Heat Capacity of Solid Halide Eutectics and Their Enthalpy at Melting Point
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The molar heat capacity of all mixtures under study was found to be close to the additive sum of that of pure salts.
- The enthalpy of the solid eutectic mixtures closes to melting temperature was directly dependent on the melting point.
- The results obtained allow for the estimation of the amount of energy that the eutectic mixture can accumulate for storage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Redkin, A.; Korzun, I.; Yaroslavtseva, T.; Reznitskikh, O.; Zaikov, Y. Isobaric heat capacity of molten halide eutectics. J. Therm. Anal. Calorim. 2017, 128, 621–626. [Google Scholar] [CrossRef]
- Fernandez, A.; Velis, S.; Galleguillos, H. Thermal characterization of solar salts from north of Chile and variations of their properties over time at high temperature. J. Therm. Anal. Calorim. 2017, 128, 1241–1249. [Google Scholar] [CrossRef]
- Fernández, A.; Vidal, J.; Oró, E.; Kruisenga, A.; Solé, A.; Cabeza, L. Mainstreaming commercial CSP systems: A Technology Review. Renew. Energy 2019, 140, 152–176. [Google Scholar] [CrossRef]
- Badenhorst, H.; Böhmer, T. Enthalpy of fusion prediction for the economic optimisation of salt based latent heat thermal energy stores. J. Energy Storage 2018, 20, 459–472. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Peng, O.; Ding, J.; Yang, J. Quaternary chloride eutectic mixture for thermal energy storage at high temperature. Energy Procedia 2015, 75, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Song, M.; Peng, O.; Ding, J.; Yang, J. A new ternary chloride eutectic mixture and its thermo-physical properties for solar thermal energy storage. Energy Procedia 2014, 61, 1314–1317. [Google Scholar] [CrossRef] [Green Version]
- Masset, P.; Guidotti, R. Thermal activated (thermal) battery technology: Part II. Molten Salt Electrolytes. J. Power Sources 2007, 164, 397–414. [Google Scholar] [CrossRef]
- Glushko, V. Thermodynamic Properties of Individual Substances; Book 2; Nauka: Moscow, Russia, 1982; Volume 4, 559p. [Google Scholar]
- Grimes, W. Reactor Chemistry Division Annual Progress Report for Period Ending: December 31, 1965; ORNL-3913; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1966; p. 29.
- Gaune-Escard, M.; Bogacz, M.; Rycerz, L.; Szczepaniak, W. Heat capacity of LaCI3, CeC13, PrC13, NdCI3, GdC13, DyC13. J. Alloys Compd. 1996, 235, 176–181. [Google Scholar] [CrossRef]
- Beilmann, M.; Beneš, O.; Capelli, E.; Reuscher, V.; Konings, R.; Fanghänel, T. Excess Heat Capacity in Liquid Binary Alkali-Fluoride Mixtures. Inorg. Chem. 2013, 52, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.; Yoko, T.; Janz, G. Fusion properties and heat capacities of the eutectic LiF-NaF-KF. J. Chem. Eng. Data 1982, 27, 366–367. [Google Scholar] [CrossRef]
