Riboflavin as a Coloring Agent of Tablets Affects the Photostability of Manidipine after the Change of Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. UV Irradiation Experiment
2.4. Evaluation of the Residual Amounts of MP in UV-Irradiated Samples
2.5. Statistical Analysis
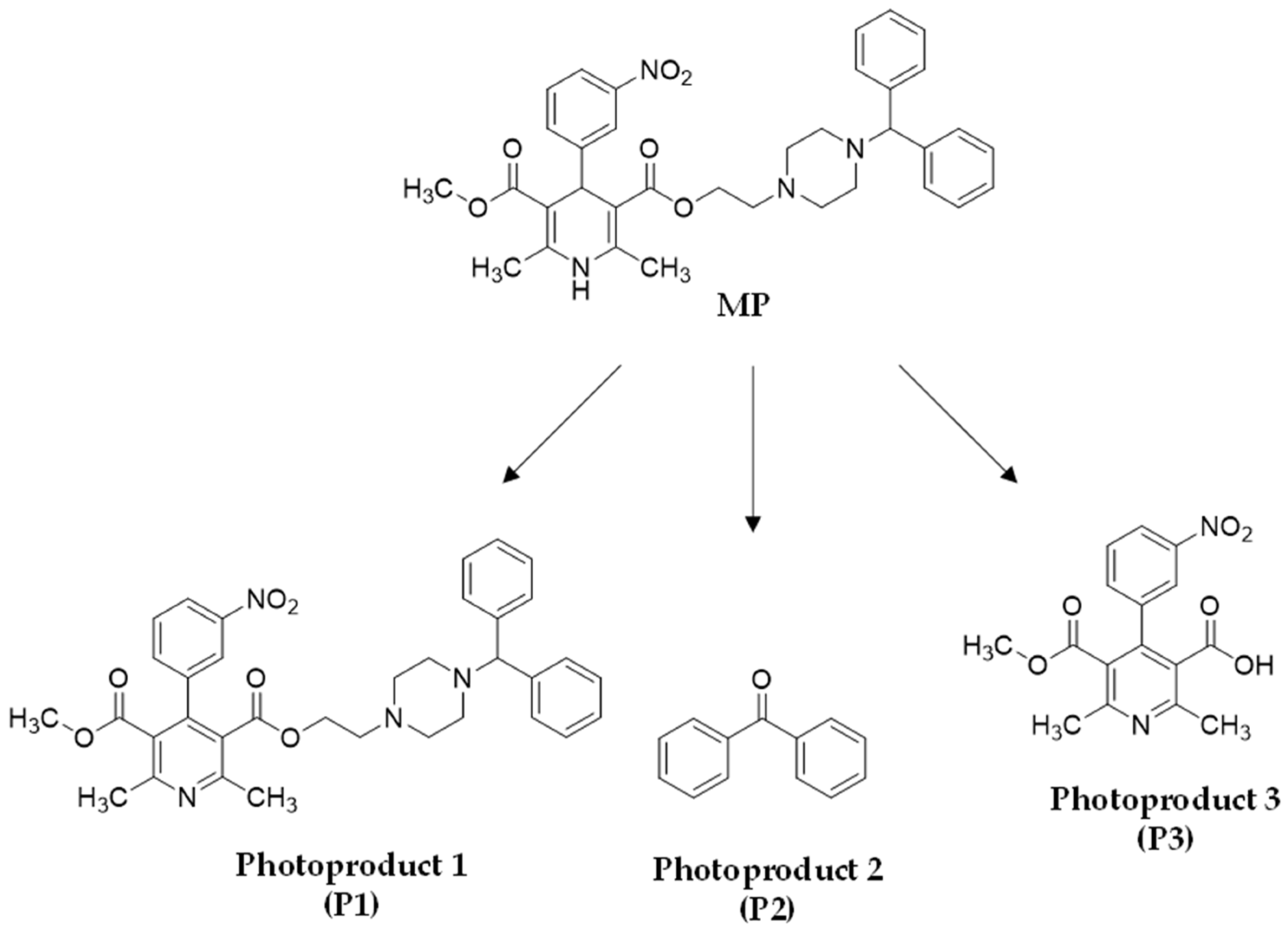
3. Results
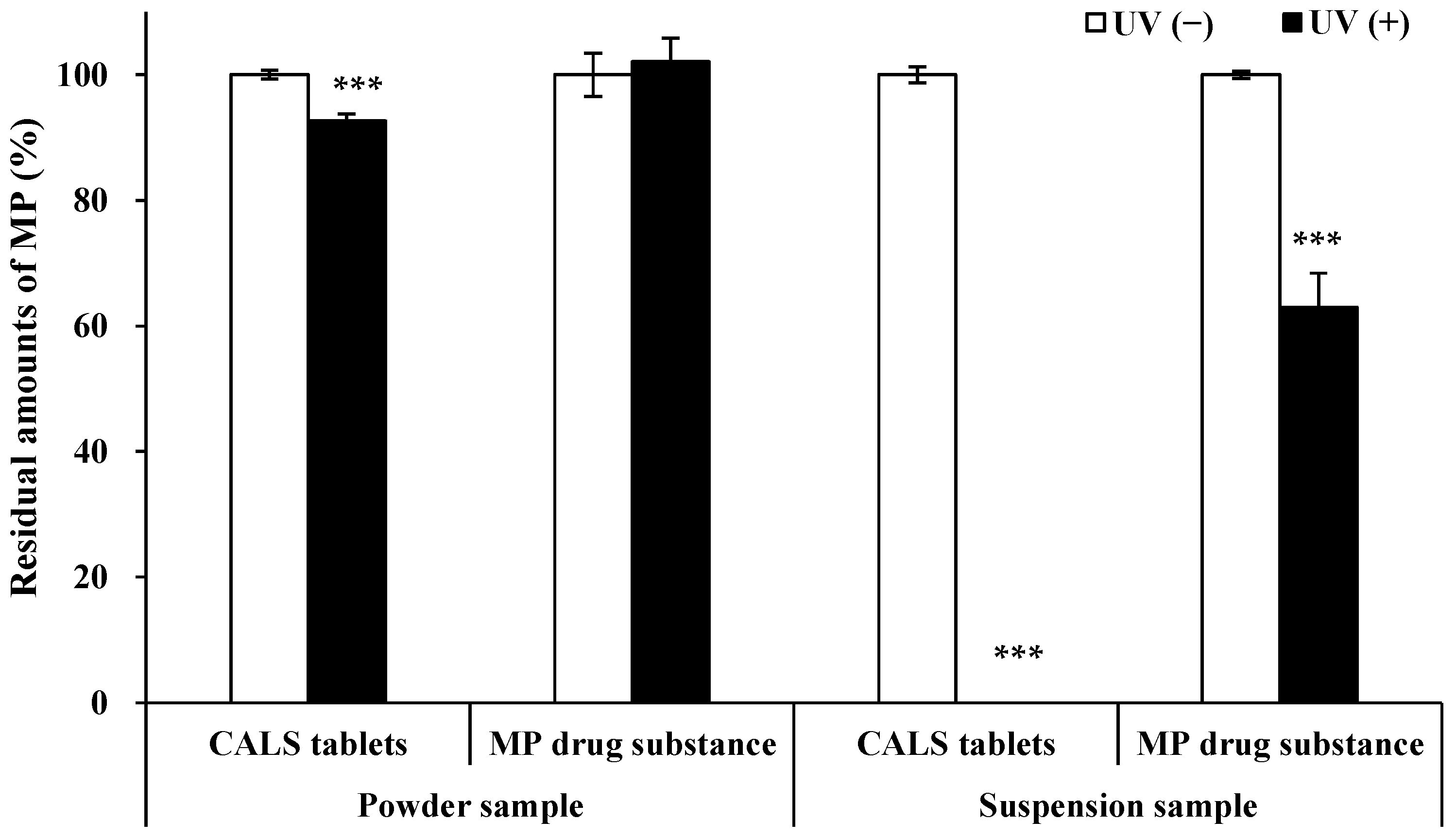
3.1. Photostability Evaluation of Altered Forms of CALS Tablets and MP Drug Substances
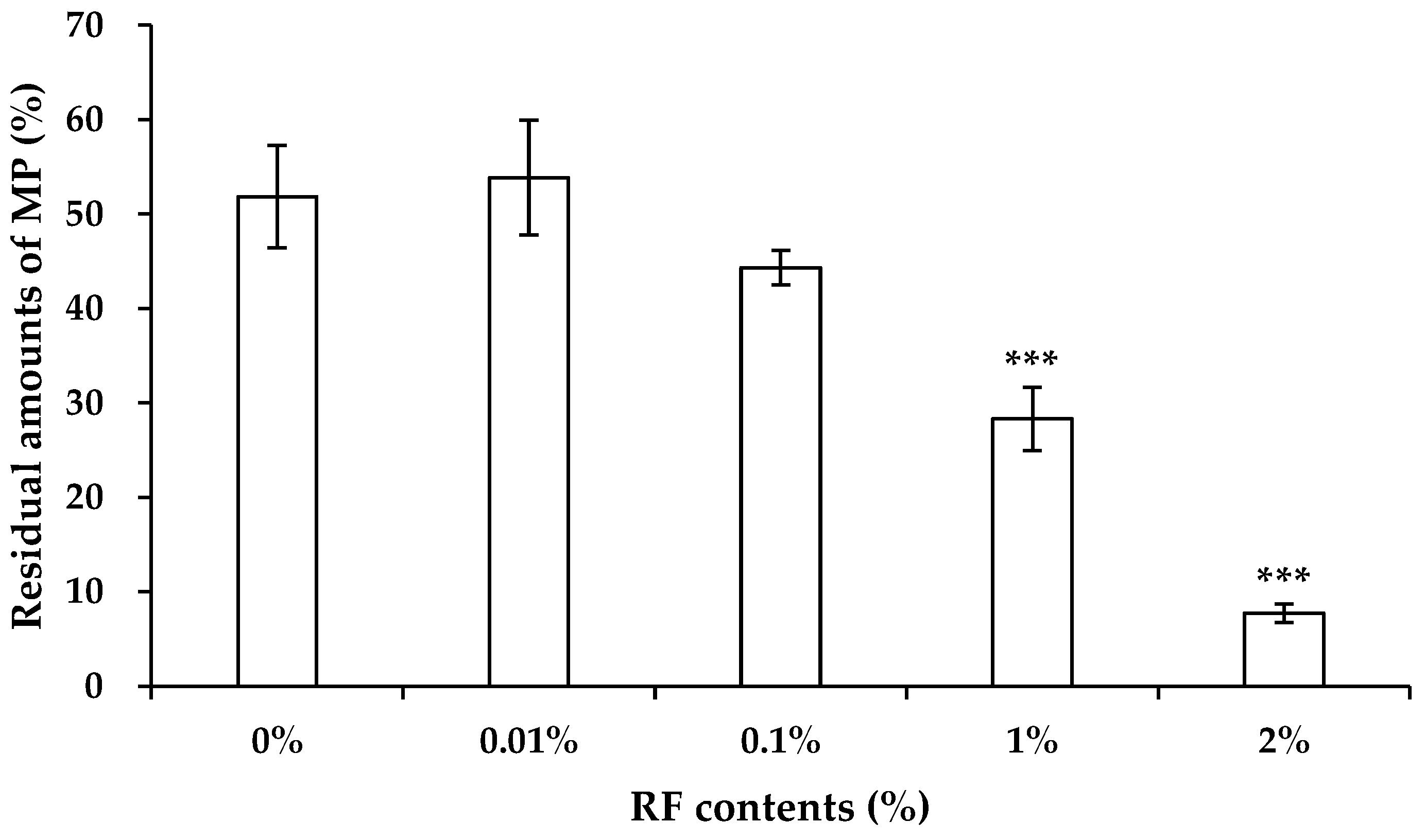
3.2. Evaluation of Photocatalysis Activity of RF
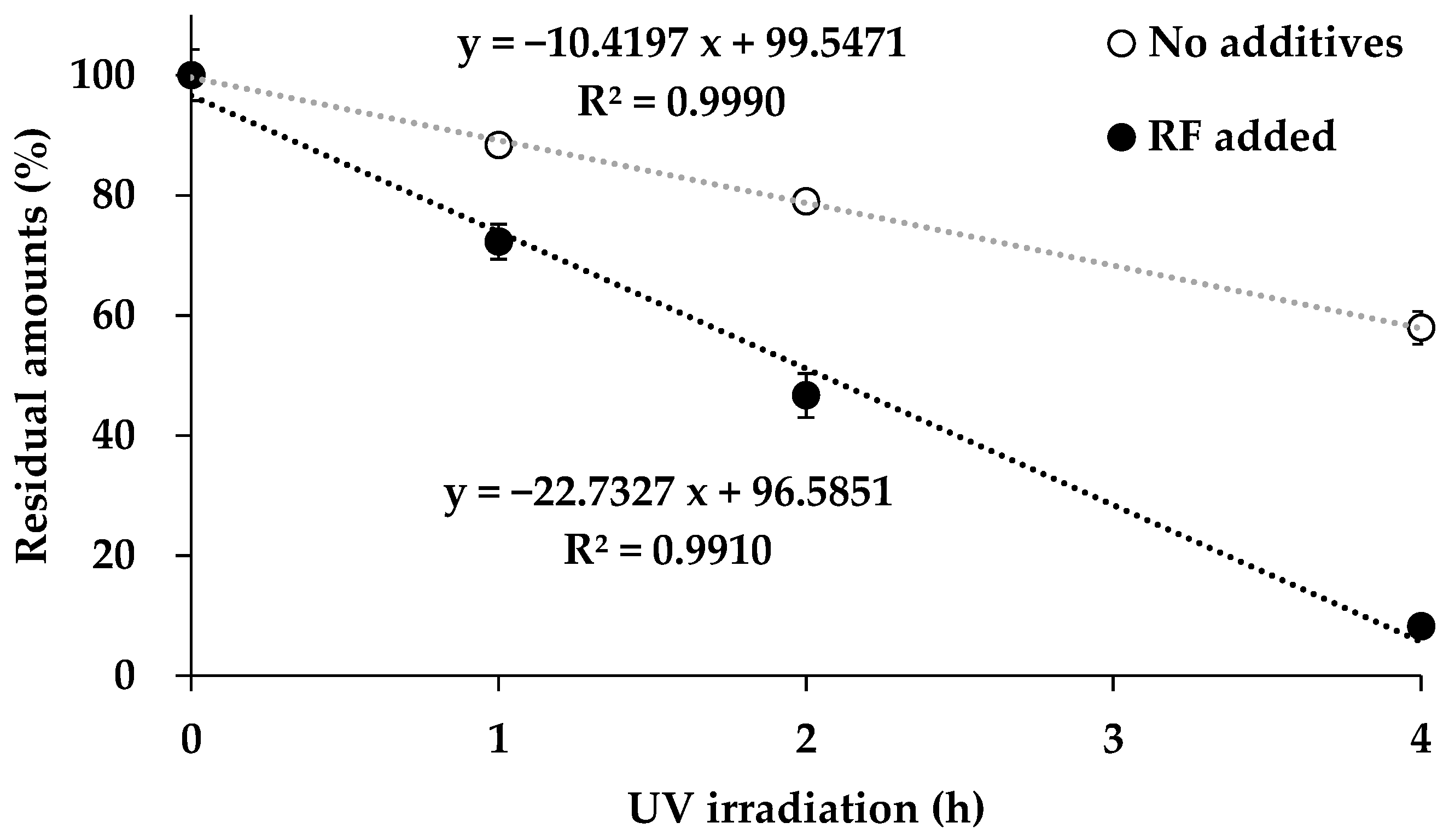
3.3. Study on the Photostabilization of CALS Suspensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheer, S.M.; McClellan, K. Manidipine: A review of its use in hypertension. Drugs 2001, 61, 1777–1799. [Google Scholar] [CrossRef] [PubMed]
- Jakimska, A.; Śliwka-Kaszyńska, M.; Nagórski, P.; Namieśnik, J.; Kot-Wasik, A. Phototransformation of amlodipine: Degradation kinetics and identification of its photoproducts. PLoS ONE 2014, 9, e109206. [Google Scholar] [CrossRef] [PubMed]
