Synthesis of Metallic and Metal Oxide Nanoparticles Using Homopolymers as Solid Templates: Luminescent Properties of the Eu+3 Nanoparticle Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Macromolecular Metal Complex of Type MXn-Polymer
3. Results and Discussions
3.1. Macromolecular Complexes (1a-Pt), (1a-Zn), (1a-Eu), (1b-Pt), (1b-Zn), (1b-Eu), (1c-Pt), (1c-Zn), and (1c-Eu)
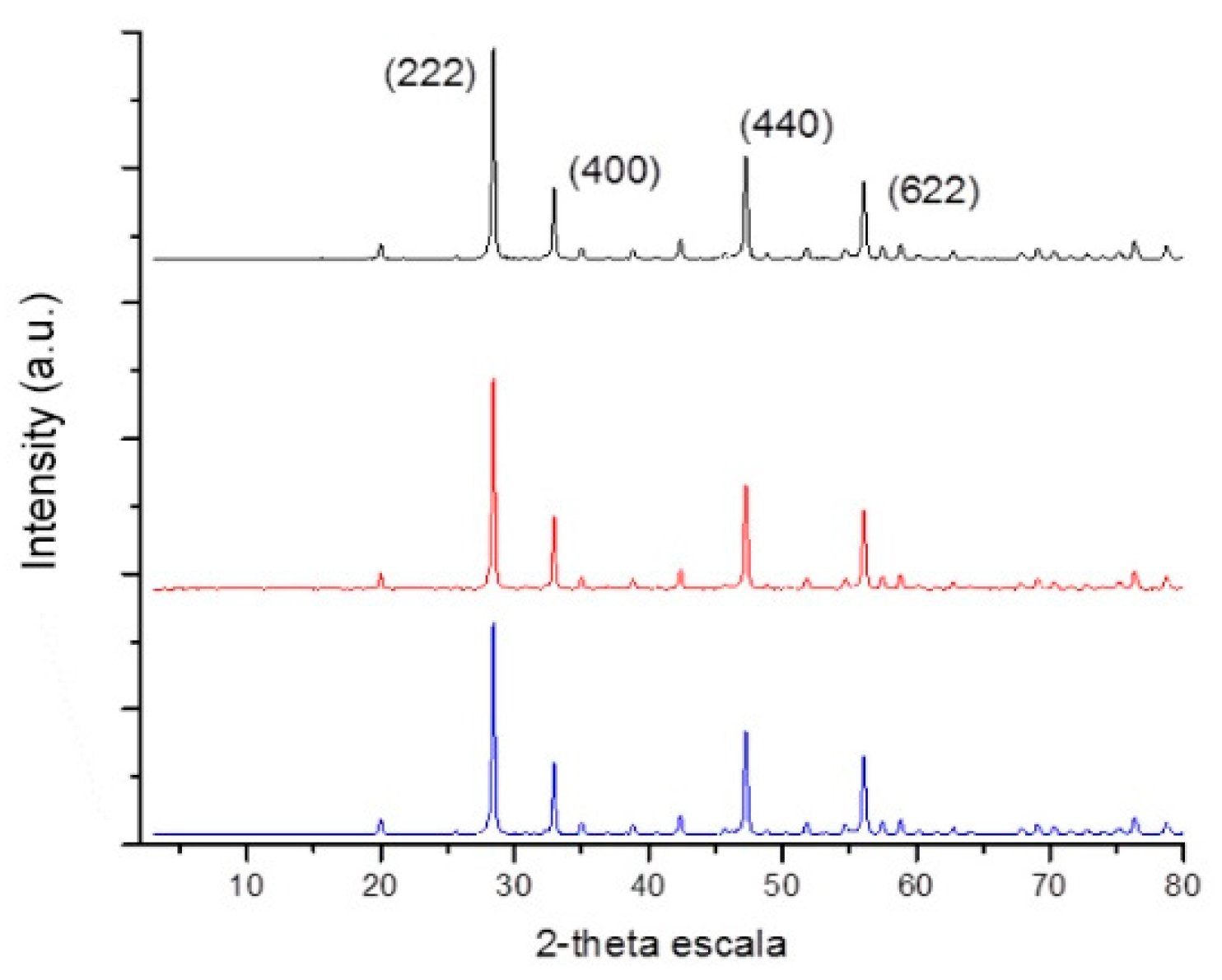
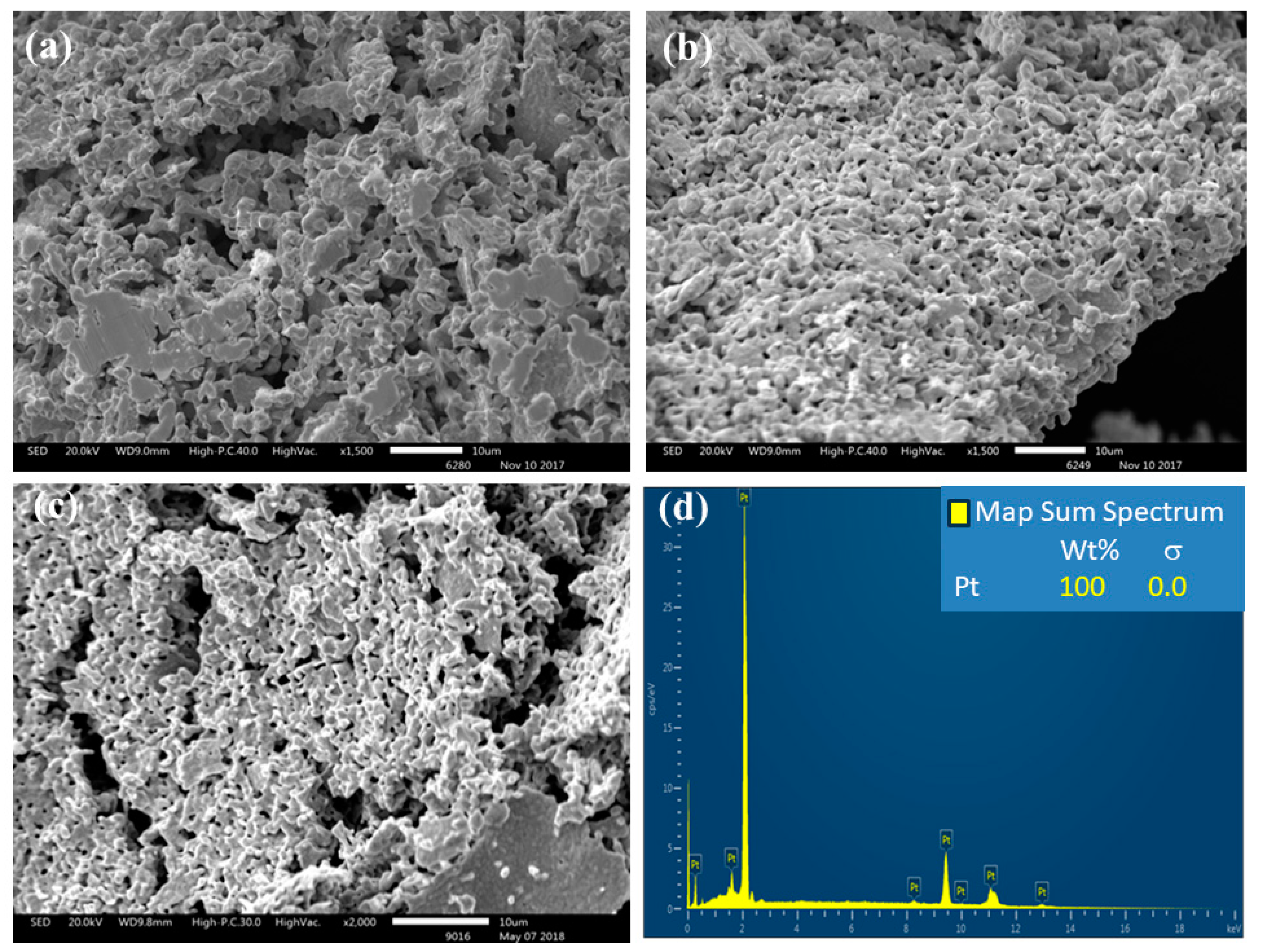
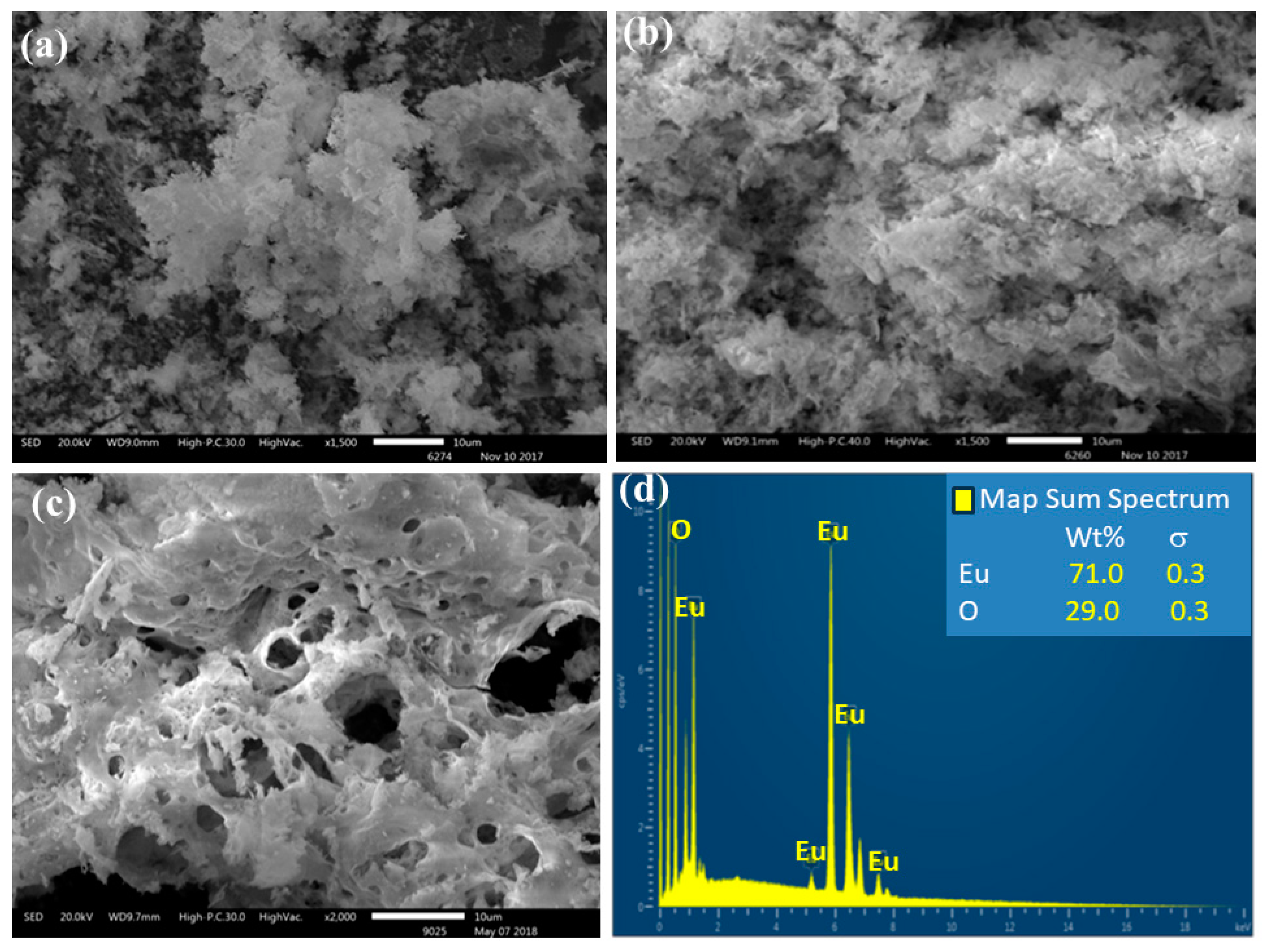
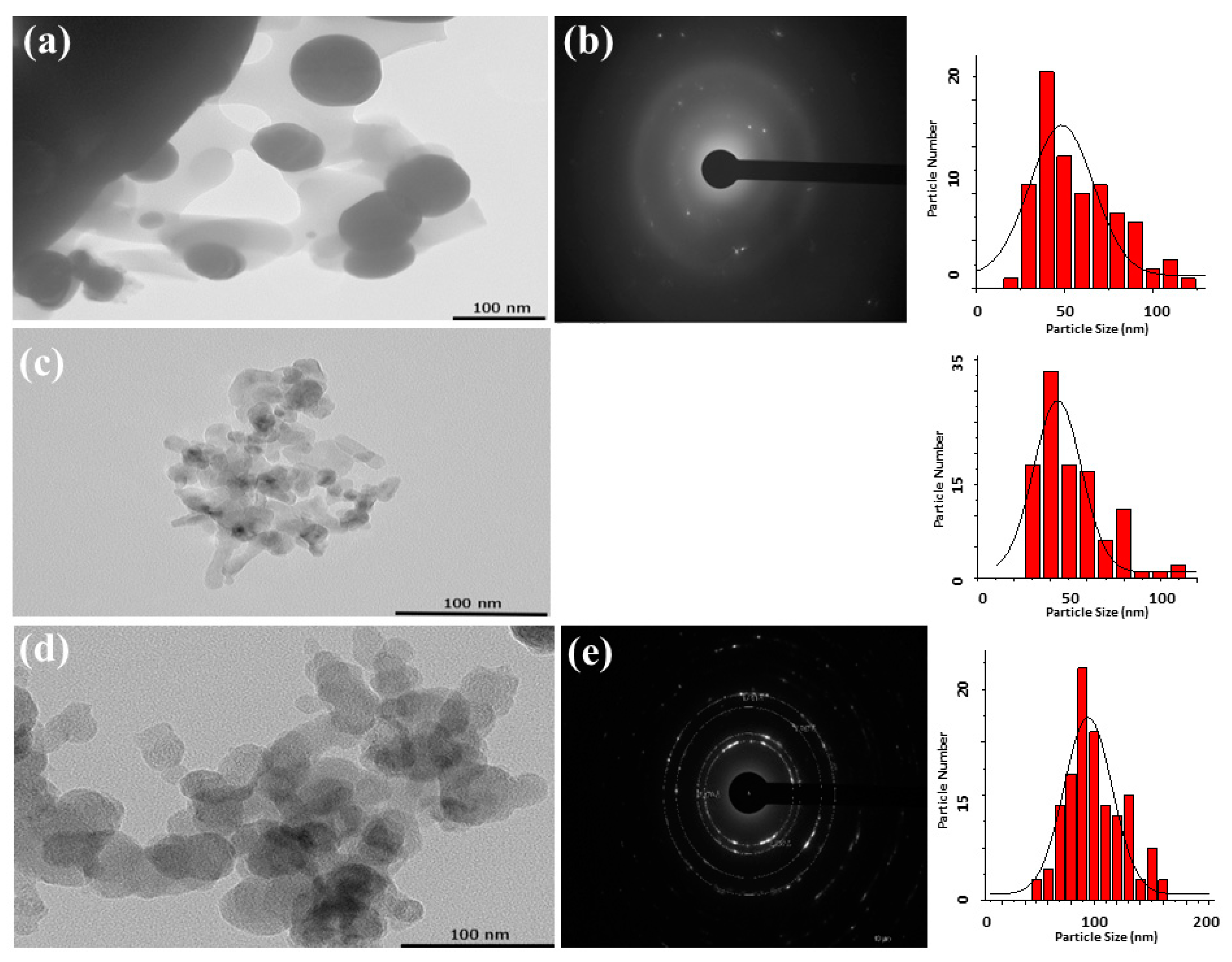
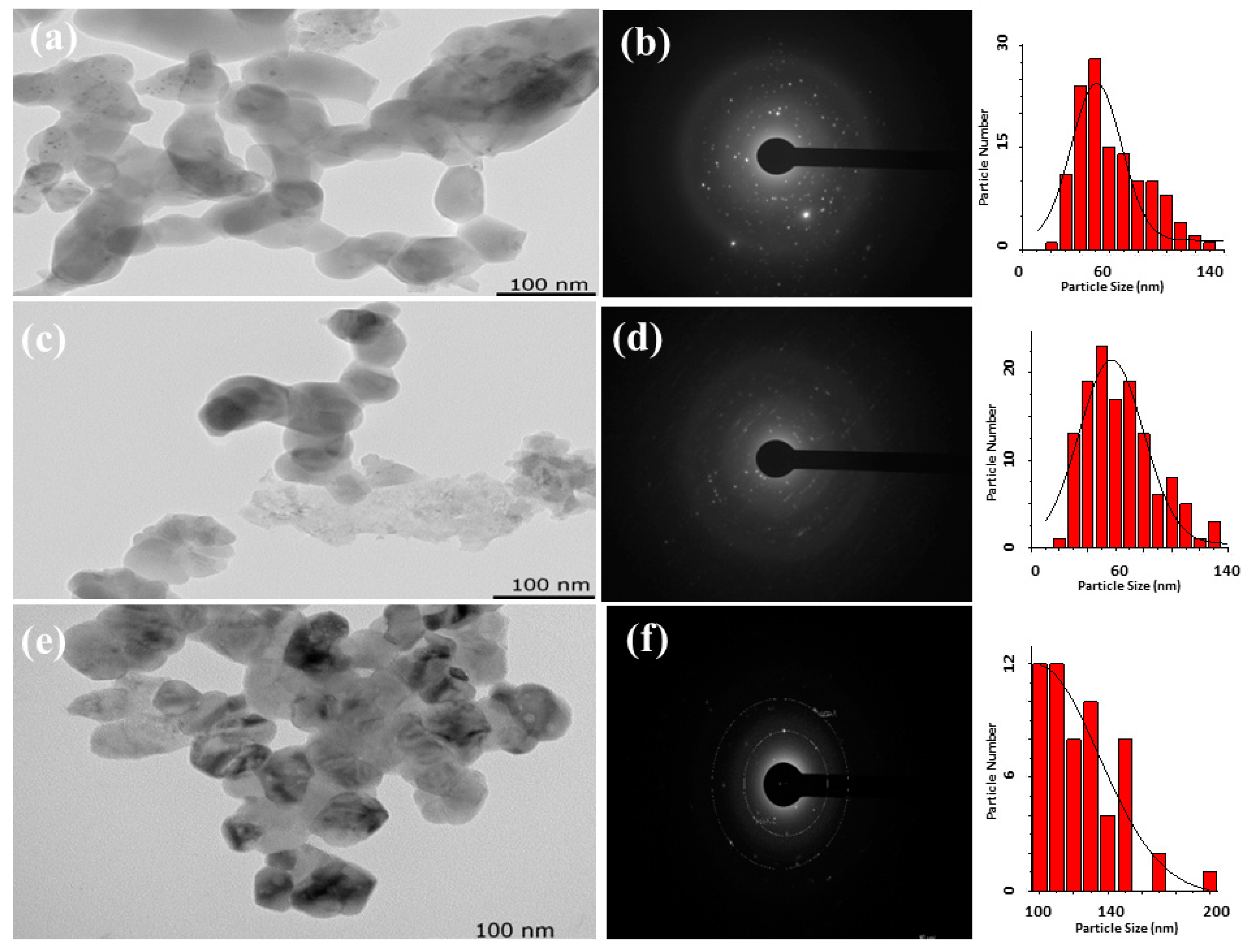
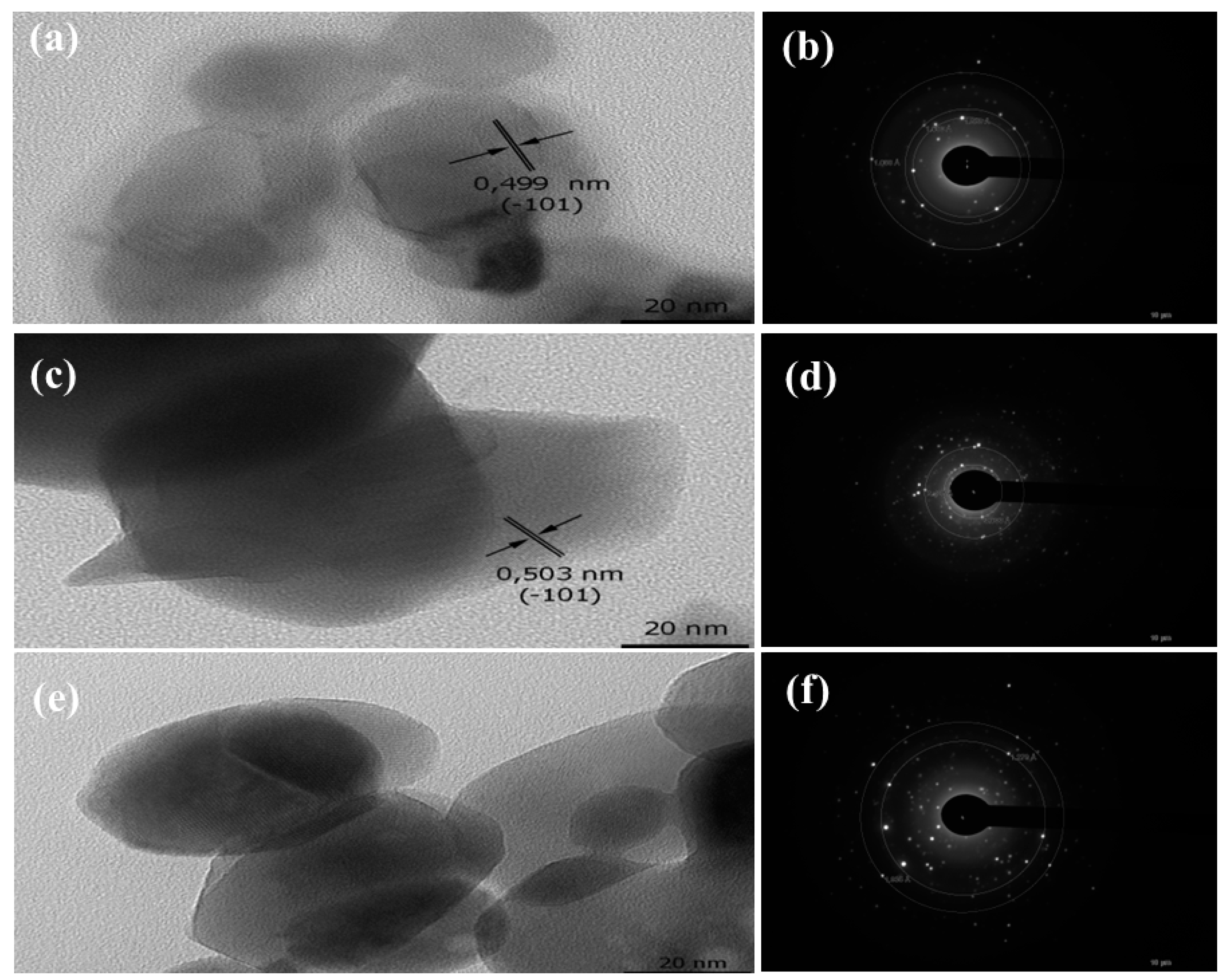
3.2. Pyrolysis of the Macromolecular Metal, (1a-Pt), (1a-Zn), (1a-Eu), (1b-Pt), (1b-Zn), (1b-Eu), (1c-Pt), (1c-Zn), and (1c-Eu), to Nanostructured Pt, ZnO, and Eu2O3
3.2.1. EDS Analysis of (1c-Zn)
3.2.2. EDS Analysis of (1c-Eu)
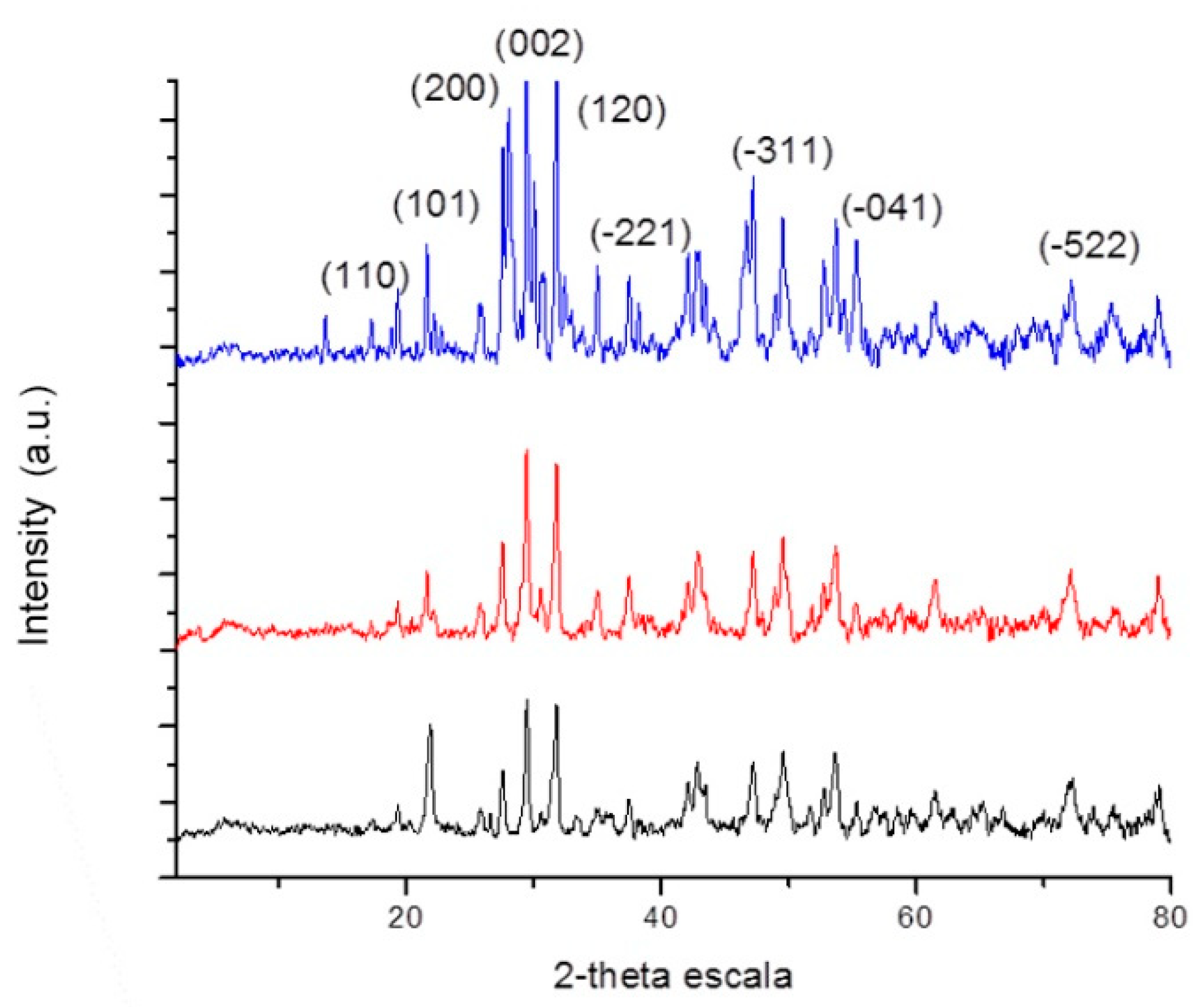
3.3. Pyrolysis of Polyphosphazene-Based Macromolecular Metal Complexes ([N=P(O2CH2CF3)]20-b-[P2VP(Eu(NO3)3)x]20 (2a-Eu), [N=P(O2CH2CF3)]60-b-[P2VP(Eu(NO3)3)x]20 (2b-Eu), and [N=P(O2CH2CF3)]100-b-[P2VP(Eu(NO3)3)x]20 (2c-Eu)) to EuPO4
3.4. Optical Properties
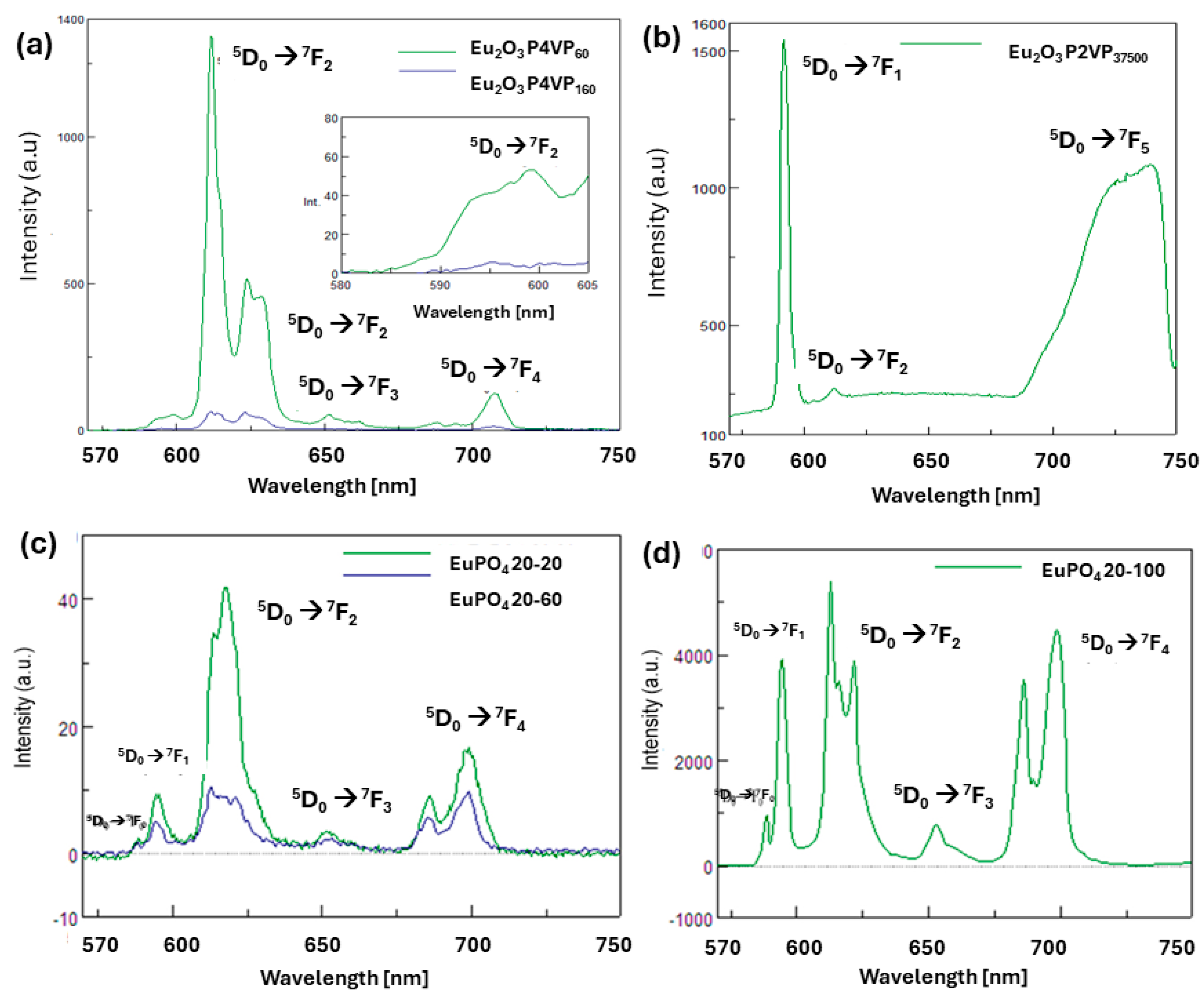
3.5. Study of the Luminescent Properties of Nanostructured Eu2O3 and EuPO4 Materials Prepared by Pyrolysis of the Macromolecular Metal Precursors (1a-Eu), (1b-Eu), and (1c-Eu) and of EuPO4 from (2a-Eu), (2b-Eu), and (2c-Eu)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allcock, H.R. Recent developments in polyphosphazene materials science. Curr. Opin. Solid State Mater. Sci. 2006, 10, 231–240. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kugel, R.L. Synthesis of high polymeric alkoxy- and aryloxy-phosphonitriles. J. Am. Chem. Soc. 1965, 87, 4216–4217. [Google Scholar] [CrossRef]
- Linhardt, A.; König, M.; Schöfberger, W.; Brüggemann, O.; Andrianov, A.K.; Teasdale, I. Biodegradable Polyphosphazene Based Peptide-Polymer Hybrids. Polymers 2016, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Weidel, A.L.; Krogman, N.R.; Nguyen, N.Q.; Nair, L.S.; Laurencin, C.T.; Allcock, H.R. Polyphosphazenes That Contain Dipeptide Side Groups: Synthesis, Characterization, and Sensitivity to Hydrolysis. Macromolecules 2009, 42, 636–639. [Google Scholar]
- Zhou, X.; Qiu, S.; Mu, X.; Zhou, M.; Cai, W.; Song, L.; Xing, W.; Hu, Y. Polyphosphazenes-based flame retardants: A review. Compos. Part B Eng. 2020, 202, 108397. [Google Scholar] [CrossRef]