- Kosaka, M.; Asahina, T.; Taoda, H.; Kushi, A. Heat of fusion and heat capacities of MX and M2Y (M=Li, Na, K; X=F, Cl, I; Y=CO3, SO4) ternary eutectic salts. Nippon Kagaku Kayshi 1982, 6, 977–982. [Google Scholar] [CrossRef]
- Mullabaev, A.; Kovrov, V.; Kholkina, A.A.; Zaikov, Y. Anode processes on Pt and ceramic anodes in chloride and oxide-chloride melts. Nucl. Eng. Technol. 2022, 54, 965–974. [Google Scholar] [CrossRef]
- Nikolaev, A.; Mullabaev, A.; Suzdaltsev, A.; Kovrov, V.; Kholkina, A.; Shishkin, V.; Zaikov, Y. Purification alkali metal chlorides by zone recrystallisation for use in pyrochemical processing of spent nuclear fuel. At. Energy 2022, 131, 195–201. [Google Scholar] [CrossRef]
- Leitner, J.; Vonka, P.; Sedmidubsky, D.; Svoboda, P. Application of Neumann-Kopp rule for estimation of heat capacity of mixed oxides. Thermochim. Acta 2010, 497, 7–13. [Google Scholar] [CrossRef]
- Il’ina, E.; Raskovalov, A.; Reznitskikh, O. Thermodynamic properties of solid electrolyte Li7La3Zr2O12. J. Chem. Thermodyn. 2019, 128, 68–73. [Google Scholar] [CrossRef]
- Redkin, A.; Zaykov, Y. Relation between the thermal expansion coefficient and the heat capacity in halide melts. Russ. Metall. 2017, 2017, 75–78. [Google Scholar] [CrossRef]