- Rango, G.; Garofalo, A.; Vetuschi, C. Photodegradation monitoring of amlodipine by derivative spectrophotometry. J. Pharm. Biomed. Anal. 2002, 27, 19–24. [Google Scholar]
- Chen, S.M.; Hsieh, M.C.; Chao, S.H.; Chang, E.E.; Wang, P.Y.; Wu, A.B. Separation and structure determination of nicardipine photoproducts by LC-ESI-MS. Biomed. Chromatogr. 2008, 22, 1008–1012. [Google Scholar] [CrossRef]
- Horinouchi, Y.; Tsuchiya, K.; Taoka, C.; Tajima, S.; Kihira, Y.; Matsuda, Y.; Shishido, K.; Yoshida, M.; Hamano, S.; Kawazoe, K.; et al. Antioxidant effects of photodegradation product of nifedipine. Chem. Pharm. Bull. 2011, 59, 208–214. [Google Scholar] [CrossRef]
- Asahi, M.; Matsushita, R.; Kawahara, M.; Ishida, T.; Emoto, C.; Suzuki, N.; Kataoka, O.; Mukai, C.; Hanaoka, M.; Ishizaki, J.; et al. Causative agent of vascular pain among photodegradation products of dacarbazine. J. Pharm. Pharmacol. 2002, 54, 1117–1122. [Google Scholar] [CrossRef]
- Della Greca, M.; Iesce, M.R.; Previtera, L.; Rubino, M.; Temussi, F. A new photoproduct of the drug furosemide in aqueous media. Environ. Chem. Lett. 2004, 2, 155–158. [Google Scholar] [CrossRef]
