Power and Energy Requirements for Carbon Capture and Sequestration
Abstract
:1. Introduction
2. Properties of CO2 Pertinent to CCS
2.1. Thermodynamic Properties
2.2. Transport and Storage Properties
3. Methods of CO2 Capture and Energy Requirements
3.1. Methods of Capture
3.2. Minimum Mechanical Work
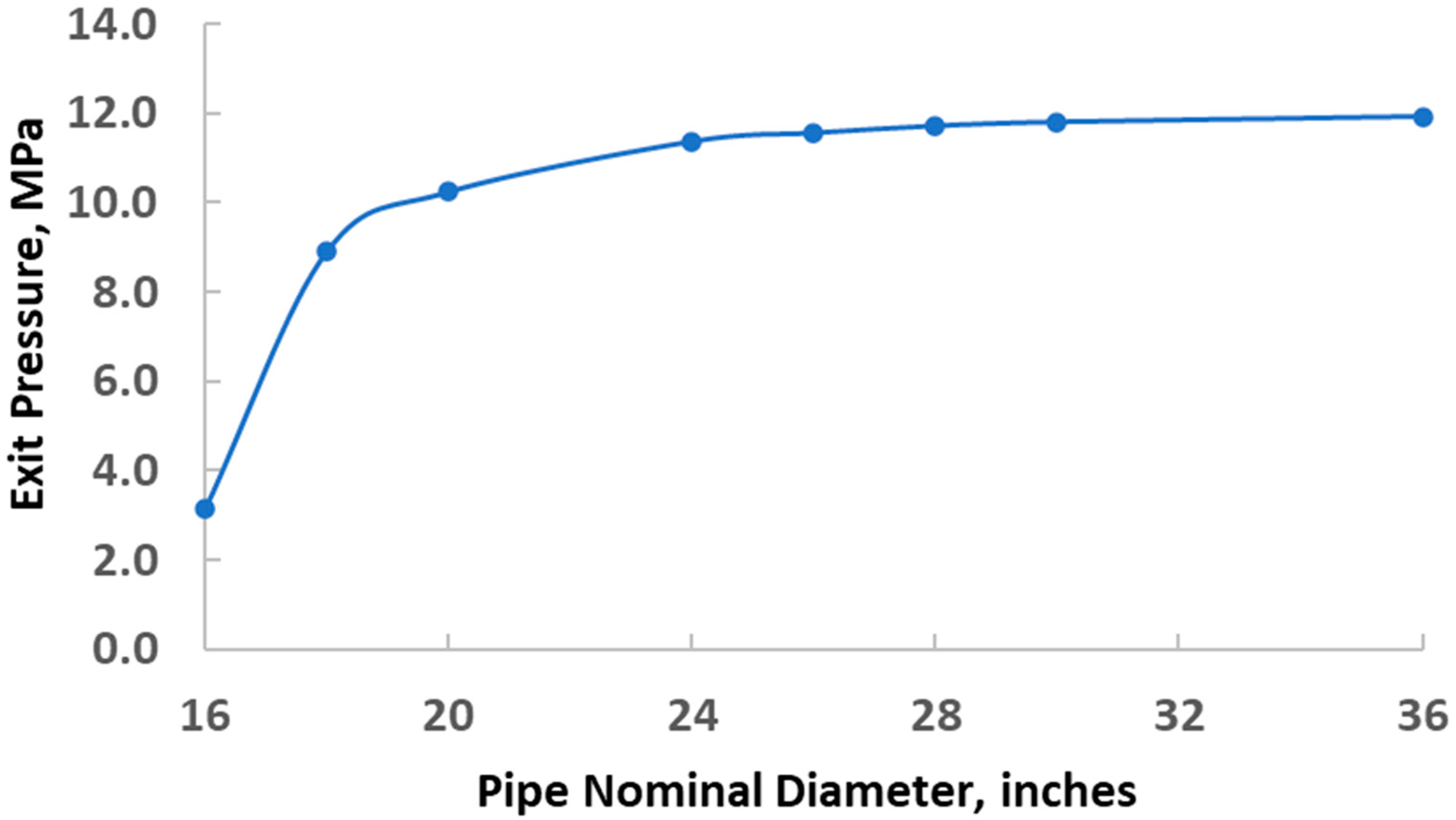
4. Compression and Transportation
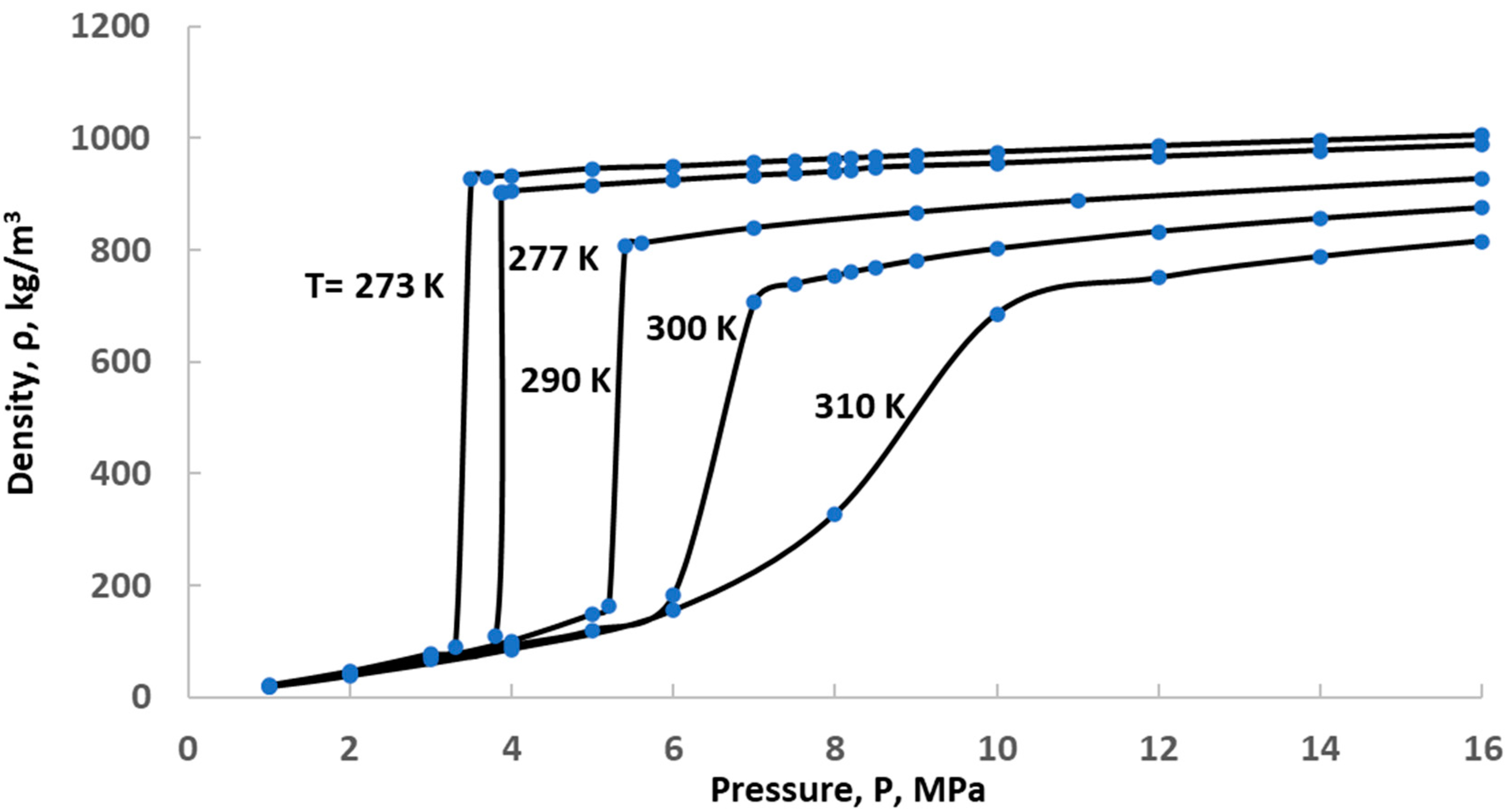
Effect of Temperature
5. Injection, Storage, and Monitoring
- Leakage of CO2 into the atmosphere.
- Environmental effects (local and regional) of the stored CO2.
6. Total Power for Sequestration—The Cases of a Coal Power Plant and a Cement Plant
6.1. A Cement Production Plant in Midlothian Texas
6.2. CCS for the Welsh Coal Power Plant in Pittsburgh, Texas
6.3. Power and Energy Needed for the CCS of 90% of the Produced CO2
6.4. A Note on the Cost of CCS
- The CO2 separation and capture facility.