- Berg, W.T.; Morrison, J.A. The Thermal Properties of Alkali Halide Crystals. I. The Heat Capacity of Potassium Chloride, Potassium Bromide, Potassium Iodide and Sodium Iodide between 2, 8 and 270 °K. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1957, 242, 467–477. [Google Scholar]
- Atkinson, K.E. An Introduction to Numerical Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1989; 693p. [Google Scholar]
- Galwey, A. A view and a review of the melting of alkali metal halide crystals: Part 1. A melt model based on density and energy changes. J. Therm. Anal. Calorim. 2005, 82, 23–40. [Google Scholar] [CrossRef]
- Jaulin, L.; Kieffer, M.; Didrit, O.; Walter, E. Applied Interval Analysis; Springer: New York, NY, USA, 2001; 379p. [Google Scholar]
- Kumkov, S.I.; Nikitin, V.S.; Ostanina, T.N.; Rudoy, V.M. Interval processing of electrochemical data. J. Comput. Appl. Math. 2020, 380, 112961. [Google Scholar] [CrossRef]
- Redkin, A.; Korzun, I.; Reznitskikh, O.; Yaroslavtseva, T.; Zaikov, Y.; Kumkov, S. Heat of fusion of halide salts and their eutectics. J. Therm. Anal. Calorim. 2018, 131, 2021–2026. [Google Scholar] [CrossRef]

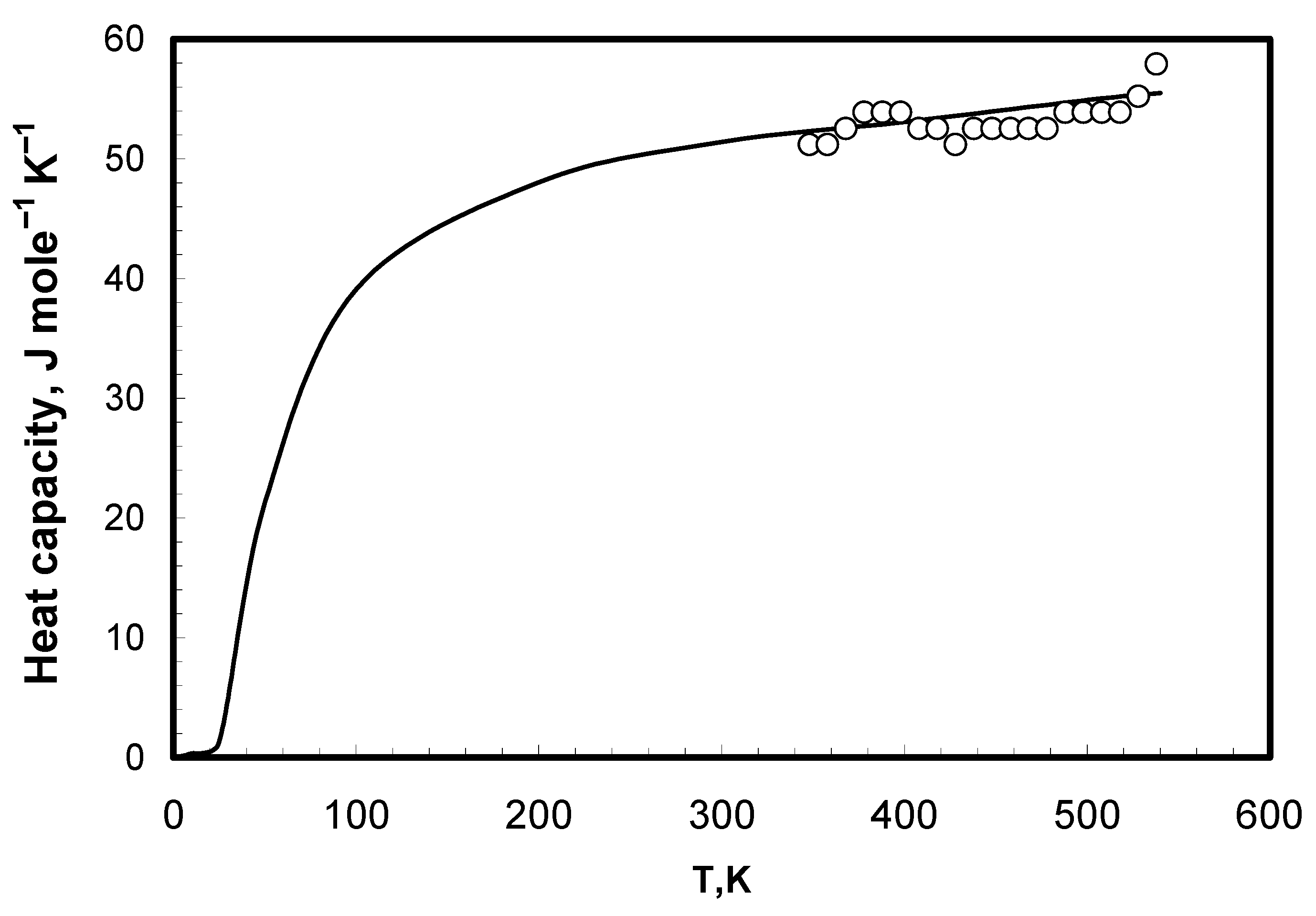


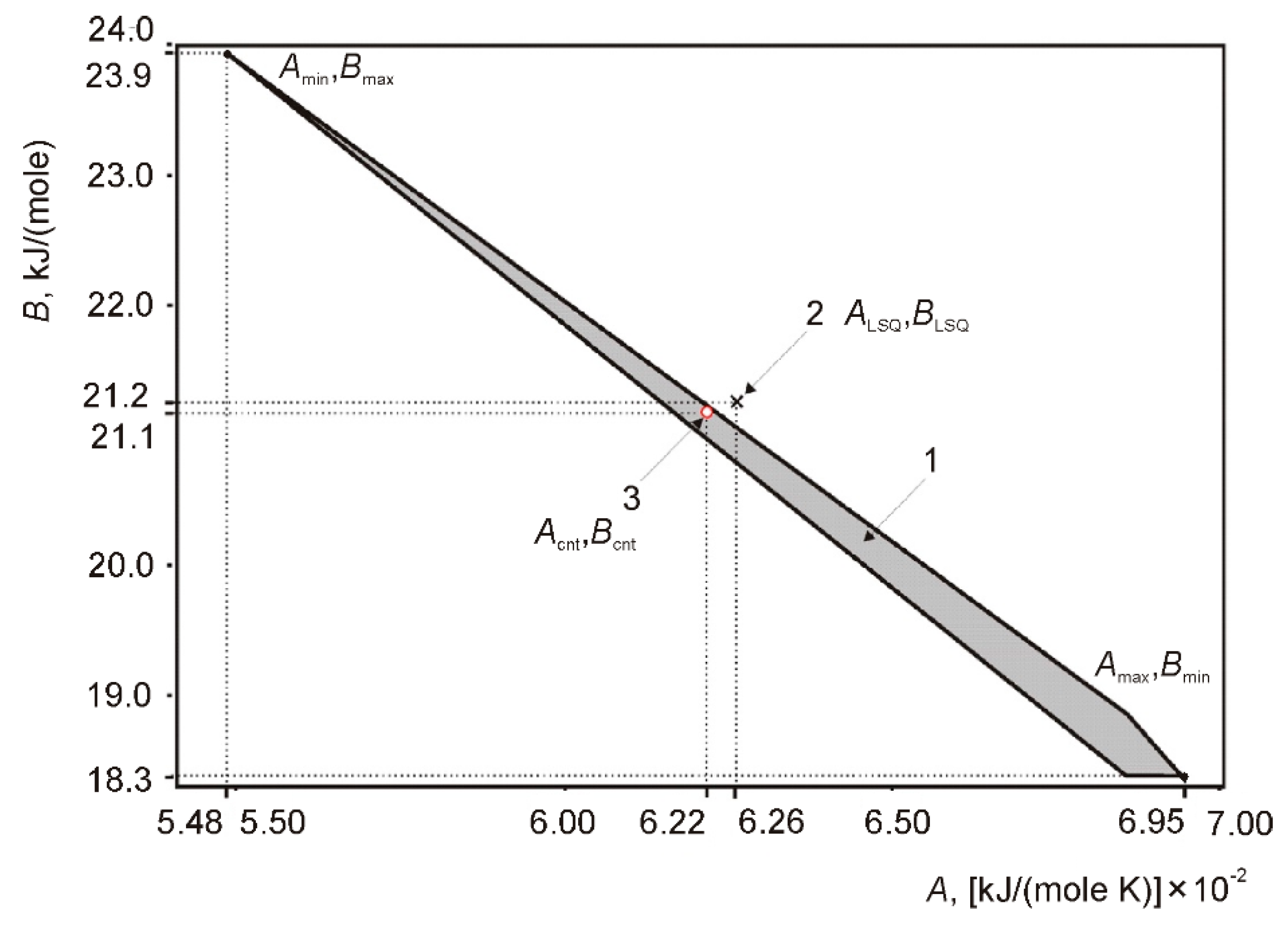
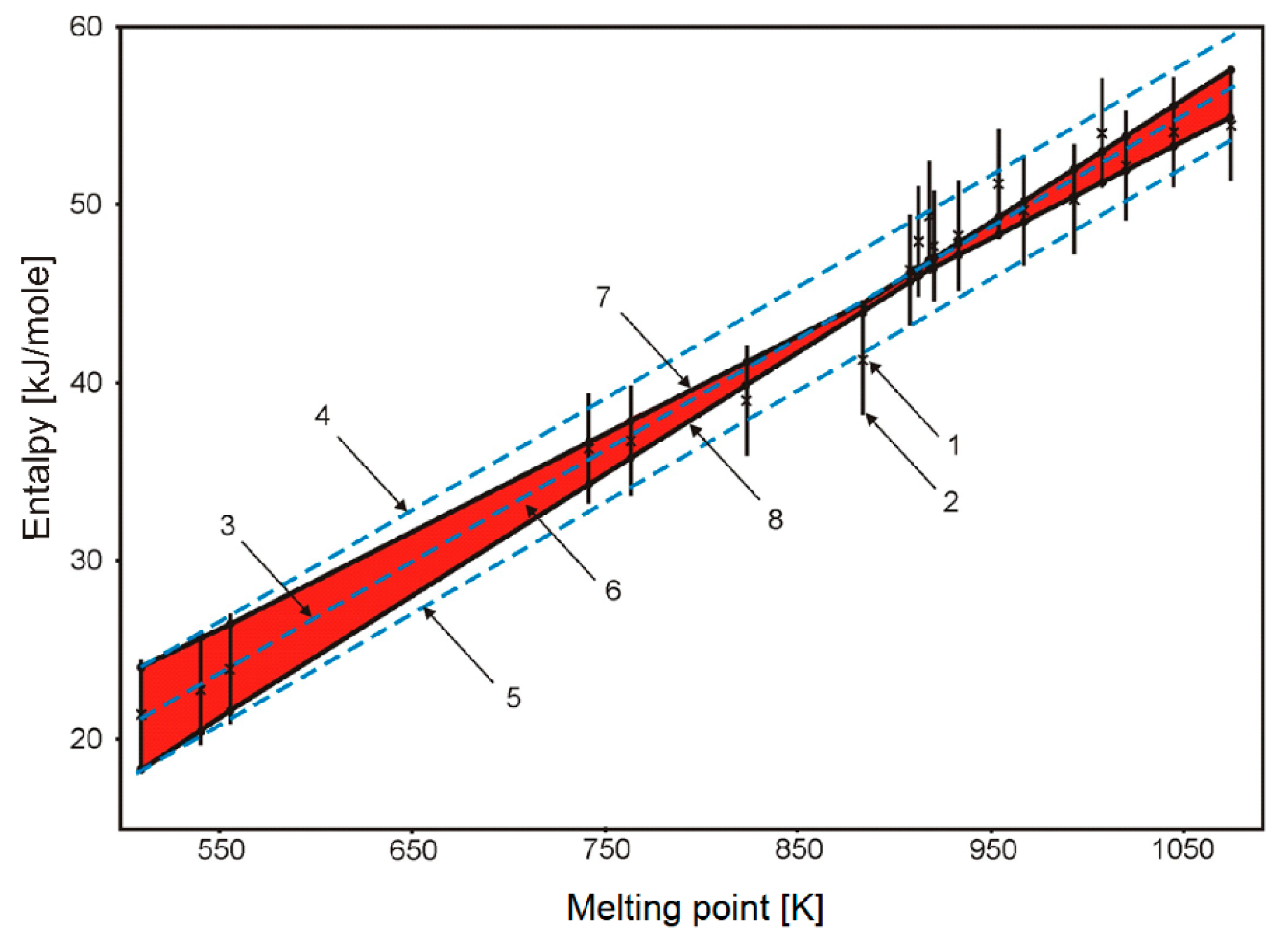
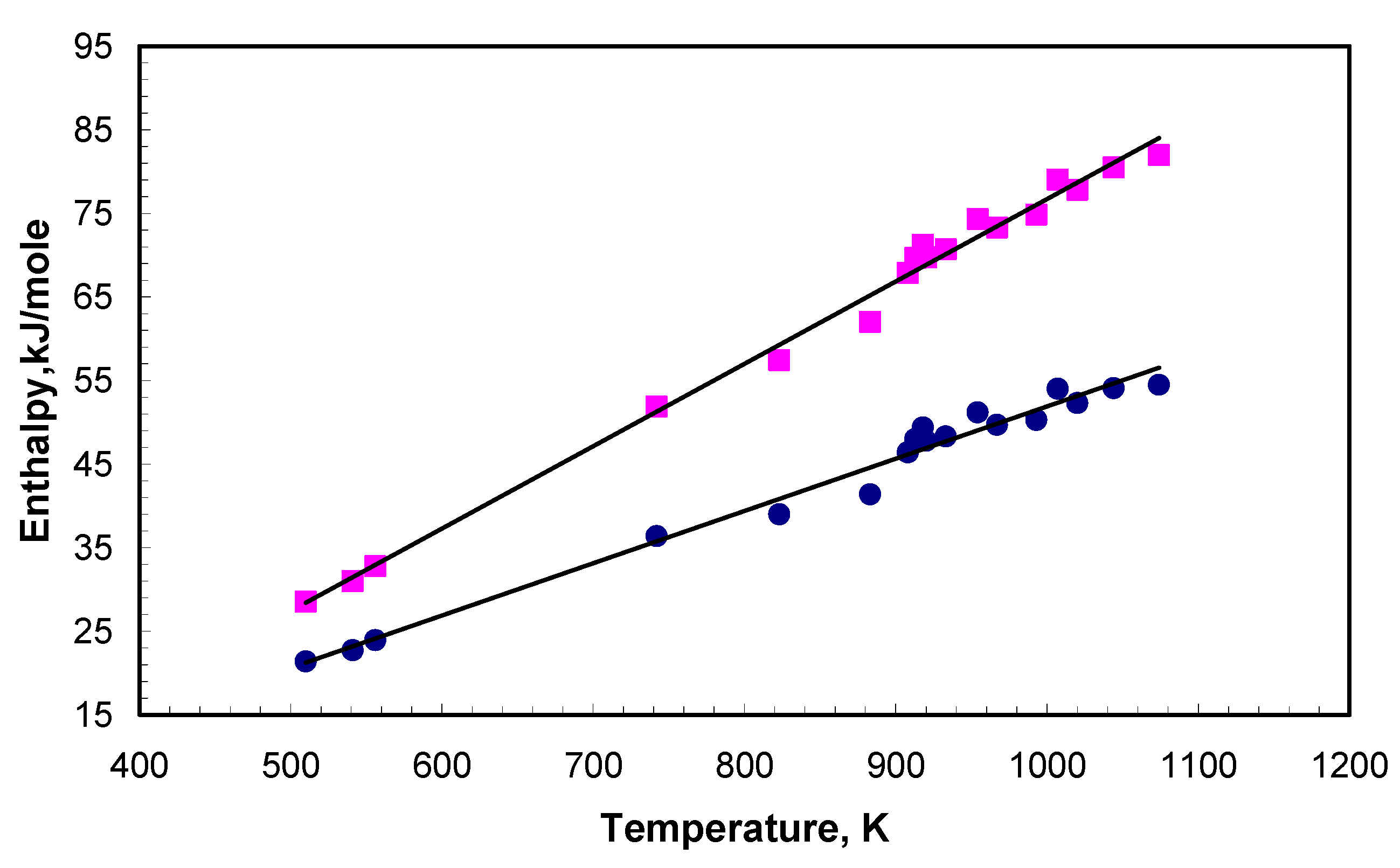
| Molar Composition | Melting Point, K [1] | Molar Weight |
