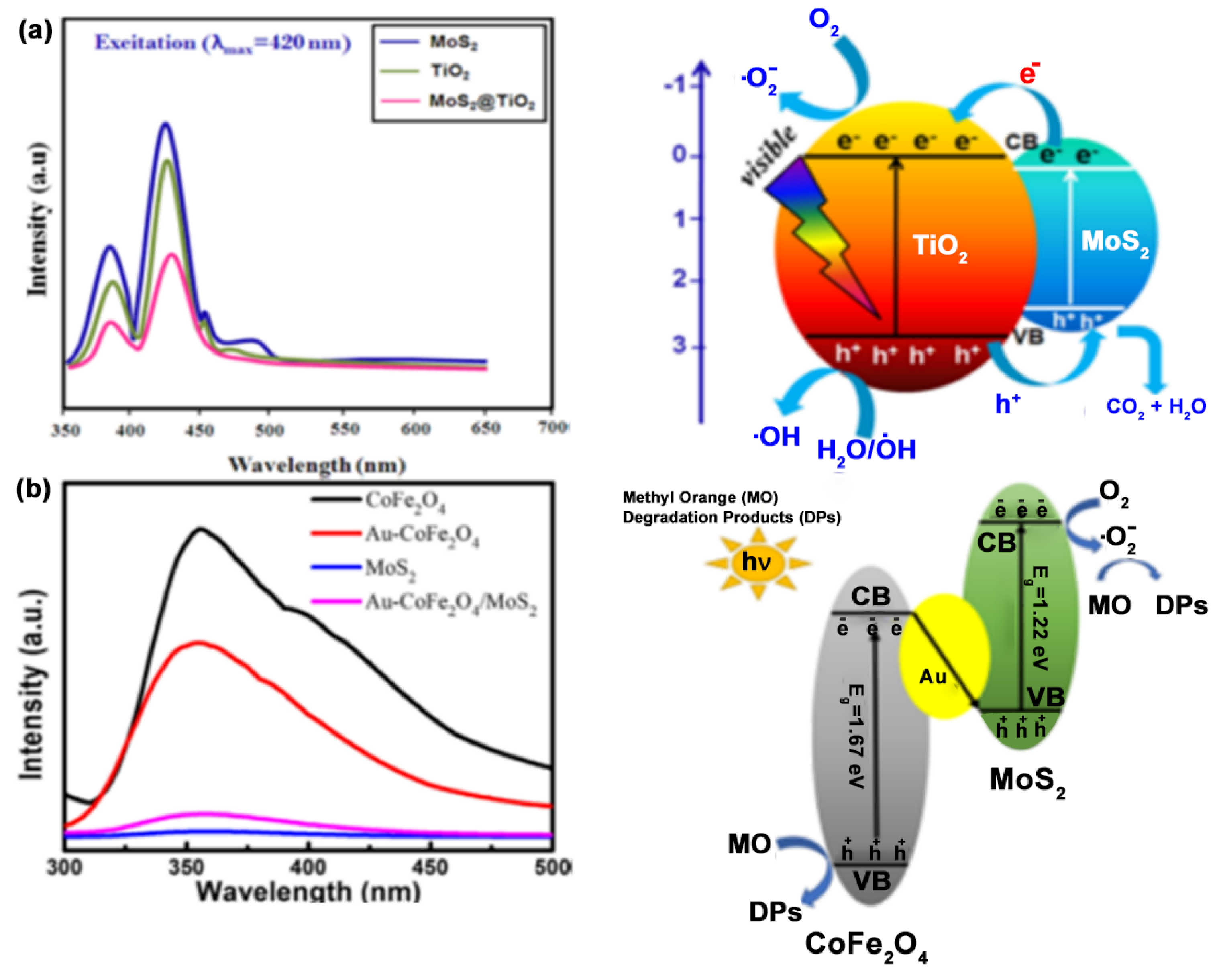
6. Mechanism of Dye Degradation Using MoS2-Based Systems
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-Catalysed Growth of Carbon Nanotubes with Single-Atomic-Layer Walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Xie, X.; Kretschmer, K.; Wang, G. Advances in Graphene-Based Semiconductor Photocatalysts for Solar Energy Conversion: Fundamentals and Materials Engineering. Nanoscale 2015, 7, 13278–13292. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.A.; Yoffe, A.D. The Transition Metal Dichalcogenides Discussion and Interpretation of the Observed Optical, Electrical and Structural Properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Late, D.J.; Kumar, A.; Patel, S.; Singh, J. 2D Layered Transition Metal Dichalcogenides (MoS2): Synthesis, Applications and Theoretical Aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef]
- Monga, D.; Sharma, S.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Advances in Transition Metal Dichalcogenide-Based Two-Dimensional Nanomaterials. Mater. Today Chem. 2021, 19, 100399. [Google Scholar] [CrossRef]
- Agarwal, V.; Chatterjee, K. Recent Advances in the Field of Transition Metal Dichalcogenides for Biomedical Applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-Dimensional Layered MoS2: Rational Design, Properties and Electrochemical Applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C.N.R. Hydrogen Generation by Water Splitting Using MoS2 and Other Transition Metal Dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Lu, H.W.; Yu, Z.T.; Zou, Z.G. Noble-Metal-Free Molybdenum Disulfide Cocatalyst for Photocatalytic Hydrogen Production. ChemSusChem 2015, 8, 4113–4127. [Google Scholar] [CrossRef]
- Jin, X.; Fan, X.; Tian, J.; Cheng, R.; Li, M.; Zhang, L. MoS2 Quantum Dot Decorated g-C3N4 Composite Photocatalyst with Enhanced Hydrogen Evolution Performance. RSC Adv. 2016, 6, 52611–52619. [Google Scholar] [CrossRef]
- Dhiman, V.; Kondal, N. MoS2–ZnO Nanocomposites for Photocatalytic Energy Conversion and Solar Applications. Phys. B 2022, 628, 413569. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, R.; Zhou, L.; Li, Z.; Tian, L.; Cao, R. Phase Engineering-Defective 1T @ 2H MoS2 Nanoflowers as Excellent Full Spectrum Photocatalyst. J. Alloys Compd. 2022, 910, 164898. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive Photodetectors Based on Monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Liu, J.; Kang, J.S.; Matthews, T.S.; You, L.; Li, J.; Grossman, J.C.; Wu, J. Broad-Range Modulation of Light Emission in Two-Dimensional Semiconductors by Molecular Physisorption Gating. Nano Lett. 2013, 13, 2831–2836. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug Delivery with PEGylated MoS2 Nano-Sheets for Combined Photothermal and Chemotherapy of Cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef]
- Tian, Y.; Ge, L.; Wang, K.; Chai, Y. Synthesis of Novel MoS2/g-C3N4 Heterojunction Photocatalysts with Enhanced Hydrogen Evolution Activity. Mater. Charact. 2014, 87, 70–73. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Paredes, J.I.; Munuera, J.M.; Villar-Rodil, S.; Ayán-Varela, M.; Martínez-Alonso, A.; Tascón, J.M.D. Chemically Exfoliated MoS2 Nanosheets as an Efficient Catalyst for Reduction Reactions in the Aqueous Phase. ACS Appl. Mater. Interfaces 2014, 6, 21702–21710. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.H.; Zhang, Y.; Whitten, D.G. Aggregation of Cationic P-Phenylene Ethynylenes on Laponite Clay in Aqueous Dispersions and Solid Films. J. Colloid Interface Sci. 2015, 449, 347–356. [Google Scholar] [CrossRef]
- Das, P.; Malho, J.M.; Rahimi, K.; Schacher, F.H.; Wang, B.; Demco, D.E.; Walther, A. Nacre-Mimetics with Synthetic Nanoclays up to Ultrahigh Aspect Ratios. Nat. Commun. 2015, 6, 5967. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Fang, W.; Yuan, H.; Xia, W.; Zeng, X.; Shangguan, W. Few-Layered MoS2/ZnCdS/ZnS Heterostructures with an Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 4893–4902. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Di Bartolomeo, A. MoS2/Ni3S2/Reduced Graphene Oxide Nanostructure as an Electrocatalyst for Alcohol Fuel Cells. ACS Appl. Nano Mater. 2022, 5, 3361–3373. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Construction of Multifunctional Hydrogel Based on the Tannic Acid-Metal Coating Decorated MoS2 Dual Nanozyme for Bacteria-Infected Wound Healing. Bioact. Mater. 2022, 9, 461–474. [Google Scholar] [CrossRef]
