BDP1 Expression Correlates with Clinical Outcomes in Activated B-Cell Diffuse Large B-Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oncomine Analyses
2.2. BDP1 Promoter Analysis
3. Results
3.1. BDP1 Expression Is Significantly Altered in a Subset of Human Cancers
3.2. BDP1 mRNA Is Significantly and Specifically Underexpressed in Lymphoma
3.3. Heat Map Identifies BDP1 Expression as Significantly Underexpressed in BL and ALK+ ALCL
3.4. In Silico Identification of Putative Transcription Factor Binding Sites in the BDP1 Promoter for Transcription Factors Deregulated in NHL
3.5. Decreased BDP1 Expression Correlates with FOXP1 and BCL6 Expression
3.6. Decreased BDP1 Expression Correlates with Clinical Outcomes in Activated B-Cell Diffuse Large B-Cell Lymphoma
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S. Diagnosis and classification of lymphoma: Impact of technical advances. Semin. Hematol. 2019, 56, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L.; Hernandez, N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 2002, 16, 2593–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, L.; Pendergrast, P.S.; Sun, Y.; Hernandez, N. Different human TFIIIB activities direct RNA polymerase III transcription from TATA-containing and TATA-less promoters. Genes Dev. 2000, 14, 2650–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teichmann, M.; Wang, Z.; Roeder, R.G. A stable complex of a novel transcription factor IIB- related factor, human TFIIIB50, and associated proteins mediate selective transcription by RNA polymerase III of genes with upstream promoter elements. Proc. Natl. Acad. Sci. USA 2000, 97, 14200–14205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelter, A.R.; Herchenbach, J.; Wirth, B. The transcription factor-like nuclear regulator (TFNR) contains a novel 55-amino-acid motif repeated nine times and maps closely to SMN1. Genomics 2000, 70, 315–326. [Google Scholar] [CrossRef]
- Gouge, J.; Guthertz, N.; Kramm, K.; Dergai, O.; Abascal-Palacios, G.; Satia, K.; Cousin, P.; Hernandez, N.; Grohmann, D.; Vannini, A. Molecular mechanisms of Bdp1 in TFIIIB assembly and RNA polymerase III transcription initiation. Nat. Commun. 2017, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- White, R.J. RNA polymerase III transcription and cancer. Oncogene 2004, 23, 3208–3216. [Google Scholar] [CrossRef] [Green Version]