- Allcock, H.R.; Smith, D.E.; Kim, Y.B.; Fitzgerald, J.J. Poly(organophosphazenes) Containing Allyl Side Groups: Cross-Linking and Modification by hydrosilylation. Macromolecules 1994, 27, 5207–5215. [Google Scholar] [CrossRef]
- Akram, M.; Wang, L.; Yu, H.; Amer, W.A.; Khalid, H.; Abbasi, N.M.; Chen, Y.; Saleem, M.; Tong, R. Polyphophazenes as anti-cancer drug carriers: From synthesis to application. Prog. Polym. Sci. 2014, 39, 1987–2009. [Google Scholar] [CrossRef]
- Allcock, H.R.; Chen, C. Polyphosphazenes: Phosphorus in inorganic−organic polymers. J. Org. Chem. 2020, 85, 14286–14297. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Teasdale, I. Preparation of polyphosphazenes: A tutorial review. Chem. Soc. Rev. 2016, 45, 5200–5215. [Google Scholar] [CrossRef]
- Allcock, H.R. The expanding field of polyphosphazene high polymers. Dalton Trans. 2016, 45, 1856–1862. [Google Scholar] [CrossRef]
- Gleria, M.; De Jaeger, R. (Eds.) Synthesis Characterization and Applications of Phosphazenes; Nova Science: Hauppauge, NY, USA, 2014; Volume 3, p. 83. [Google Scholar]
- Allcock, H.R. A Perspective of Polyphosphazene Research. J. Inorg. Organomet. Polym. Mater. 2006, 16, 277–294. [Google Scholar] [CrossRef]
- Honeyman, C.H.; Manners, I.; Morrissey, C.T.; Allcock, H.R. Ambient Temperature Synthesis of Poly(dichlorophosphazene) with Molecular Weight Control. J. Am. Chem. Soc. 1995, 117, 7035–7036. [Google Scholar] [CrossRef]
- Carriedo, G.A.; De la Campa, R.; Presa Soto, A. Polyphosphazenes—Synthetically Versatile Block Copolymers (“Multi-Tool”) for Self-Assembly. Eur. J. Inorg. Chem. 2018, 2018, 2484–2499. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L. Metallic Nanostructures Using Oligo and Polyphosphazenes as Template or Stabilizer in Solid State. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2010; Chapter 16. [Google Scholar]
- Díaz, C.; Castillo, P.; Valenzuela, M.L. Thermolytic Transformations of Organometallic Polymer Containing the Cr(CO)5 Precursor into Nanostructured Chromium Oxide. J. Clust. Sci. 2005, 16, 515–522. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L. Synthesis of Nanostructured Materials by a New Solid State Pyrolysis Organometallic Method. J. Chil. Chem. Soc. 2005, 50, 417–419. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L. Small Molecules and High Polymeric Phosphazenes Containing Oxypyridine Side Groups and Their Organometallic Derivatives: Useful Precursors to Nanostructured Materials. Macromolecules 2006, 39, 103–111. [Google Scholar] [CrossRef]