- Shen, H.; Liao, S.; Jiang, C.; Zhang, J.; Wei, Q.; Ghiladi, R.A.; Wang, Q. In Situ Grown Bacterial Cellulose/ MoS2 Composites for Multi-Contaminant Wastewater Treatment and Bacteria Inactivation. Carbohydr. Polym. 2022, 277, 118853. [Google Scholar] [CrossRef]
- Sivaranjani, P.R.; Janani, B.; Thomas, A.; Raju, L.L.; Khan, S.S. Recent Development in MoS2-Based Nano-Photocatalyst for the Degradation of Pharmaceutical Active Compounds. J. Clean. Prod. 2022, 352, 131506. [Google Scholar] [CrossRef]
- Szkoda, M.; Zarach, Z.; Nadolska, M.; Trykowski, G.; Trzciński, K. SnO2 Nanoparticles Embedded onto MoS2 Nanoflakes-an Efficient Catalyst for Photodegradation of Methylene Blue and Photoreduction of Hexavalent Chromium. Electrochim. Acta 2022, 414, 140173. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Dwivedi, A.K. Researches in Water Pollution: A Review. Int. Res. J. Nat. Appl. Sci. 2017, 4, 118–142. [Google Scholar]
- Pirilä, M.; Saouabe, M.; Ojala, S.; Rathnayake, B.; Drault, F.; Valtanen, A.; Huuhtanen, M.; Brahmi, R.; Keiski, R.L. Photocatalytic Degradation of Organic Pollutants in Wastewater. Top. Catal. 2015, 58, 1085–1099. [Google Scholar] [CrossRef]
- Wu, F.; Guan, P.; Xu, X.; Kong, Y.; Cao, D.; Yang, J. High Aspect-Ratio of MoS2 Nanoribbons via a Single-Source Precursor Route for Photocatalytic Degradation. Mater. Lett. 2022, 315, 131987. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L. A New Class of Semiconducting Polymers for Bulk Heterojunction Solar Cells with Exceptionally High Performance. Acc. Chem. Res. 2010, 43, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Kumar, D.P.; Kim, E.H.; Park, H.; Chun, S.Y.; Gopannagari, M.; Bhavani, P.; Reddy, D.A.; Song, J.K.; Kim, T.K. Tuning Band Alignments and Charge-Transport Properties through MoSe2 Bridging between MoS2 and Cadmium Sulfide for Enhanced Hydrogen Production. ACS Appl. Mater. Interfaces 2018, 10, 26153–26161. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.; Hong, L.; Ji, X.; Li, Z.; Lin, Z.; Pan, W. Recent Advances and Perspectives of MoS2-Based Materials for Photocatalytic Dyes Degradation: A Review. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125836. [Google Scholar] [CrossRef]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef] [Green Version]
- Wypych, F.; Weber, T.; Prins, R. Scanning Tunneling Microscopic Investigation of Kx(H2O)Y MoS2. Surf. Sci. 1997, 380, L474–L478. [Google Scholar] [CrossRef]
- El-Mahalawy, S.H.; Evans, B.L. The Thermal Expansion of 2H-MoS2, 2H-MoSe2 and 2H-WSe2 between 20 and 800 °C. J. Appl. Crystallogr. 1976, 9, 403–406. [Google Scholar] [CrossRef]
- Arif Khalil, R.M.; Hussain, F.; Manzoor Rana, A.; Imran, M.; Murtaza, G. Comparative Study of Polytype 2H-MoS2 and 3R-MoS2 Systems by Employing DFT. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 106, 338–345. [Google Scholar] [CrossRef]
- Lin, L.; Miao, N.; Huang, J.; Zhang, S.; Zhu, Y.; Horsell, D.D.; Ghosez, P.; Sun, Z.; Allwood, D.A. A Photocatalyst of Sulphur Depleted Monolayered Molybdenum Sulfide Nanocrystals for Dye Degradation and Hydrogen Evolution Reaction. Nano Energy 2017, 38, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Kampouri, S.; Valizadeh, B.; Luo, W.; Ongari, D.; Planes, O.M.; Züttel, A.; Smit, B.; Stylianou, K.C. Photocatalytic Hydrogen Generation from a Visible-Light-Responsive Metal-Organic Framework System: Stability versus Activity of Molybdenum Sulfide Cocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 30035–30039. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary Attributes of 2-Dimensional MoS2 Nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhu, H.; Dargusch, M.; Huang, Y. A Reliable and Highly Efficient Exfoliation Method for Water-Dispersible MoS2 Nanosheet. J. Colloid Interface Sci. 2018, 514, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Funke, S.; Miller, B.; Parzinger, E.; Thiesen, P.; Holleitner, A.W.; Wurstbauer, U. Imaging Spectroscopic Ellipsometry of MoS2. J. Phys. Condens. Matter 2016, 28, 385301. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, F.; Abdollahi, A.; Mohajerzadeh, S. Controlled Plasma Thinning of Bulk MoS2 Flakes for Photodetector Fabrication. ACS Omega 2019, 4, 19693–19704. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yan, X.; Lu, Z.; Qiu, H.; Xu, G.; Zhou, X.; Wang, P.; Pan, X.; Liu, K.; Jiao, L. High-Mobility Multilayered MoS2 Flakes with Low Contact Resistance Grown by Chemical Vapor Deposition. Adv. Mater. 2017, 29, 1604540. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, S.; Hu, D.; Lin, L.; Yan, Z.; Gao, X.; Zhang, Z.; Liu, J.-M. Ultrathin MoS2-Coated Ag@Si Nanosphere Arrays as an Efficient and Stable Photocathode for Solar-Driven Hydrogen Production. Nanotechnology 2018, 29, 105402. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, V.; Nargelienė, V.; Balakauskas, S.; Bukauskas, V.; Kamarauskas, M.; Lukša, A.; Mironas, A.; Rėza, A.; Šetkus, A. Single Variable Defined Technology Control of the Optical Properties in MoS2 Films with Controlled Number of 2D-Layers. Nanotechnology 2020, 31, 025602. [Google Scholar] [CrossRef] [PubMed]