- Athineos, D.; Marshall, L.; White, R.J. Regulation of TFIIIB during F9 cell differentiation. BMC Mol. Biol. 2010, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Felton-Edkins, Z.A.; Kenneth, N.S.; Brown, T.R.; Daly, N.L.; Gomez-Roman, N.; Grandori, C.; Eisenman, R.N.; White, R.J. Direct regulation of RNA polymerase III transcription by RB, p53 and c-Myc. Cell Cycle 2003, 2, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Cairns, C.A.; White, R.J. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 1998, 17, 3112–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabarcas, S.; Watabe, K.; Schramm, L. Inhibition of U6 snRNA Transcription by PTEN. Online J. Biol. Sci. 2010, 10, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woiwode, A.; Johnson, S.A.; Zhong, S.; Zhang, C.; Roeder, R.G.; Teichmann, M.; Johnson, D.L. PTEN represses RNA polymerase III-dependent transcription by targeting the TFIIIB complex. Mol. Cell Biol 2008, 28, 4204–4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veras, I.; Rosen, E.M.; Schramm, L. Inhibition of RNA polymerase III transcription by BRCA1. J. Mol. Biol. 2009, 387, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J.E.; Cairns, C.A.; McLees, A.; Allison, S.J.; Tosh, K.; White, R.J. RNA polymerase III transcription factor IIIB is a target for repression by pocket proteins p107 and p130. Mol. Cell Biol. 1999, 19, 4255–4261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockwood, W.W.; Chari, R.; Coe, B.P.; Thu, K.L.; Garnis, C.; Malloff, C.A.; Campbell, J.; Williams, A.C.; Hwang, D.; Zhu, C.Q.; et al. Integrative genomic analyses identify BRF2 as a novel lineage-specific oncogene in lung squamous cell carcinoma. PLoS Med. 2010, 7, e1000315. [Google Scholar] [CrossRef] [Green Version]
- Girotto, G.; Abdulhadi, K.; Buniello, A.; Vozzi, D.; Licastro, D.; d'Eustacchio, A.; Vuckovic, D.; Alkowari, M.K.; Steel, K.P.; Badii, R.; et al. Linkage study and exome sequencing identify a BDP1 mutation associated with hereditary hearing loss. PLoS ONE 2013, 8, e80323. [Google Scholar] [CrossRef]
- Zhao, D.; Lu, X.; Wang, G.; Lan, Z.; Liao, W.; Li, J.; Liang, X.; Chen, J.R.; Shah, S.; Shang, X.; et al. Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer. Nature 2017, 542, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Felton-Edkins, Z.A.; White, R.J. Multiple mechanisms contribute to the activation of RNA polymerase III transcription in cells transformed by papovaviruses. J. Biol. Chem. 2002, 277, 48182–48191. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. Oncomine: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brune, V.; Tiacci, E.; Pfeil, I.; Döring, C.; Eckerle, S.; van Noesel, C.J.; Klapper, W.; Falini, B.; von Heydebreck, A.; Metzler, D.; et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008, 205, 2251–2268. [Google Scholar] [CrossRef] [PubMed]