- Isidori, M.; Nardelli, A.; Parrella, A.; Pascarella, L.; Previtera, L. A multispecies study to assess the toxic and genotoxic effect of pharmaceuticals: Furosemide and its photoproduct. Chemosphere 2006, 63, 785–793. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugihara, K.; Sanoh, S.; Kitamura, S.; Ohta, S. Ultraviolet-photoproduct of acetaminophen: Structure determination and evaluation of ecotoxicological effect. J. Photochem. Photobiol. A Chem. 2012, 249, 29–35. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugihara, K.; Sanoh, S.; Kitamura, S.; Ohta, S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J. Toxicol. Sci. 2013, 38, 215–223. [Google Scholar] [CrossRef]
- Kawabata, K.; Akimoto, S.; Nishi, H. Photo-conversion of phenytoin to ecotoxicological substance benzophenone by ultraviolet light irradiation in aqueous media. Chromatography 2020, 41, 51–58. [Google Scholar] [CrossRef]
- Kawabe, Y.; Nakamura, H.; Hino, E.; Suzuki, S. Photochemical stabilities of some dihydropyridine calcium-channel blockers in powdered pharmaceutical tablets. J. Pharm. Biomed. Anal. 2008, 47, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Muraoka, H.; Miyara, M.; Kotake, Y.; Nishi, H. Photodegradation profiling