- Amani, M.; Burke, R.A.; Ji, X.; Zhao, P.; Lien, D.H.; Taheri, P.; Ahn, G.H.; Kirya, D.; Ager, J.W.; Yablonovitch, E.; et al. High Luminescence Efficiency in MoS2 Grown by Chemical Vapor Deposition. ACS Nano 2016, 10, 6535–6541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nan, H.; Xiao, S.; Wan, X.; Ni, Z.; Gu, X.; Ostrikov, K. Shape-Uniform, High-Quality Monolayered MoS2 Crystals for Gate-Tunable Photoluminescence. ACS Appl. Mater. Interfaces 2017, 9, 42121–42130. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Armstrong, D.; Alharbi, S.; Shahrjerdi, D. Physically Unclonable Cryptographic Primitives by Chemical Vapor Deposition of Layered MoS2. ACS Nano 2017, 11, 12772–12779. [Google Scholar] [CrossRef] [PubMed]
- Balendhran, S.; Ou, J.Z.; Bhaskaran, M.; Sriram, S.; Ippolito, S.; Vasic, Z.; Kats, E.; Bhargava, S.; Zhuiykov, S.; Kalantar-Zadeh, K. Atomically Thin Layers of MoS2 via a Two Step Thermal Evaporation-Exfoliation Method. Nanoscale 2012, 4, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Wang, L.; Sun, J.; Long, Y.; Hu, P.; Liu, F.; He, X. Production Methods of Van Der Waals Heterostructures Based on Transition Metal Dichalcogenides. Crystals 2018, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, T.; Wang, Q.; Wang, W.; Shi, R.; Wang, N.; Amini, A.; Cheng, C. Controlled Growth of Atomically Thin Transition Metal Dichalcogenides via Chemical Vapor Deposition Method. Mater. Today Adv. 2020, 8, 100098. [Google Scholar] [CrossRef]
- Jaleel, U.C.; Devi, K.R.; Madhushree, P. Statistical and Experimental Studies of MoS2/g-C3N4/TiO2: A Ternary Z-Scheme Hybrid Composite. J. Mater. Sci. 2021, 56, 6922–6944. [Google Scholar] [CrossRef]
- Chaudhary, N.; Khanuja, M.; Abid; Islam, S.S. Hydrothermal Synthesis of MoS2 Nanosheets for Multiple Wavelength Optical Sensing Applications. Sens. Actuators A Phys. 2018, 277, 190–198. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Tang, H.; Cao, K.; Chen, J.; Wang, F.; Jin, Y. Synthesis and Characterization of Hollow MoS2 Microspheres Grown from MoO3 Precursors. J. Alloys Compd. 2010, 501, 275–281. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Z.; Wu, Z.; Wang, Z.; Liu, Z. Hydrothermal Synthesis of MoS2 Nanoflowers as Highly Efficient Hydrogen Evolution Reaction Catalysts. J. Power Sources 2014, 264, 229–234. [Google Scholar] [CrossRef]
- Song, X.C.; Zheng, Y.F.; Zhao, Y.; Yin, H.Y. Hydrothermal Synthesis and Characterization of CNT@ MoS2 Nanotubes. Mater. Lett. 2006, 60, 2346–2348. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Jia, F.; Song, S. Adsorption of Heavy Metals on Molybdenum Disulfide in Water: A Critical Review. J. Mol. Liq. 2019, 292, 111390. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, J.; Zhao, X.; Zhang, Z. MoS2/RGO Composites for Photocatalytic Degradation of Ranitidine and Elimination of NDMA Formation Potential under Visible Light. Chem. Eng. J. 2020, 383, 123084. [Google Scholar] [CrossRef]
- Anwer, S.; Huang, Y.; Li, B.; Govindan, B.; Liao, K.; Cantwell, W.J.; Wu, F.; Chen, R.; Zheng, L. Nature-Inspired, Graphene-Wrapped 3D MoS2 Ultrathin Microflower Architecture as a High-Performance Anode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 22323–22331. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, B.; Lin, Z.; Shu, D.; Ma, L. Hydrothermal Synthesis of Flower-like MoS2 nanospheres for Electrochemical Supercapacitors. J. Nanosci. Nanotechnol. 2014, 14, 7250–7254. [Google Scholar] [CrossRef]
- Xia, J.; Ren, X.; Zhao, L.; Tang, M.; Tan, L.; Fu, C.; Wu, Q.; Ren, J.; Ding, M.; Meng, X. Synthesis of MoS2 Nanoflowers on CdS Nanorods with a Simple Route and Their Application in Removal of Dyes. J. Nanoparticle Res. 2022, 24, 1–9. [Google Scholar] [CrossRef]
- Jian, R.; Liu, J.; Wu, Z.; Zheng, H.; Zhu, K.; Sun, Z. Constructing Z-Scheme Structure by Loading BiOBr with (010) Exposure on the Surface of MoS2 and Its Enhanced Photocatalytic Property for Degrading RhB. J. Mater. Sci. Mater. Electron. 2022, 33, 6722–6733. [Google Scholar] [CrossRef]