- Eckerle, S.; Brune, V.; Döring, C.; Tiacci, E.; Bohle, V.; Sundström, C.; Kodet, R.; Paulli, M.; Falini, B.; Klapper, W.; et al. Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: Insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia 2009, 23, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaknovich, R.; Geng, H.; Johnson, N.A.; Tsikitas, L.; Cerchietti, L.; Greally, J.M.; Gascoyne, R.D.; Elemento, O.; Melnick, A. DNA methylation signatures define molecular subtypes of diffuse large B-cell lymphoma. Blood 2010, 116, e81–e89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreos, R.; Ambrosini, G.; Groux, R.; Cavin Périer, R.; Bucher, P. The eukaryotic promoter database in its 30th year: Focus on non-vertebrate organisms. Nucleic Acids Res. 2017, 45, D51–D55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellido, F.; Sowada, N.; Mur, P.; Lázaro, C.; Pons, T.; Valdés-Mas, R.; Pineda, M.; Aiza, G.; Iglesias, S.; Soto, J.L.; et al. Association Between Germline Mutations in BRF1, a Subunit of the RNA Polymerase III Transcription Complex, and Hereditary Colorectal Cancer. Gastroenterology 2018, 154, 181–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabarcas, S.; Jacob, J.; Veras, I.; Schramm, L. Differential expression of the TFIIIB subunits Brf1 and Brf2 in cancer cells. BMC Mol. Biol. 2008, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Yi, Y.; Shi, G.; Li, S.; Chen, S.; Lin, Y.; Li, Z.; He, Z.; Li, W.; Zhong, S. Role of Brf1 interaction with ERα, and significance of its overexpression, in human breast cancer. Mol. Oncol. 2017, 11, 1752–1767. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhang, Y.; Zhong, S. Alcohol Intake and Abnormal Expression of Brf1 in Breast Cancer. Oxid. Med. Cell Longev. 2019, 2019, 4818106. [Google Scholar] [CrossRef]
- Lei, J.; Chen, S.; Zhong, S. Abnormal expression of TFIIIB subunits and RNA Pol III genes is associated with hepatocellular carcinoma. Liver Res. 2017, 1, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Cabarcas-Petroski, S.; Meneses, P.I.; Schramm, L. A meta-analysis of BRF2 as a prognostic biomarker in invasive breast carcinoma. BMC Cancer 2020, 20, 1093. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Cabarcas-Petroski, S.; Petrie, J.L.; Diette, N.; White, R.J.; Schramm, L. Induction of proto-oncogene BRF2 in breast cancer cells by the dietary soybean isoflavone daidzein. BMC Cancer 2015, 15, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Tian, H.; Yue, W.; Li, L.; Li, S.; Qi, L.; Hu, W.; Gao, C.; Si, L. Overexpression of TFIIB-related factor 2 is significantly correlated with tumor angiogenesis and poor survival in patients with esophageal squamous cell cancer. Med. Oncol 2013, 30, 553. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tian, H.; Yue, W.; Li, L.; Li, S.; Qi, L.; Hu, W.; Gao, C.; Si, L. TFIIB-related factor 2 over expression is a prognosis marker for early-stage non-small cell lung cancer correlated with tumor angiogenesis. PLoS ONE 2014, 9, e88032. [Google Scholar] [CrossRef]