of nitrendipine: Evaluation of active pharmaceutical ingredient, tablets and its altered forms. Anal. Sci. 2023, 39, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Hirai, K.; Akimoto, S.; Inagaki, M.; Nishi, H. Photostability evaluation of manidipine tablets and structural determination of its photoproducts. Anal Sci. 2024, 40, 1733–1747. [Google Scholar] [CrossRef]
- Kawabata, K.; Takato, A.; Oshima, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Protective Effect of Selected Antioxidants on Naproxen Photodegradation in Aqueous Media. Antioxidants 2019, 8, 424. [Google Scholar] [CrossRef]
- Kawabata, K.; Miyoshi, A.; Nishi, H. Photoprotective effects of selected polyphenols and antioxidants on naproxen photodegradability in the solid-state. Photochem 2022, 2, 880–890. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef]
- Meng, J.; Xu, F.; Yuan, S.; Mu, Y.; Wang, W.; Hu, Z.H. Photocatalytic oxidation of roxarsone using riboflavin-derivative as a photosensitizer. Chem. Eng. J. 2019, 355, 130–136. [Google Scholar] [CrossRef]
- Castillo, C.; Criado, S.; Díaz, M.; García, N.A. Riboflavin as a sensitiser in the photodegradation of tetracyclines. Kinetics, mechanism and microbiological implications. Dye. Pigment. 2007, 72, 178–184. [Google Scholar] [CrossRef]
- Suga, M.; Makino, K.; Tabata, H.; Oshitari, T.; Natsugari, H.; Takahashi, H. Photoisomerization of sulindac and ozagrel hydrochloride by vitamin B2 catalyst under visible light irradiation. Pharm. Res. 2022, 39, 577–586. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Dzakpasu, M.; Wang, X.C. Evaluation of ecotoxicological effects of benzophenone UV filters: Luminescent bacteria toxicity, genotoxicity and hormonal activity. Ecotoxicol. Environ. Saf. 2017, 142, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Fent, K. Estrogenic activity of UV filter mixtures. Toxicol. Appl. Pharmacol. 2006, 217, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Takeyoshi, M.; Yakabe, Y.; Sawaki, M.; Takatsuki, M. Comparison of the reporter gene assay for ER-alpha antagonists with the immature rat uterotrophic assay of 10 chemicals. Toxicol. Let. 2003, 142, 119–131. [Google Scholar] [CrossRef] [PubMed]

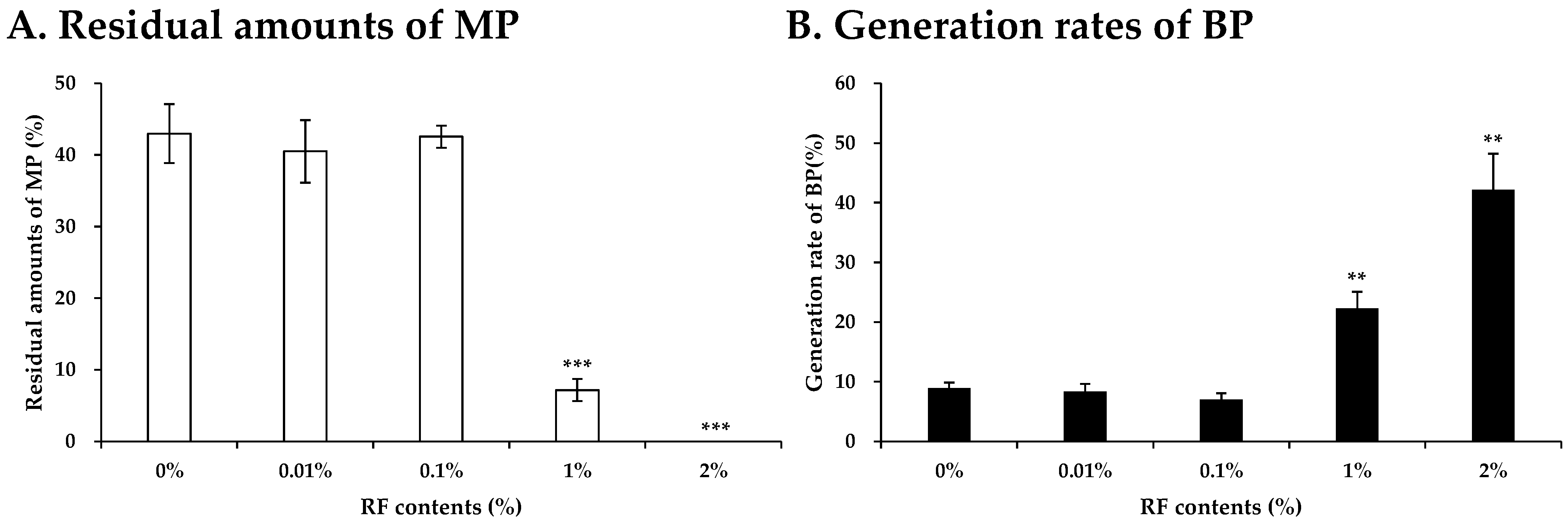