- Zhao, T.; Xing, Z.; Xiu, Z.; Li, Z.; Chen, P.; Zhu, Q.; Zhou, W. Synergistic Effect of Surface Plasmon Resonance, Ti3+ and Oxygen Vacancy Defects on Ag/ MoS2/TiO2-x Ternary Heterojunctions with Enhancing Photothermal Catalysis for Low-Temperature Wastewater Degradation. J. Hazard. Mater. 2019, 364, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Aliofkhazraei, M. Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-15337-7. [Google Scholar]
- Bai, J.; Meng, T.; Guo, D.; Wang, S.; Mao, B.; Cao, M. Co9S8@ MoS2 Core-Shell Heterostructures as Trifunctional Electrocatalysts for Overall Water Splitting and Zn-Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Selkirk, A.; Zhang, S.; Huang, J.; Li, Y.; Xie, Y.; Dong, N.; Cui, Y.; Zhang, L.; Blau, W.J.; et al. MoS2/Carbon Nanotube Core-Shell Nanocomposites for Enhanced Nonlinear Optical Performance. Chem.-A Eur. J. 2017, 23, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.R.; Liang, J.X.; Zheng, Y.R.; Xu, Y.F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.H. An Efficient Molybdenum Disulfide/Cobalt Diselenide Hybrid Catalyst for Electrochemical Hydrogen Generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, L.; Zhu, Z.; Chen, J.; Kang, J.; Huang, Z.; Yang, D. MoS2 Nanosheet Arrays Rooted on Hollow RGO Spheres as Bifunctional Hydrogen Evolution Catalyst and Supercapacitor Electrode. Nano-Micro Lett. 2018, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Antonelou, A.; Syrrokostas, G.; Sygellou, L.; Leftheriotis, G.; Dracopoulos, V.; Yannopoulos, S.N. Facile, Substrate-Scale Growth of Mono- and Few-Layer Homogeneous MoS2 Films on Mo Foils with Enhanced Catalytic Activity as Counter Electrodes in DSSCs. Nanotechnology 2015, 27, 45404. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, L.; Yan, A.; Bai, C.; Xie, Y. Ultrasound-Assisted Cracking Process to Prepare MoS2 Nanorods. Ultrason. Sonochem. 2004, 11, 83–88. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. Exfoliation of MoS2 and H-BN Nanosheets by Hydrolysis of LiBH4. Nanotechnology 2017, 28, 115604. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Zhu, Z.; Wang, Y.; Xia, H.; You, Z.; Lee, C.; Tu, C. Comparison of MoS2 Nanosheets and Hierarchical Nanospheres in the Application of Pulsed Solid-State Lasers. Opt. Mater. Express 2015, 5, 2924. [Google Scholar] [CrossRef]
- Visic, B.; Dominko, R.; Gunde, M.K.; Hauptman, N.; Skapin, S.D.; Remskar, M. Optical Properties of Exfoliated MoS2 Coaxial Nanotubes-Analogues of Graphene. Nanoscale Res. Lett. 2011, 6, 593. [Google Scholar] [CrossRef] [Green Version]
- Mahyavanshi, R.D.; Kalita, G.; Sharma, K.P.; Kondo, M.; Dewa, T.; Kawahara, T.; Tanemura, M. Synthesis of MoS2 Ribbons and Their Branched Structures by Chemical Vapor Deposition in Sulfur-Enriched Environment. Appl. Surf. Sci. 2017, 409, 396–402. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lin, M.-N.; Wang, L.-D.; Zhang, T. Photoluminescence of MoS2 Prepared by Effective Grinding-Assisted Sonication Exfoliation. J. Nanomater. 2014, 2014, 1–7. [Google Scholar]
- Shi, Y.; Li, H.; Wong, J.I.; Zhang, X.; Wang, Y.; Song, H.; Yang, H.Y. MoS2 Surface Structure Tailoring via Carbonaceous Promoter. Sci. Rep. 2015, 5, 10378. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Si, M.; Li, J.; Zhang, J.; Zhang, Z.; Yang, Z.; Xue, D. Ferromagnetism in Freestanding MoS2 Nanosheets. Nanoscale Res. Lett. 2013, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Mahalakshmi, G.; Rajeswari, M.; Ponnarasi, P. Fabrication of Dandelion Clock-Inspired Preparation of Core-Shell TiO2 @ MoS2 Composites for Unprecedented High Visible Light-Driven Photocatalytic Performance. J. Mater. Sci. Mater. Electron. 2020, 31, 22252–22264. [Google Scholar] [CrossRef]
- Bahuguna, A.; Kumar, S.; Sharma, V.; Reddy, K.L.; Bhattacharyya, K.; Ravikumar, P.C.; Krishnan, V. Nanocomposite of MoS2-RGO as Facile, Heterogeneous, Recyclable, and Highly Efficient Green Catalyst for One-Pot Synthesis of Indole Alkaloids. ACS Sustain. Chem. Eng. 2017, 5, 8551–8567. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Shanmugapriya, K.; Sriram, S. Photocatalytic Activity of MoS2 Nanoparticles: An Experimental and DFT Analysis. Appl. Phys. A Mater. Sci. Process. 2019, 125, 817. [Google Scholar] [CrossRef]
- Rasamani, K.D.; Alimohammadi, F.; Sun, Y. Interlayer-Expanded MoS2. Mater. Today 2017, 20, 83–91. [Google Scholar] [CrossRef]