- Melchor, L.; Garcia, M.J.; Honrado, E.; Pole, J.C.; Alvarez, S.; Edwards, P.A.; Caldas, C.; Brenton, J.D.; Benítez, J. Genomic analysis of the 8p11-12 amplicon in familial breast cancer. Int. J. Cancer 2007, 120, 714–717. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, M.; Yue, W.; Li, L.; Li, S.; Gao, C.; Si, L.; Qi, L.; Hu, W.; Tian, H. TFIIB-related factor 2 is associated with poor prognosis of nonsmall cell lung cancer patients through promoting tumor epithelial-mesenchymal transition. Biomed. Res. Int. 2014, 2014, 530786. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Lu, M. BRF2 as a promising indicator for radical lymph-node dissection surgery in patients with cN0 squamous cell carcinoma of the middle thoracic esophagus. Surg. Today 2019, 49, 158–169. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, T.; Ushikoshi-Nakayama, R.; Suenaga, A.; Ide, K.; Yumoto, N.; Naruo, Y.; Takahashi, K.; Saeki, Y.; Taiji, M.; Tanaka, H.; et al. Mutation of epidermal growth factor receptor is associated with MIG6 expression. FEBS J. 2009, 276, 5239–5251. [Google Scholar] [CrossRef]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, K.; Brehm, A. Chromatin regulation: How complex does it get? Epigenetics 2014, 9, 1485–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloghini, A.; Buglio, D.; Khaskhely, N.M.; Georgakis, G.; Orlowski, R.Z.; Neelapu, S.S.; Carbone, A.; Younes, A. Expression of histone deacetylases in lymphoma: Implication for the development of selective inhibitors. Br. J. Haematol. 2009, 147, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Van Bergen, M.G.J.M.; van der Reijden, B.A. Targeting the GFI1/1B-CoREST Complex in Acute Myeloid Leukemia. Front. Oncol. 2019, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Kowenz-Leutz, E.; Laumonnier, Y.; Xu, H.; Leutz, A. Histone H3 tail positioning and acetylation by the c-Myb but not the v-Myb DNA-binding SANT domain. Genes Dev. 2005, 19, 2447–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kalkum, M.; Chait, B.T.; Roeder, R.G. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol. Cell 2002, 9, 611–623. [Google Scholar] [CrossRef]