- The gas compression system, which includes intercoolers.
- The power needed for all the processes.
- The entire pipeline, including the injection system at the end. This includes permitting, materials and labor, rights of way, surveying, and trench construction.
- Any monitoring costs, which extend to the entire life of the systems.
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CO2-Earth—Numbers for Living Earth. Available online: https://www.co2.earth/daily-co2 (accessed on 28 December 2024).
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- IEA. Energy Efficiency Market Report—2013; IEA Publications: Paris, France, 2013. [Google Scholar]
- Michaelides, E.E. Energy, the Environment and Sustainability; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Michaelides, E.E.; Michaelides, D.N. Impact of nuclear energy on fossil fuel substitution. Nucl. Eng. Des. 2020, 366, 110742. [Google Scholar] [CrossRef]
- IEA. Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach—2023 Update; IEA Publications: Paris, France, 2023. [Google Scholar]
- US-EPA. Sources of Greenhouse Gas Emissions. 2020. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 1 December 2024).
- Carbon Capture Utilization and Storage—Special Report. Oil Gas Journal Ebook, March 2024.
- Available online: https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/co2-capture-and-utilisation (accessed on 1 December 2024).
- Kim, G.W.; Alam, M.A.; Lee, J.J.; Kim, G.Y.; Kim, P.J.; Khan, M.I. Assessment of direct carbon dioxide emission factor from urea fertilizer in temperate upland soil during warm and cold cropping season. Eur. J. Soil Biol. 2017, 83, 76–83. [Google Scholar] [CrossRef]
- Commercial Carbon Dioxide Uses: Carbon Dioxide Enhanced Oil Recovery, National Energy Technology Laboratory. Available online: https://netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/eor (accessed on 5 December 2024).
- Paltsev, S.; Morris, J.; Kheshgi, H.; Herzog, H. Hard-to-Abate Sectors: The role of industrial carbon capture and storage (CCS) in emission mitigation. Appl. Energy 2021, 300, 117322. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Koo, Y. Economic impacts of carbon capture and storage on the steel industry–A hybrid energy system model incorporating technological change. Appl. Energy 2022, 317, 119208. [Google Scholar] [CrossRef]
- Al Baroudi, H.; Awoyomi, A.; Patchigolla, K.; Jonnalagadda, K.; Anthony, E.J. A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilisation and storage. Appl. Energy 2022, 287, 116510. [Google Scholar] [CrossRef]
- REFPROP, version 2.1. Reference Thermodynamic and Fluid Transport Properties, Nat. Institute of Standards and Technology: Boulder, CO, USA, 2013.
- Wallace, M.; Goudarzi, L.; Callahan, K.; Wallace, R. A Review of the CO2 Pipeline Infrastructure in the U.S.; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2015; DOE/NETL Report-2014/1681. [Google Scholar]
- Philips, P. The viscosity of Carbon Dioxide. Proc. R. Soc. 1912, 87, 48–61. [Google Scholar]
- Available online: https://www.engineeringtoolbox.com/carbon-dioxide-dynamic-kinematic-viscosity-temperature-pressure-d_2074.html (accessed on 28 January 2025).