- Balakumar, S.; Rakkesh, R.A.; Prasad, A.K.; Dash, S.; Tyagi, A.K. Nanoplatelet Structures of MoO3 for H2 Gas Sensors. In Proceedings of the International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), Chennai, India, 28–30 November 2011; IEEE: Chennai, India, 2011; pp. 514–517. [Google Scholar]
- Chakrabarty, S.; Mukherjee, A.; Basu, S. RGO- MoS2 Supported NiCo2O4 Catalyst toward Solar Water Splitting and Dye Degradation. ACS Sustain. Chem. Eng. 2018, 6, 5238–5247. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, G.; Parida, K. Enhanced Photocatalytic Activities of RhB Degradation and H2 Evolution from in Situ Formation of the Electrostatic Heterostructure MoS2/NiFe LDH Nanocomposite through the Z-Scheme Mechanism via p–n Heterojunctions. ACS Appl. Mater. Interfaces 2019, 11, 20923–20942. [Google Scholar] [CrossRef]
- Rubio, E.J.; Ramana, C.V. Tungsten-Incorporation Induced Red-Shift in the Bandgap of Gallium Oxide Thin Films. Appl. Phys. Lett. 2013, 102, 191913. [Google Scholar] [CrossRef]
- Zhang, W.; Chuu, C.P.; Huang, J.K.; Chen, C.H.; Tsai, M.L.; Chang, Y.H.; Liang, C.T.; Chen, Y.Z.; Chueh, Y.L.; He, J.H.; et al. Ultrahigh-Gain Photodetectors Based on Atomically Thin Graphene- MoS2 Heterostructures. Sci. Rep. 2015, 4, 3826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Chen, H.Y.; Penumatcha, A.V.; Appenzeller, J. High Performance Multilayer MoS2 Transistors with Scandium Contacts. Nano Lett. 2013, 13, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Su, S.H.; Chang, J.K.; Tsai, D.S.; Chen, C.H.; Wu, C.I.; Li, L.J.; Chen, L.J.; He, J.H. Monolayer MoS2 Heterojunction Solar Cells. ACS Nano 2014, 8, 8317–8322. [Google Scholar] [CrossRef]
- Late, D.J.; Huang, Y.K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, N.; Yang, Y.; Wang, G.; Ng, D.H.L. High Efficiency Photocatalysis for Pollutant Degradation with MoS2/C3N4 Heterostructures. Langmuir 2014, 30, 8965–8972. [Google Scholar] [CrossRef]
- Benavente, E.; Durán, F.; Sotomayor-Torres, C.; González, G. Heterostructured Layered Hybrid ZnO/MoS2 Nanosheets with Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. Solids 2018, 113, 119–124. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, Y.; Wang, Z. TiO2, MoS2, and TiO2/MoS2 Heterostructures for Use in Organic Dyes Degradation. ChemistrySelect 2018, 3, 1713–1718. [Google Scholar] [CrossRef]
- Selvaraj, R.; Kalimuthu, K.R.; Kalimuthu, V. A Type-II MoS2 /ZnO Heterostructure with Enhanced Photocatalytic Activity. Mater. Lett. 2019, 243, 183–186. [Google Scholar] [CrossRef]
- Bargozideh, S.; Tasviri, M.; Kianifar, M. Construction of Novel Magnetic BiFeO3/MoS2 Composite for Enhanced Visible-Light Photocatalytic Performance towards Purification of Dye Pollutants. Int. J. Environ. Anal. Chem. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Nie, W.; Chen, P. MoS2–GO Nanocomposites Synthesized via a Hydrothermal Hydrogel Method for Solar Light Photocatalytic Degradation of Methylene Blue. Appl. Surf. Sci. 2015, 357, 1606–1612. [Google Scholar] [CrossRef]
- Tang, J.; Shi, Y.; Cai, W.; Liu, F. Construction of Embedded Heterostructured SrZrO3 /Flower-like MoS2 with Enhanced Dye Photodegradation under Solar-Simulated Light Illumination. ACS Omega 2020, 5, 9576–9584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harish, S.; Archana, J.; Navaneethan, M.; Shimomura, M.; Ikeda, H. Applied Surface Science Synergistic Interaction of 2D Layered MoS2/ZnS Nanocomposite for Highly Efficient Photocatalytic Activity under Visible Light Irradiation. Appl. Surf. Sci. 2019, 488, 36–45. [Google Scholar] [CrossRef]
- Liu, X.; Meng, F.; Yu, B.; Wu, H. Self-Assembly Synthesis of Flower-like CeO2/MoS2 Heterojunction with Enhancement of Visible Light Photocatalytic Activity for Methyl Orange. J. Mater. Sci. Mater. Electron. 2020, 31, 6690–6697. [Google Scholar] [CrossRef]
- Sharma, Y. Biosynthesis of 3D/2D CeO2/MoS2 Nanocomposites with Enhanced Photocatalytic Activity to Degrade Organic Dye in Wastewater and Statistical Optimization of Reaction Parameters. Res. Sq. 2021, 1, 1–21. [Google Scholar]