- Carriedo, G.; Garcia Alonso, F.J.; Díaz, C.; Valenzuela, M.L. Synthesis and thermal decarbonylation of W(CO)5 complexes supported by nitrile, pyridine, or phosphine ligands to poly-spirophosphazene random copolymers carrying O-C6H5CO2Pr groups. Polyhedron 2006, 25, 105–112. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L. Photoluminiscent Manganese nanoparticles from solid state Polyphosphazenes organometallic derivatives. J. Inorg. Organomet. Polym. 2006, 16, 123–128. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Carriedo, G.; Garcia Alonso, F.J.; Presa-Soto, A. Neutral AuCl complexes supported in linear high molecular weight, poly-spirophosphazene-phosphine copolymer and its conversion to nanostructured gold materials. Polym. Bull. 2006, 57, 913–920. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L. Organometallic Derivatives of Polyphosphazenes as Precursors for Metallic Nanostructured Materials. J. Inorg. Organomet. Polym. 2006, 16, 419–435. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Spodine, E.; Moreno, Y.; Peña, O. A Cyclic and Polymeric Phosphazene as Solid State Template for the Formation of RuO2 Nanoparticles. J. Clust. Sci. 2007, 18, 831–844. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Yutronic, N. Polyphosphazenes as Solid Templates for the Formation of Monometallic and Bimetallic Nanostructures. J. Inorg. Organomet. Polym. 2007, 17, 577–582. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Jimenez, J.; Laguna, A. Microsize and Nanosize BPO4 from Pyrolysis of a Carborane Substituted Polyphosphazene. J. Inorg. Organomet. Polym. 2006, 16, 211–218. [Google Scholar] [CrossRef]
- Carriedo, G.; Díaz, C.; Valenzuela, M.L.; Ushak, S. Synthesis and pyrolysis of silicon and tin containing poly(2,2′-dioxy-1,1′-biphenoxy-phosphazenes). Eur. Polym. J. 2008, 44, 686–693. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Ushak, S. Synthesis and characterization of N3P3(O2C12H8)2(OC6H4Si(CH3)3)(OC6H4Br) and its conversion to nanostructured Si materials. J. Clust. Sci. 2008, 19, 471–479. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Bravo, D.; Lavayen, V.; O’Dwyer, C. Synthesis and Characterization of Cyclotriphosphazenes Containing Silicon as Single Solid-State Precursors for the Formation of Silicon/Phosphorus Nanostructured Materials. Inorg. Chem. 2008, 47, 11561–11569. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Ushak, S.; Lavayen, V.; O’Dwyer, C. Nanostructured Silicon containing materials derived from solid state pyrolysis of sililated polyphosphazene derivatives. J. Nanosci. Nanotechnol. 2009, 9, 1825–1831. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Ushak, S.; Zuñiga, L.; O’Dwyer, C. Organometallic Derivatives of Cyclotriphosphazenes as precursors of Nanostructured Metallic Materials: A new solid-state method. J. Inorg. Organomet. Polym. 2009, 19, 507–520. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Carriedo, G. Polymer and Oligomer Phosphazene Cymantrene derivatives as solid-state precursors of nanostructured manganese pyrophosphate. Polym. Bull. 2009, 63, 829–835. [Google Scholar] [CrossRef]
- Jiménez, J.; Laguna, A.; Sanz, J.A.; Díaz, C.; Valenzuela, M.L.; Jones, P.G. Metallocyclo-and Polyphosphazenes Containing Gold or Silver: Thermolytic Transformations into Nanostructured Materials. Chem. Eur. J. 2009, 15, 13509–13520. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Laguna, A.; Lavayen, V.; Jimenez, J.; Power, L.; O’Dwyer, C. Metallophosphazene Precursors Routes to the Solid-State Deposition of Metallic and Dielectric Microstructures and Nanostructures on Si and SiO2. Langmuir 2010, 26, 10223–10233. [Google Scholar] [CrossRef]
- Díaz, C.; Lavayen, V.; O’Dwyer, C. Single-Crystal Micro/Nanostructures and Thin Films of Lamellar Molybdenum Oxide by Solid-State Pyrolysis of Organometallic Derivatives of a Cyclotriphosphazene. J. Solid-State Chem. 2010, 20, 1595–1603. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Bravo, D.; Dickinson, C.; O’Dwyer, C. Solid-State Synthesis of Embedded Single-Crystal Oxide and Phosphate Nanoparticles and In-Situ Crystallization. J. Colloid Interface Sci. 2011, 362, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Valenzuela, M.L.; Villalobos, V.; Yutronic, N.; Barrera, G. Nanostructured VOx/VO(PO4)n. Using Solid-State Vanadium Containing Phosphazene Precursors: A Useful Potential Bi-Catalyst System. J. Clust. Sci. 2011, 22, 693–704. [Google Scholar] [CrossRef]
- Díaz, C.; Carriedo, G.A.; Valenzuela, M.L.; Zuñiga, L.; O’Dwyer, C. Polymer/Trimer/Metal Complex Mixtures as Precursors of Gold Nanoparticles: Tuning the Morphology in the Solid-State. J. Inorg. Organomet. Polym. Mater. 2012, 22, 447–454. [Google Scholar]