- Zeng, L.S.; Yang, X.Z.; Wen, Y.F.; Mail, S.J.; Wang, M.H.; Zhang, M.Y.; Zheng, X.F.; Wang, H.Y. Overexpressed HDAC4 is associated with poor survival and promotes tumor progression in esophageal carcinoma. Aging 2016, 8, 1236–1249. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, S.K.; Volinia, S.; Costinean, S.; Galasso, M.; Neinast, R.; Santhanam, R.; Parthun, M.R.; Perrotti, D.; Marcucci, G.; Garzon, R.; et al. miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Eμ-miR-155 transgenic mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 20047–20052. [Google Scholar] [CrossRef] [Green Version]
- Chan, F.C.; Telenius, A.; Healy, S.; Ben-Neriah, S.; Mottok, A.; Lim, R.; Drake, M.; Hu, S.; Ding, J.; Ha, G.; et al. An RCOR1 loss-associated gene expression signature identifies a prognostically significant DLBCL subgroup. Blood 2015, 125, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, G.; Chowdhury, A.H.; Vaidyanathan, B.; Kim, D.; Saleque, S. Antagonistic actions of Rcor proteins regulate LSD1 activity and cellular differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 8071–8076. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.; Xie, L.; Leithäuser, F.; Flossbach, L.; Möller, P.; Wirth, T.; Ushmorov, A. KLF4 is a tumor suppressor in B-cell non-Hodgkin lymphoma and in classic Hodgkin lymphoma. Blood 2010, 116, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Mossafa, H.; Damotte, D.; Jenabian, A.; Delarue, R.; Vincenneau, A.; Amouroux, I.; Jeandel, R.; Khoury, E.; Martelli, J.M.; Samson, T.; et al. Non-Hodgkin’s lymphomas with Burkitt-like cells are associated with c-Myc amplification and poor prognosis. Leuk. Lymphoma 2006, 47, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.D.; Ahearne, M.; Ko Ferrigno, P. The role of BCL6 in lymphomas and routes to therapy. Br. J. Haematol 2011, 152, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, I.; Veyt, E.; De Paepe, P.; Vandenberghe, P.; Nooijen, P.; Theate, I.; Michaux, L.; Sagaert, X.; Marynen, P.; Hagemeijer, A.; et al. FOXP1, a gene highly expressed in a subset of diffuse large B-cell lymphoma, is recurrently targeted by genomic aberrations. Leukemia 2005, 19, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Molina-Privado, I.; Jiménez, P.R.; Montes-Moreno, S.; Chiodo, Y.; Rodríguez-Martínez, M.; Sánchez-Verde, L.; Iglesias, T.; Piris, M.A.; Campanero, M.R. E2F4 plays a key role in Burkitt lymphoma tumorigenesis. Leukemia 2012, 26, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Medeiros, L.J.; Young, K.H. Diagnostic and predictive biomarkers for lymphoma diagnosis and treatment in the era of precision medicine. Mod. Pathol. 2016, 29, 1118–1142. [Google Scholar] [CrossRef] [Green Version]