- Wang, H.; Wen, F.; Li, X.; Gan, X.; Yang, Y.; Chen, P.; Zhang, Y. Cerium-Doped MoS2 Nanostructures: Efficient Visible Photocatalysis for Cr(VI) Removal. Sep. Purif. Technol. 2016, 170, 190–198. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon N. Y. 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Jo, W.K.; Adinaveen, T.; Vijaya, J.J.; Sagaya Selvam, N.C. Synthesis of MoS2 Nanosheet Supported Z-Scheme TiO2/g-C3N4 Photocatalysts for the Enhanced Photocatalytic Degradation of Organic Water Pollutants. RSC Adv. 2016, 6, 10487–10497. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; Li, Y.; Zeng, X.; Zheng, L.; Wan, C. Liquid-Exfoliation of Layered MoS2 for Enhancing Photocatalytic Activity of TiO2/g-C3N4 Photocatalyst and DFT Study. Appl. Surf. Sci. 2016, 389, 496–506. [Google Scholar] [CrossRef]
- Mahalakshmi, G.; Rajeswari, M.; Ponnarasi, P. Synthesis of Few-Layer g-C3N4 Nanosheets-Coated MoS2/TiO2 Heterojunction Photocatalysts for Photo-Degradation of Methyl Orange (MO) and 4-Nitrophenol (4-NP) Pollutants. Inorg. Chem. Commun. 2020, 120, 108146. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Sun, R.-Q.; Wang, X. A Facile Band Alignment of Polymeric Carbon Nitride Semiconductors to Construct Isotype Heterojunctions. Angew. Chemie Int. Ed. 2012, 51, 10145–10149. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, H.; Zhao, X.; Kondamareddy, K.K.; Ding, J.; Li, C.; Fang, P. Highly Efficient Visible-Light-Induced Photoactivity of Z-Scheme g-C3N4/Ag/ MoS2 Ternary Photocatalysts for Organic Pollutant Degradation and Production of Hydrogen. ACS Sustain. Chem. Eng. 2017, 5, 1436–1445. [Google Scholar] [CrossRef]
- Beyhaqi, A.; Zeng, Q.; Chang, S.; Wang, M.; Taghi Azimi, S.M.; Hu, C. Construction of g-C3N4/WO3/MoS2 Ternary Nanocomposite with Enhanced Charge Separation and Collection for Efficient Wastewater Treatment under Visible Light. Chemosphere 2020, 247, 125784. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.J. MoS2/CdS/TiO2 Ternary Composite Incorporated into Carbon Nanofibers for the Removal of Organic Pollutants from Water. Inorg. Chem. Commun. 2019, 102, 113–119. [Google Scholar] [CrossRef]
- Shanmugam, V.; Jeyaperumal, K.S.; Mariappan, P.; Muppudathi, A.L. Fabrication of Novel g-C3N4 Based MoS2 and Bi2O3 Nanorod Embedded Ternary Nanocomposites for Superior Photocatalytic Performance and Destruction of Bacteria. New J. Chem. 2020, 44, 13182–13194. [Google Scholar] [CrossRef]
- Ikram, M.; Khan, M.I.; Raza, A.; Imran, M.; Ul-Hamid, A.; Ali, S. Outstanding Performance of Silver-Decorated MoS2 Nanopetals Used as Nanocatalyst for Synthetic Dye Degradation. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114246. [Google Scholar] [CrossRef]
- Mohammed, R.; Eid, M.; Ali, M.; Abdel-moniem, S.M.; Ibrahim, H.S. Nano-Structures & Nano-Objects Reusable and Highly Stable MoS2 Nanosheets for Photocatalytic, Sonocatalytic and Thermocatalytic Degradation of Organic Dyes: Comparative Study. Nano-Struct. Nano-Objects 2022, 31, 100900. [Google Scholar]
- Sindhu, A.S.; Shinde, N.B.; Harish, S.; Navaneethan, M.; Eswaran, S.K. Recoverable and Reusable Visible-Light Photocatalytic Performance of CVD Grown Atomically Thin MoS2 Films. Chemosphere 2022, 287, 132347. [Google Scholar] [CrossRef]
- Rani, A.; Singh, K.; Sharma, P. Investigation of Visible Light Photocatalytic Degradation of Organic Dyes by MoS2 Nanosheets Synthesized by Different Routes. Bull. Mater. Sci. 2022, 45, 63. [Google Scholar] [CrossRef]
- Yuan, B.; Yang, L.; Yang, H.; Bai, L.; Wang, W.; Wei, D.; Liang, Y.; Chen, H. Flexible Vacancy-Mediated MoS2-x Nanosheet Arrays for Solar-Driven Interfacial Water Evaporation, Photothermal-Enhanced Photodegradation, and Thermoelectric Generation. Energy Convers. Manag. 2022, 252, 115070. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Duan, L.; Liu, R.; Li, Q.; Zhang, Q.; Zhao, X. Enhanced Photocatalytic Activity and Synthesis of ZnO Nanorods/MoS2 Composites. Superlattices Microstruct. 2018, 117, 336–341. [Google Scholar] [CrossRef]
- Abinaya, R.; Archana, J.; Harish, S.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Shimomura, M.; Hayakawa, Y. Ultrathin Layered MoS2 Nanosheets with Rich Active Sites for Enhanced Visible Light Photocatalytic Activity. RSC Adv. 2018, 8, 26664–26675. [Google Scholar] [CrossRef] [Green Version]
- Sabarinathan, M.; Harish, S.; Archana, J.; Navaneethan, M.; Ikeda, H.; Hayakawa, Y. Highly Efficient Visible-Light Photocatalytic Activity of MoS2-TiO2 Mixtures Hybrid Photocatalyst and Functional Properties. RSC Adv. 2017, 7, 24754–24763. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Wang, S.; Li, X.; Han, Z.; Zhao, C.; Di, T.; Liu, S.; Cheng, Z. Controllable Growth of MoS2 nanosheets on TiO2 burst Nanotubes and Their Photocatalytic Activity. RSC Adv. 2020, 10, 40904–40915. [Google Scholar] [CrossRef]