- Díaz, C.; Valenzuela, M.L.; Lavayen, V.; O’Dwyer, C. Layered Graphitic Carbon Host Formation during Liquid-free Solid Stat Growth of Metal Pyrophosphates. Inorg. Chem. 2012, 51, 6228–6236. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Garrido, D.; Aguirre, P. Sol-Gel Incorporation of Organometallic Compounds into Silica. Useful Precursors to Metallic Nanostructured Materials. J. Chil. Chem. Soc. 2012, 57, 1155–1160. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Garrido, D.; Riquelme, J.; Díaz, R. The inclusion of Organometallic Derivatives of Cyclotriphosphazenes inside SiO2 Matrix and Their Conversion to Nanostructured Metal-oxides and Phosphates. J. Inorg. Organomet. Polym. Mater. 2012, 22, 1101–1112. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Garrido, D.; Zuñiga, L.; O’Dwyer, C. Solid State Pathways to Complex Shape Evolution and Tunable Porosity during Metallic Crystal Growth. Sci. Rep. 2013, 3, 2642. [Google Scholar]
- Díaz, C.; Valenzuela, M.L.; Lavayen, V.; Mendoza, K.; Peña, O.; O’Dwyer, C. Nanostructured copper oxides and phosphates from a new solid-state route. Inorganica Chim. Acta 2011, 377, 5–11. [Google Scholar] [CrossRef]
- Díaz, C.; Barrera, G.; Segovia, M.; Valenzuela, M.L.; Osiak, M.; O’Dwyer, C. Crystallizing Vanadium Pentoxide Nanostructures in the Solid State using Modified Block Co-Polymer and Chitosan Complexes. J. Nanomater. 2015, 2015, 105157. [Google Scholar] [CrossRef]
- Díaz, C.; Barrera, G.; Segovia, M.; Valenzuela, M.L.; Osiak, M.; O’Dwyer, C. Solvent-less method for efficient photocatalytic α-Fe2O3 nanoparticles for using macromolecular polymeric precursors. New J. Chem. 2016, 40, 6768–6776. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Laguna, M.A.; Orera, A.; Bobadilla, D.; Abarca, S.; Peña, O. Synthesis and Magnetic Properties of Nanostructured metallic Co, Mn and Ni oxide materials obtained from solid-state macromolecular complex precursors. RSC Adv. 2017, 7, 27729–27736. [Google Scholar] [CrossRef]
- Diaz, C.; Valenzuela, M.L.; Bobadilla, D.; Laguna-Bercero, M.A. Bimetallic Au//Ag Alloys Inside SiO2 using a solid-state method. J. Clust. Chem. 2017, 28, 2809–2815. [Google Scholar] [CrossRef]
- Diaz, C.; Valenzuela, M.L.; Garcia, C.; De la Campa, R.; Presa-Soto, A. Solid-state synthesis of pure and doped lanthanides oxide nanomaterials by using polymer templates. Study of their luminescent properties. Mater. Lett. 2017, 209, 111–114. [Google Scholar] [CrossRef]