|---|---|---|
| 0.575 LiCl–0.165 KCl–0.26 CsCl | 541 | 78.6 |
| 0.62 LiBr–0.38 CsBr | 556 | 134.7 |
| 0.561 LiBr–0.189 KBr–0.25 CsBr | 510 | 124.45 |
| LiCl-KCl-CsCl | LiBr-CsBr | LiBr-KBr-CsBr | |||
|---|---|---|---|---|---|
| T,K | Cp, J mole−1 | T,K | Cp, J mole−1 | T,K | Cp, J mole−1 |
| 303 | 48.3 | 348 | 51.2 | 316 | 49.8 |
| 323 | 50.7 | 358 | 51.2 | 326 | 49.8 |
| 333 | 49.1 | 368 | 52.5 | 336 | 50.4 |
| 341 | 50.5 | 378 | 53.9 | 346 | 50.4 |
| 350 | 51.7 | 388 | 53.9 | 356 | 51.0 |
| 359 | 52.3 | 398 | 53.9 | 366 | 51.0 |
| 370 | 52.3 | 408 | 52.5 | 376 | 51.0 |
| 380 | 53.1 | 428 | 51.2 | 386 | 51.0 |
| 390 | 53.1 | 438 | 52.5 | 396 | 51.0 |
| 400 | 52.3 | 448 | 52.5 | 406 | 51.6 |
| 410 | 52.3 | 458 | 52.5 | 416 | 51.6 |
| 420 | 53.8 | 468 | 52.5 | 426 | 52.3 |
| 430 | 53.6 | 478 | 52.5 | 436 | 52.3 |
| 440 | 53.1 | 488 | 53.9 | 446 | 52.3 |
| 450 | 52.3 | 498 | 53.9 | 456 | 53.5 |
| 460 | 51.5 | 508 | 53.9 | 466 | 53.5 |
| 470 | 52.3 | 518 | 53.9 | 476 | 54.8 |
| 528 | 55.2 | 481 | 54.8 | ||
| 538 | 57.9 | ||||
| Molar Composition | Melting Point, K [1] | Enthalpy at Melting Point kJ/mole |
|---|---|---|
| 0.575 LiCl–0.165 KCl–0.26 CsCl | 541 | 21.90 |
| 0.62 LiBr–0.38 CsBr | 556 | 23.95 |
| 0.561 LiBr–0.189 KBr–0.25 CsBr | 510 | 21.40 |
| Salt | Tm, K | H, kJ mole−1 | Salt | Tm, K | H, kJ mole−1 |
|---|---|---|---|---|---|