- Tama, A.M.; Das, S.; Dutta, S.; Bhuyan, M.D.I.; Islam, M.N.; Basith, M.A. MoS2 Nanosheet Incorporated α-Fe2O3/ZnO Nanocomposite with Enhanced Photocatalytic Dye Degradation and Hydrogen Production Ability. RSC Adv. 2019, 9, 40357–40367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Mohapatra, P.K.; Bahadur, D. Improved Photocatalytic Degradation of Organic Dye Using Ag3PO4/MoS2 Nanocomposite. Front. Mater. Sci. 2017, 11, 366–374. [Google Scholar] [CrossRef]
- Raja, V.R.; Rosaline, D.R.; Suganthi, A.; Rajarajan, M. Facile Fabrication of PbS/ MoS2 Nanocomposite Photocatalyst with Efficient Photocatalytic Activity under Visible Light. Solid State Sci. 2017, 67, 99–108. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic Wastewater Purification with Simultaneous Hydrogen Production Using MoS2 QD-Decorated Hierarchical Assembly of ZnIn2S4 on Reduced Graphene Oxide Photocatalyst. Water Res. 2017, 121, 11–19. [Google Scholar] [CrossRef]
- Rani, A.; Singh, K.; Patel, A.S.; Chakraborti, A.; Kumar, S.; Ghosh, K.; Sharma, P. Visible Light Driven Photocatalysis of Organic Dyes Using SnO2 Decorated MoS2 Nanocomposites. Chem. Phys. Lett. 2020, 738, 136874. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, L.; Liu, C. Magnetically Separable and Recyclable Photocatalyst MoS2-SrFe12O19 with p-n Heterojunction: Fabrication, Characterization, and Photocatalytic Mechanism. Appl. Organomet. Chem. 2020, 34, 1–15. [Google Scholar] [CrossRef]
- Gawari, D.; Pandit, V.; Jawale, N.; Kamble, P. Proceedings Layered MoS2 for Photocatalytic Dye Degradation. Mater. Today Proc. 2022, 53, 10–14. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, H.; Liu, C. Au Nanoparticles Enhanced Z-Scheme Au-CoFe2O4/MoS2 Visible Light Photocatalyst with Magnetic Retrievability. Appl. Surf. Sci. 2019, 463, 854–862. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Wang, Y.; Zheng, L.; He, G.; Deng, J.; Liu, M.; Li, Y.; Liu, Y.; Zhou, H. Fabrication of Z-Scheme TiO2/SnS2/MoS2 Ternary Heterojunction Arrays for Enhanced Photocatalytic and Photoelectrochemical Performance under Visible Light. J. Solid State Chem. 2022, 307, 122737. [Google Scholar] [CrossRef]
- Khaing, K.K.; Yin, D.; Ouyang, Y.; Xiao, S.; Liu, B.; Deng, L.; Li, L.; Guo, X.; Wang, J.; Liu, J.; et al. Fabrication of 2D–2D Heterojunction Catalyst with Covalent Organic Framework (COF) and MoS2 for Highly Efficient Photocatalytic Degradation of Organic Pollutants. Inorg. Chem. 2020, 59, 6942–6952. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Tang, H.; Zhang, P.; Wang, J. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic Activity of BiPO4 Nanoparticles. Appl. Surf. Sci. 2017, 425, 100–106. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Hu, X.; Wang, Y.; Liu, E.; Fan, J. A Novel S-Scheme MoS2 /CdIn2S4 Flower-like Heterojunctions with Enhanced Photocatalytic Degradation and H2 Evolution Activity. J. Phys. D Appl. Phys. 2020, 53, 205101. [Google Scholar] [CrossRef]
- Ren, B.; Shen, W.; Li, L.; Wu, S.; Wang, W. 3D CoFe2O4 Nanorod/Flower-like MoS2 Nanosheet Heterojunctions as Recyclable Visible Light-Driven Photocatalysts for the Degradation of Organic Dyes. Appl. Surf. Sci. 2018, 447, 711–723. [Google Scholar] [CrossRef]
- Lu, M.; Xiao, X.; Wang, Y.; Chen, J. Construction of Novel BiOIO3/MoS2 2D/2D Heterostructures with Enhanced Photocatalytic Activity. J. Alloys Compd. 2020, 831, 154789. [Google Scholar] [CrossRef]
- Rathnasamy, R.; Santhanam, M.; Alagan, V. Anchoring of ZnO Nanoparticles on Exfoliated MoS2 Nanosheets for Enhanced Photocatalytic Decolorization of Methyl Red Dye. Mater. Sci. Semicond. Process. 2018, 85, 59–67. [Google Scholar] [CrossRef]
- Talukdar, K.; Saravanakumar, K.; Kim, Y.; Fayyaz, A.; Kim, G.; Yoon, Y.; Park, C.M. Rational Construction of CeO2–ZrO2@ MoS2 Hybrid Nanoflowers for Enhanced Sonophotocatalytic Degradation of Naproxen: Mechanisms and Degradation Pathways. Compos. Part B Eng. 2021, 215, 108780. [Google Scholar] [CrossRef]
- Ji, R.; Zhu, Z.; Ma, W.; Tang, X.; Liu, Y.; Huo, P. A Heterojunction Photocatalyst Constructed by the Modification of 2D-CeO2 on 2D-MoS2 Nanosheets with Enhanced Degrading Activity. Catal. Sci. Technol. 2020, 10, 788–800. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Wang, F.; Lei, W. Cu2O/ MoS2 Composites: A Novel Photocatalyst for Photocatalytic Degradation of Organic Dyes under Visible Light. Ionics 2020, 26, 6359–6369. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Liu, Q.; Yang, X.; Tang, H. Construction of Carbon Nitride and MoS2 Quantum Dot 2D/0D Hybrid Photocatalyst: Direct Z-Scheme Mechanism for Improved Photocatalytic Activity. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 2160–2170. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, W.; Zhang, X.; Zhang, S.; Liu, B.; Zhen, D. Synthesis of Z-Scheme Mn-CdS/ MoS2/TiO2 Ternary Photocatalysts for High-Efficiency Sunlight-Driven Photocatalysis. Adv. Compos. Lett. 2019, 28, 1–10. [Google Scholar] [CrossRef] [Green Version]