- Diaz, C.; Valenzuela, M.L.; Segovia, M.; De la Campa, R.; Presa-Soto, A. Solution, Solid-State Two Step Synthesis and Optical Properties of ZnO and SnO Nanoparticles and Their Nanocomposites with SiO2. J. Clust. Sci. 2018, 29, 251–266. [Google Scholar] [CrossRef]
- Allende, P.; Laguna, M.; Barrientos, L.; Valenzuela, M.L.; Diaz, C. Solid State Tuning Morphology, Crystal Phase and Size through Metal Macromolecular Complexes and Its Significance in the Photocatalytic Response. ACS Appl. Energy Mater. 2018, 1, 3159–3170. [Google Scholar] [CrossRef]
- Díaz, C.; Carrillo, D.; De la Campa, R.; Pres-Soto, A.; Valenzuela, M.L. Solid-State synthesis of LnOCl/Ln2O3 (Ln = Eu,Nd) by using chitosan and PS-co-P4VP as polymeric supports. J. Rare Earth 2018, 36, 1326–1332. [Google Scholar] [CrossRef]
- Allende, P.; Barrientos, L.; Orera, A.; Laguna-Bercero, M.A.; Salazar, N.; Valenzuela, M.L.; Díaz, C. TiO2/SiO2 Composite for Efficient Protection of UVA and UVB Rays Through of a Solvent-Less Synthesis. J. Clust. Sci. 2019, 30, 1511–1517. [Google Scholar] [CrossRef]
- Cortes, M.A.; De la Campa, R.; Valenzuela, M.L.; Díaz, C.; Carriedo, G.A.; Presa-Soto, A. Cylindrical Micelles by the Self-Assembly of Crystalline-b-Coil Polyphosphazene-b-P2VP Block Copolymers. Stabilization of Gold Nanoparticles. Molecules 2019, 24, 1772. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Cifuentes-Vaca, O.; Segovia, M.; Laguna-Bercero, M.A. Incorporation of Nanostructured ReO3 in Silica Matrix and Their Activity Toward Photodegradation of Blue Methylene. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1726–1734. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Cifuentes-Vaca, O.; Segovia, M.; Laguna-Bercero, M.A. Iridium nanostructured metal oxide, its inclusion in Silica matrix and their activity toward Photodegradation of Methylene Blue. Mater. Chem. Phys. 2020, 252, 123276. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Cifuentes-Vaca, O.; Segovia, M. Polymer precursors effect in the macromolecular metal-polymer on the Rh/RhO2/Rh2O3 phase using solvent-less synthesis and its photocatalytic activity. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4702–4708. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Cifuentes-Vaca, O.; Segovia, M.; Laguna-Bercero, M.A. Incorporation of NiO into SiO2, TiO2, Al2O3, and Na4.2Ca2.8(Si6O18) Matrices: Medium Effect on the Optical Properties and Catalytic Degradation of Methylene Blue. Nanomaterials 2020, 10, 2470. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Laguna-Bercero, M.A.; Mendoza, K.; Cartes, P. Solventless preparation of thoria, their inclusion inside SiO2 and TiO2, their luminescent properties and their photocatalytic behavior. ACS Omega 2021, 6, 9391–9400. [Google Scholar] [CrossRef] [PubMed]
- Allende, P.; Orera, A.; Díaz, C.; Valenzuela, M.L.; Laguna-Bercero, M.A.; Barrientos, L. Insights of the formation mechanism of nanostructured titanium oxide polymorphs from different macromolecular metal-complex precursors. Heliyon 2021, 7, e07684. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Valenzuela, M.L.; Baez, R.; Segovia, M. Solid State Morphology and Size Tuning of Nanostructured Platinum Using Macromolecular Complexes. J. Chil. Chem. Soc. 2015, 60, 2986–2990. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, S.; You, H.; Wu, H.; Peng, O.; Li, W.; Cao, K.; Zhou, Y. Submicrometre-sized EuPO4 hollow spheres: Template-directed synthesis and luminescent properties. Micro Nano Lett. 2019, 14, 309–312. [Google Scholar] [CrossRef]
- Tappan, B.; Steiner, S.; Luther, E. Nanoporous Metal Foams. Angew. Chem. 2010, 49, 4544–4565. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band Gap Narrowing and Widening of ZnO Nanostructures and Doped Material. Nanoscale Res. Lett. 2015, 10, 346–358. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Shelykh, A.I.; Melekh, B.T. Periodicity in the band gap variation of Ln2 × 3 (X = O, S, Se) in the lanthanide series. J. Alloys Compd. 1996, 242, 41–44. [Google Scholar] [CrossRef]
- Wakefield, G.; Keron, H.A.; Dobson, P.J.; Hutchison, J.L. Synthesis and Properties of Sub-50-nm Europium Oxide Nanoparticles. J. Colloid Interface Sci. 1999, 215, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.S.; Wu, A.; Zhao, X.; Yan, Y.; Guo, X.; Bian, Y.; Bao, J.; Lia, W.; Zhu, X. Controlled synthesis of EuPO4 nano/microstructures and core–shell SiO2@EuPO4 nanostructures with improved photoluminescence. RSC Adv. 2017, 7, 52238–52244. [Google Scholar] [CrossRef]
- Yang, Y. Synthesis and luminescent properties of LaPO4:Eu3+ microspheres. Mater. Sci. Eng. B 2013, 178, 807–810. [Google Scholar] [CrossRef]