- Li, Y.-H.; Tsui, T.-F. The Solubility of CO2 in Water and Sea Water. J. Geoph. Res. 1971, 76, 4203–4207. [Google Scholar] [CrossRef]
- Song, Y.; Chen, B.; Nishio, B.; Akai, B. The study on density change of carbon dioxide seawater solution at high pressure and low temperature. Energy 2005, 30, 2298–2307. [Google Scholar] [CrossRef]
- Hamond, H.F.; Fechner-Levy, E.J. Chemical Fate and Transport in the Environment; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Valenti, G.; Bonalumi, D.; Macchi, E. Energy and exergy analyses for the carbon capture with the Chilled Ammonia Process (CAP). Energy Procedia 2009, 1, 1059–1066. [Google Scholar] [CrossRef]
- Bonalumi, D.; Lillia, S.; Valenti, G. Rate-based simulation and techno-economic analysis of coal-fired power plants with aqueous ammonia carbon capture. Energy Convers. Manag. 2019, 199, 111966. [Google Scholar] [CrossRef]
- Weiland, R.H.; Dingman, J.C.; Cronin, D.B. Heat Capacity of Aqueous Monoethanolamine, Diethanolamine, Nmethyldiethanolamine, and N-methyldiethanolamine-Based Blends with Carbon Dioxide. J. Chem. Eng. Data 1997, 42, 1004–1006. [Google Scholar] [CrossRef]
- Aliyon, K.; Hajinezhad, A.; Mehrpooya, M. Energy assessment of coal-fired steam power plant, carbon capture, and carbon liquefaction process chain as a whole. Energy Convers. Manag. 2019, 199, 111994. [Google Scholar] [CrossRef]
- Carapellucci, R.; Di Battista, D.; Cipollone, R. The retrofitting of a coal-fired subcritical steam power plant for carbon dioxide capture: A comparison between MCFC-based active systems and conventional MEA. Energy Convers. Manag. 2019, 194, 124–139. [Google Scholar] [CrossRef]
- Rao, A.; Rubin, E. Identifying cost-effective CO2 control levels for amine-based CO2 capture systems. Ind. Eng. Chem. Res. 2006, 45, 2421–2429. [Google Scholar] [CrossRef]
- Tong, Z.; Ho, W.S.W. Facilitated transport membranes for CO2 separation and capture. Sep. Sci. Technol. 2017, 52, 156–167. [Google Scholar] [CrossRef]
- Furcasa, F.E.; Wanawan, P.; Chacartegui, R.; Afzal, W. Sodium carbonate-based post combustion carbon capture utilising trona as main sorbent feed stock. Energy Convers. Manag. 2020, 208, 112484. [Google Scholar] [CrossRef]
- Strube, R.; Pellegrini, G.; Manfrida, G. The environmental impact of post-combustion CO2 capture with MEA, with aqueous ammonia, and with an aqueous ammonia—Ethanol mixture for a coal-fired power plant. Energy 2011, 36, 3763–3770. [Google Scholar] [CrossRef]
- Michaelides, E.E. Exergy and the Coversion of Energy; Cambidge University Press: Cambridge, UK, 2021. [Google Scholar]
- Lee, W.-S.; Lee, J.-C.; Oh, H.-T.; Baek, S.-W.; Oh, M.; Lee, C.-H. Performance, economic and exergy analyses of carbon capture processes for a 300 MW class integrated gasification combined cycle power plant. Energy 2017, 134, 731–742. [Google Scholar] [CrossRef]
- Kunze, C.; Riedl, K.; Spliethoff, H. Structured exergy analysis of an integrated gasification combined cycle (IGCC) plant with carbon capture. Energy 2011, 36, 1480–1487. [Google Scholar] [CrossRef]
- Rosner, F.; Qin, C.; Rao, A.; Samuelsen, S. Thermo-economic analyses of concepts for increasing carbon capture in high-methane syngas integrated gasification combined cycle power plants. Energy Convers. Manag. 2019, 199, 112020. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Boyano, A.; Cabrera, M.; Tsatsaronis, G. Exergoeconomic and exergoenvironmental analyses of a combined cycle power plant with chemical looping technology. Int. J. Greenh. Gas Control 2011, 5, 475–482. [Google Scholar] [CrossRef]
- Akinola, T.E.; Bonilla Prado, P.L.; Wang, M. Experimental studies, molecular simulation and process modelling\simulation of adsorption-based post-combustion carbon capture for power plants: A state-of-the-art review. Appl. Energy 2022, 317, 119156. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Gundersen, T. Post-combustion Carbon Capture with a Gas Separation Membrane: Parametric Study, Capture Cost, and Exergy Analysis. Energy Fuels 2013, 27, 3021798. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, L. Membranes for CO2 capture and separation: Progress in research and development for industrial applications. Sep. Purif. Technol. 2024, 335, 126022. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Li, C.; Guo, S.; Ye, X.; Fu, W. Performance and thermoeconomics of solar-aided double-reheat coal-fired power systems with carbon capture. Energy 2019, 177, 1–15. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L.; Vaccarelli, M. Analysis of CO2 post-combustion capture in coal-fired power plants integrated with renewable energies. Energy Procedia 2015, 82, 350–357. [Google Scholar] [CrossRef]