| System | Dye | Reaction Conditions | Degradation (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Time (Min) | Light Source | Catalyst Amount (mg) | Conc of Dye (ppm) | ||||
| MoS2/SnO2 | MB | 90 | Visible | 20 | 95.0 | [30] | |
| MoS2 nanosheets | MB | 120 | Fluorescent lamp | 10 | 100 | 49.3 | [121] |
| CeO2-ZrO2@MoS2 | NPX | 40 | Visible light | 50 | 96.0 | [142] | |
| CeO2/MoS2 | MO | 90 | Visible light | 25 | 20 | 96.1 | [106] |
| 3D/2D CeO2/MoS2 | MV | 30 | Visible light | 20 | 20 | 96.25 | [107] |
| Cerium-doped MoS2 | Cr(IV) | 30 | Visible light | 6 | 20 | 40 | [108] |
| MoS2/BiOBr | RhB | 30 | Xenon arc | 20 | 10 | 96.0 | [70] |
| MoS2-x nanosheet arrays | RhB | 60 | Visible | 5 | 97.2 | [122] | |
| 10% g-C3N4/TiO2/MoS2(0.2) | MB | 60 | Visible | 30 | 10 | 99.5 | [110] |
| Layered MoS2 | MB | 90 | Visible | 50 | 10 | 71.0 | [133] |
| TiO2/SnS2/MoS2 | MB | 90 | Visible | 5 | 81.8 | [135] | |
| TiO2/g-C3N4/MoS2 | MO | 60 | Visible | 100 | 20 | 90.6 | [111] |
| g-C3N4/MoS2/TiO2 | MO | 60 | Visible | 50 | 0.03 | 88.0 | [112] |
| MoS2/g-C3N4/TiO2 | MG | 60 | Visible | 50 | 10 | 86.0 | [59] |
| TiO2/MoS2 | RhB MB MO | 60 | Visible | 50 | 10 | 99.6 96.4 87.5 | [100] |
| RGO-MoS2 supported NiCo2O4 | RhB | 90 | Visible | 50 | 10 | 95.0 | [91] |
| SrZrO3/Flower-likeMoS2 | RhB | 80 | solar | 15 | 25 | 99.7 | [104] |
| g-C3N4/Ag/MoS2 | RhB | 90 | Xenon arc | 100 | 20 | 95.8 | [114] |
| MoS2/NiFe LDH | RhB | 120 | Solar | 20 | 20 | 90.0 | [92] |
| MoS2/COF | RhB | 30 | Solar | 10 | 20 | 98.0 | [136] |
| MoS2–GO | MB | 60 | solar | 10 | 10 | 99.0 | [103] |
| BiPO4, MoS2 and graphene | RhB | 90 | Mercury | 100 | 5 | - | [137] |
| g-C3N4/WO3/MoS2 | RhB MO MB | Xenon arc | 100 | 50 20 20 | 99.9 83.4 91.8 | [115] | |
| MoS2/CdS/TiO2 | MB | 15 | Visible | 25 | 10 | 100 | [116] |
| g-C3N4-based MoS2 and Bi2O3 | MB | 90 | Visible | 50 | 20 | 98.5 | [117] |
| MoS2/ZnS | MB | 32 | Visible | 50 | 10 | 99.9 | [105] |
| MoS2/CdIn2S4 | RhB | 30 | Visible | 10 | - | [138] | |
| CoFe2O4/MoS2 | CRMBMO | 60 | Visible | 30 | 20 | 94.9 67.8 | [139] |
| ZnO/MoS2 | MB | 300 | Visible | 10 | 1 | 75.0 | [99] |
| Type II MoS2/ZnO | MB | 120 | UV | 10 | 99.0 | [101] | |
| BiOIO3/MoS2 (BM-x) 2D/2D | RhB | 90 | 500 W Xenon lamp | 50 | 10 | 98.7 | [140] |
| ZnO-MoS2 | MR | 60 | Solar | 10 | 10 | 89.0 | [141] |
| BiFeO3/MoS2 | RhB | 200 | Visible | 50 | 10 | 89.0 | [102] |
| ZnO nanorods/MoS2 | RhB | UV | - | 4 | - | [123] | |
| Ultrathin layered MoS2 | MB RhB | 36 | Visible | 100 | 5 | 95.3 41.1 | [124] |
| MoS2/TiO2 | MB | 12 | UV-Vis | 50 | 5 | 99.3 | [125] |
| TiO2/MoS2 | MB | 30 | Visible | 10 | 10 | 94.2 | [126] |
| NMS incorporateda-Fe2O3/ZnO | RhB | 240 | Visible | 40 | 91.0 | [127] | |
| Ag3PO4/MoS2 | MB | 15 | 60 W CFL | 0.20 g/L | 20 | 97.6 | [128] |
| MoS2/g-C3N4 | RhB/MO | 60 | Visible | 5 | 50 | 92.0 | [98] |
| PbS/MoS2 | MB | 180 | Visible | 1% | 30 | 83.0 | [129] |
| MoS2QDs@ZnIn2S4@RGO) | RhB/MB | 30 | 300 W Xenon lamp | 100 | 80 | 98.8 98.5 | [130] |
| SnO2-MoS2 | MR/MB | 120 | Visible | 1 | 100 | 58.5 94.0 | [131] |
| MoS2-SrFe12O19 | RhB | 120 | 300 W Xenon lamp | 10 | 96.5 | [132] | |
| Au-CoFe2O4/MoS2 | MO | 60 | 300 W iodine tungsten lamp | 70 | 50 | 96.0 | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaleel UC, J.R.; R, M.; Devi K R, S.; Pinheiro, D.; Mohan, M.K. Structural, Morphological and Optical Properties of MoS2-Based Materials for Photocatalytic Degradation of Organic Dye. Photochem 2022, 2, 628-650. https://doi.org/10.3390/photochem2030042
Jaleel UC JR, R M, Devi K R S, Pinheiro D, Mohan MK. Structural, Morphological and Optical Properties of MoS2-Based Materials for Photocatalytic Degradation of Organic Dye. Photochem. 2022; 2(3):628-650. https://doi.org/10.3390/photochem2030042
Chicago/Turabian StyleJaleel UC, Jadan Resnik, Madhushree R, Sunaja Devi K R, Dephan Pinheiro, and Mothi Krishna Mohan. 2022. "Structural, Morphological and Optical Properties of MoS2-Based Materials for Photocatalytic Degradation of Organic Dye" Photochem 2, no. 3: 628-650. https://doi.org/10.3390/photochem2030042