- Zollfrank, C.; Scheel, H.; Brungs, S.; Greil, P. Europium (III) Orthophosphates: Synthesis, Characterization, and Optical Properties. Cryst. Growth Des. 2008, 8, 766–770. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]







| Precursor/Nanoparticles | Size nm [a] | Morphology | Optical Properties | Degree Coordination (%) [b] | |
|---|---|---|---|---|---|
| Eg (eV) | Uv (nm) | ||||
| 1a-Zn/ZnO | 70 | Aggregated grains with hexagonal-like architecture | 3.23 | 310 372 | 71 |
| 1b-Zn/ZnO | 60 | Aggregated grains | 3.14 | 331 | 64 |
| 1c-Zn/ZnO | 80 | Aggregated grains with hexagonal-like architecture | 4.24 | 309 390 610 656 | 47 |
| 1a-Eu/Eu2O3 | 59 | Aggregated grains with porous surfaces | 4.52 | - | 57 |
| 1b-Eu/Eu2O3 | 61 | Aggregated grains with porous surfaces | 4.34 | - | 55 |
| 1c-Eu/Eu2O3 | 52 | Aggregated grains with porous surfaces | 4.37 | - | 57 |
| 1a-Pt/Pt | 62 | Aggregated grains | - | 292 | 85 |
| 1b-Pt/Pt | 47 | Metallic foam | - | 290 | 83 |
| 1c-Pt/Pt | 95 | Metallic foam | - | 292 | 85 |
| 2a-Eu [c]/EuPO4 | 25–40 | Porous material | - | - | 54 |
| 2b-Eu [c]/EuPO4 | 25–40 | Porous material | - | - | 53 |
| 2c-Eu [c]/EuPO4 | 25–40 | Porous material | - | - | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, M.Á.; Díaz, C.; de la Campa, R.; Presa-Soto, A.; Valenzuela, M.L. Synthesis of Metallic and Metal Oxide Nanoparticles Using Homopolymers as Solid Templates: Luminescent Properties of the Eu+3 Nanoparticle Products. Photochem 2024, 4, 302-318. https://doi.org/10.3390/photochem4030018
Cortés MÁ, Díaz C, de la Campa R, Presa-Soto A, Valenzuela ML. Synthesis of Metallic and Metal Oxide Nanoparticles Using Homopolymers as Solid Templates: Luminescent Properties of the Eu+3 Nanoparticle Products. Photochem. 2024; 4(3):302-318. https://doi.org/10.3390/photochem4030018
Chicago/Turabian StyleCortés, María Ángeles, Carlos Díaz, Raquel de la Campa, Alejandro Presa-Soto, and María Luisa Valenzuela. 2024. "Synthesis of Metallic and Metal Oxide Nanoparticles Using Homopolymers as Solid Templates: Luminescent Properties of the Eu+3 Nanoparticle Products" Photochem 4, no. 3: 302-318. https://doi.org/10.3390/photochem4030018
APA StyleCortés, M. Á., Díaz, C., de la Campa, R., Presa-Soto, A., & Valenzuela, M. L. (2024). Synthesis of Metallic and Metal Oxide Nanoparticles Using Homopolymers as Solid Templates: Luminescent Properties of the Eu+3 Nanoparticle Products. Photochem, 4(3), 302-318. https://doi.org/10.3390/photochem4030018