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabata, K.; Tsukimori, M.; Hirai, K.; Akimoto, S.; Uramaru, N.; Inagaki, M.; Nishi, H. Riboflavin as a Coloring Agent of Tablets Affects the Photostability of Manidipine after the Change of Dosage Forms. Photochem 2024, 4, 377-387. https://doi.org/10.3390/photochem4030023
Kawabata K, Tsukimori M, Hirai K, Akimoto S, Uramaru N, Inagaki M, Nishi H. Riboflavin as a Coloring Agent of Tablets Affects the Photostability of Manidipine after the Change of Dosage Forms. Photochem. 2024; 4(3):377-387. https://doi.org/10.3390/photochem4030023
Chicago/Turabian StyleKawabata, Kohei, Minami Tsukimori, Kyoka Hirai, Shiori Akimoto, Naoto Uramaru, Masanori Inagaki, and Hiroyuki Nishi. 2024. "Riboflavin as a Coloring Agent of Tablets Affects the Photostability of Manidipine after the Change of Dosage Forms" Photochem 4, no. 3: 377-387. https://doi.org/10.3390/photochem4030023
APA StyleKawabata, K., Tsukimori, M., Hirai, K., Akimoto, S., Uramaru, N., Inagaki, M., & Nishi, H. (2024). Riboflavin as a Coloring Agent of Tablets Affects the Photostability of Manidipine after the Change of Dosage Forms. Photochem, 4(3), 377-387. https://doi.org/10.3390/photochem4030023