| LiCl | 883 | 41.4 | KBr | 1007 | 54.0 |
| NaCl | 1074 | 54.5 | RbBr | 967 | 49.7 |
| KCl | 1044 | 54.1 | CsBr | 908 | 46.4 |
| RbCl | 993 | 50.3 | LiI | 742 | 36.4 |
| CsCl | 918 | 49.4 | NaI | 933 | 48.3 |
| LiBr | 823 | 39.0 | KI | 954 | 51.2 |
| NaBr | 1020 | 52.3 | RbI | 920 | 47.8 |
| CsI | 913 | 48.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redkin, A.; Korzun, I.; Yaroslavtseva, T.; Reznitskikh, O.; Zaikov, Y.; Kumkov, S.; Kodintseva, A. Heat Capacity of Solid Halide Eutectics and Their Enthalpy at Melting Point. Thermo 2023, 3, 96-103. https://doi.org/10.3390/thermo3010007
Redkin A, Korzun I, Yaroslavtseva T, Reznitskikh O, Zaikov Y, Kumkov S, Kodintseva A. Heat Capacity of Solid Halide Eutectics and Their Enthalpy at Melting Point. Thermo. 2023; 3(1):96-103. https://doi.org/10.3390/thermo3010007
Chicago/Turabian StyleRedkin, Alexander, Iraida Korzun, Tatyana Yaroslavtseva, Olga Reznitskikh, Yuriy Zaikov, Sergeiy Kumkov, and Anna Kodintseva. 2023. "Heat Capacity of Solid Halide Eutectics and Their Enthalpy at Melting Point" Thermo 3, no. 1: 96-103. https://doi.org/10.3390/thermo3010007
APA StyleRedkin, A., Korzun, I., Yaroslavtseva, T., Reznitskikh, O., Zaikov, Y., Kumkov, S., & Kodintseva, A. (2023). Heat Capacity of Solid Halide Eutectics and Their Enthalpy at Melting Point. Thermo, 3(1), 96-103. https://doi.org/10.3390/thermo3010007