- Lenz, G. Insights into the Molecular Pathogenesis of Activated B-Cell-like Diffuse Large B-Cell Lymphoma and Its Therapeutic Implications. Cancers 2015, 7, 811–822. [Google Scholar] [CrossRef]
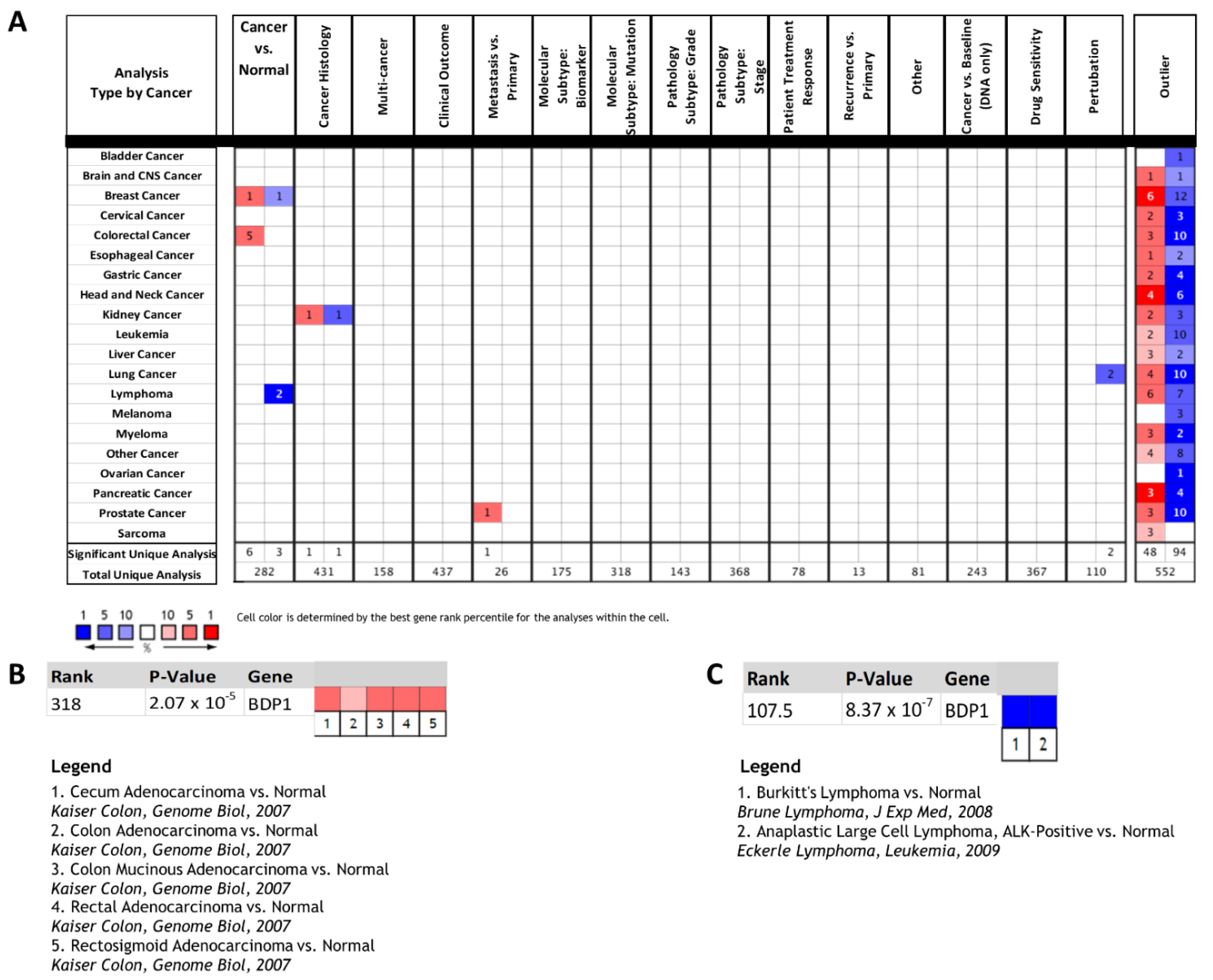
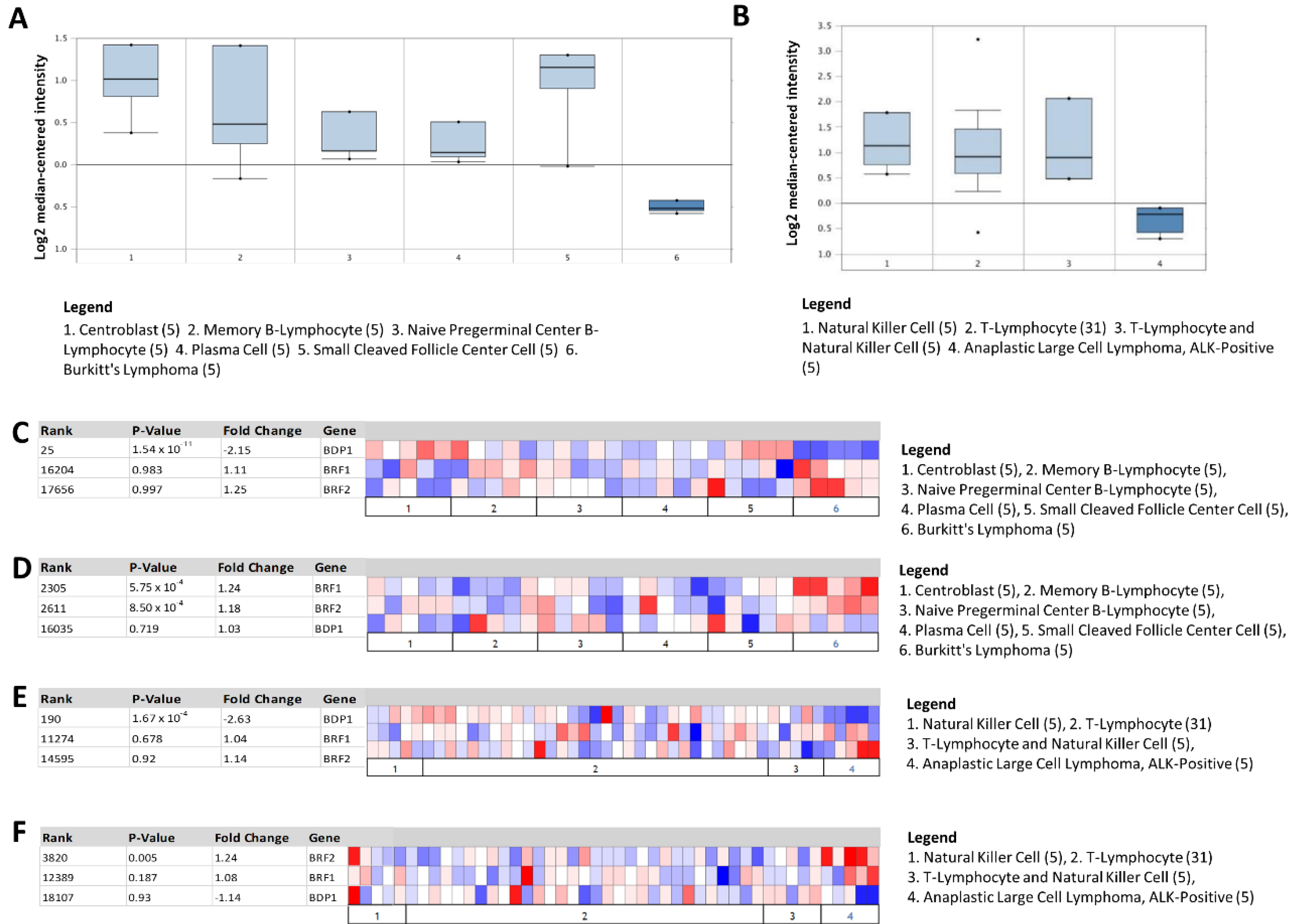





| Dataset (Hyperlink to Public Dataset) | Study Description | Reference |
|---|---|---|
| Brune | Forty-two (42) malignant lymphoma samples, including 11 Hodgkin’s lymphoma, 11 diffuse large B-cell lymphoma, 5 nodular lymphocyte predominant Hodgkin’s lymphoma, 5 follicular lymphomas, 5 Burkitt’s lymphoma, and 4 T-cell/histiocyte-rich large B-cell lymphoma samples, were analyzed. In addition, 25 normal B-cell samples of various types were included in this analysis. | [22] |
| Eckerle | Twenty-three (23) lymphoma samples, including 4 classical Hodgkin’s lymphoma, 7 primary cutaneous anaplastic large-cell lymphoma, and 12 anaplastic large-cell lymphoma samples (including 3 cell lines), were analyzed. | [23] |
| Shaknovich | Forty (40) germinal center B-cell-like diffuse large B-cell lymphoma, 20 activated B-cell-like diffuse large B-cell lymphoma, and 9 diffuse large B-cell lymphoma samples were analyzed. | [24] |
| Transcription Factor | Over- or Underexpressed | Interaction with SANT Domain | References |
|---|---|---|---|
| HDAC4 | Overexpressed | Y | [43] |
| RCOR3 | Overexpressed/Underexpressed | Y | [44] |
| Transcription Factor | Location in the BDP1 Promoter Relative to TSS | References |
|---|---|---|
| KLF4 | −734, −593, −553, −459, −291, | [51] |
| MYC | −582, −581 | [52] |
| BCL6 | −985, −936, −384, −362, −287, −276, −173 | [53] |
| FOXP1 | −876, −802, −747, −427, −388 | [54] |
| E2F4 | 526, −470, −15, 54, 74 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabarcas-Petroski, S.; Schramm, L. BDP1 Expression Correlates with Clinical Outcomes in Activated B-Cell Diffuse Large B-Cell Lymphoma. BioMedInformatics 2022, 2, 169-183. https://doi.org/10.3390/biomedinformatics2010011
Cabarcas-Petroski S, Schramm L. BDP1 Expression Correlates with Clinical Outcomes in Activated B-Cell Diffuse Large B-Cell Lymphoma. BioMedInformatics. 2022; 2(1):169-183. https://doi.org/10.3390/biomedinformatics2010011
Chicago/Turabian StyleCabarcas-Petroski, Stephanie, and Laura Schramm. 2022. "BDP1 Expression Correlates with Clinical Outcomes in Activated B-Cell Diffuse Large B-Cell Lymphoma" BioMedInformatics 2, no. 1: 169-183. https://doi.org/10.3390/biomedinformatics2010011
APA StyleCabarcas-Petroski, S., & Schramm, L. (2022). BDP1 Expression Correlates with Clinical Outcomes in Activated B-Cell Diffuse Large B-Cell Lymphoma. BioMedInformatics, 2(1), 169-183. https://doi.org/10.3390/biomedinformatics2010011