- Saghafifar, M.; Gabra, S. A critical overview of solar assisted carbon capture systems: Is solar always the solution? Int. J. Greenh. Gas Control 2020, 92, 102852. [Google Scholar] [CrossRef]
- Michaelides, E.E. Alternative Energy Sources; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Michaelides, E.E. Thermodynamic Analysis and Power Requirements of CO2 Capture, Transportation, and Storage in the Ocean. Energy 2021, 230, 120804. [Google Scholar] [CrossRef]
- Kestin, J. A Course in Thermodynamics; Hemispohere: Wastington, DC, USA, 1978; Volume I. [Google Scholar]
- Karmakar, S.; Kolar, A.K. Thermodynamic analysis of high-ash coal-fired power plant with carbon dioxide capture. Int. J. Energy Res. 2013, 37, 522–534. [Google Scholar] [CrossRef]
- Arabkhalaj, A.; Ghassemi, H.; Markadeh, R.S. Thermodynamic evaluation of integrated gasification combined cycle: Comparison between high-ash and low-ash coals. Int. J. Energy Res. 2016, 40, 1638–1651. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H.C.; Loos, M.; Meyer, L. (Eds.) IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Davison, J.E. CO2 capture and storage and the IEA Greenhouse Gas R&D Programme. In Proceedings of the Workshop on CO2 issues, Middelfart, Denmark, 24 May 2005. [Google Scholar]
- Nessi, P.; Papadopoulos, A.; Kazepidis, P.; Polichroniadis, A.; Ntourou, G.; Voutetakis, S.; Seferlis, P. Pilot Scale Assessment of a Novel Phase-change Solvent for Energy Efficient Post -combustion CO2 Capture. J. Environ. Manag. 2022, 317, 115489. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, E.E.; Crowe, C.T.; Schwarzkopf, J.D. (Eds.) Multiphase Flow Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Munson, B.R.; Young, D.F.; Okiishi, T.H.; Huwbsch, W.W. Fundamentals of Fluid Mechanics; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Paul, S.; Shepherd, R.; Woollin, P. Selection of materials for high pressure CO2 transport. In Proceedings of the 3rd International Forum on the Transportation of CO2, Newcastle, UK, 18 June 2012. [Google Scholar]
- Available online: https://www.engineersedge.com/pipe_schedules.htm (accessed on 3 February 2025).
- Available online: https://www.eia.gov/electricity/data/browser/#/plant/6139 (accessed on 26 January 2025).
- Lindsay, R. Climate Change: Atmospheric Carbon Dioxide. 2024. Available online: https://www.climate.gov/news-features (accessed on 1 January 2025).
- Lindeberg, E.; Vuillaume, J.-F.; Ghaderi, A. Determination of the CO2 storage capacity of the Utsira formation. Energ Procedia 2009, 1, 2777–2784. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/system/files/documents/2021-11/minerals_sector_profile_2020.pdf (accessed on 26 January 2025).
- U.S. Cement Industry Carbon Intensities; United States Environmental Protection Agency: Washington DC, USA, 2021; EPA report, EPA 430-F-21-004.
- Smith, C.E. Natural gas pipeline profits, construction both up. Oil Gas J. 2016, 114, 84–96. [Google Scholar]


| CO2 Mass Rate, kg/s | Separation, MW | Compression, MW | Total Power, MW | Annual Energy, GWh | Energy per Mass, MJ/kg | |
|---|---|---|---|---|---|---|
| Cement Unit | 57.3 | 20.63 | 25.35 | 45.98 | 402.7 | 0.802 |
| Power Plant | 115.2 | 82.59 | 50.96 | 133.55 | 1169.9 | 1.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaelides, E.E. Power and Energy Requirements for Carbon Capture and Sequestration. Thermo 2025, 5, 8. https://doi.org/10.3390/thermo5010008
Michaelides EE. Power and Energy Requirements for Carbon Capture and Sequestration. Thermo. 2025; 5(1):8. https://doi.org/10.3390/thermo5010008
Chicago/Turabian StyleMichaelides, Efstathios E. 2025. "Power and Energy Requirements for Carbon Capture and Sequestration" Thermo 5, no. 1: 8. https://doi.org/10.3390/thermo5010008
APA StyleMichaelides, E. E. (2025). Power and Energy Requirements for Carbon Capture and Sequestration. Thermo, 5(1), 8. https://doi.org/10.3390/thermo5010008






