Small Bowel Dose Constraints in Radiation Therapy—Where Omics-Driven Biomarkers and Bioinformatics Can Take Us in the Future
Abstract
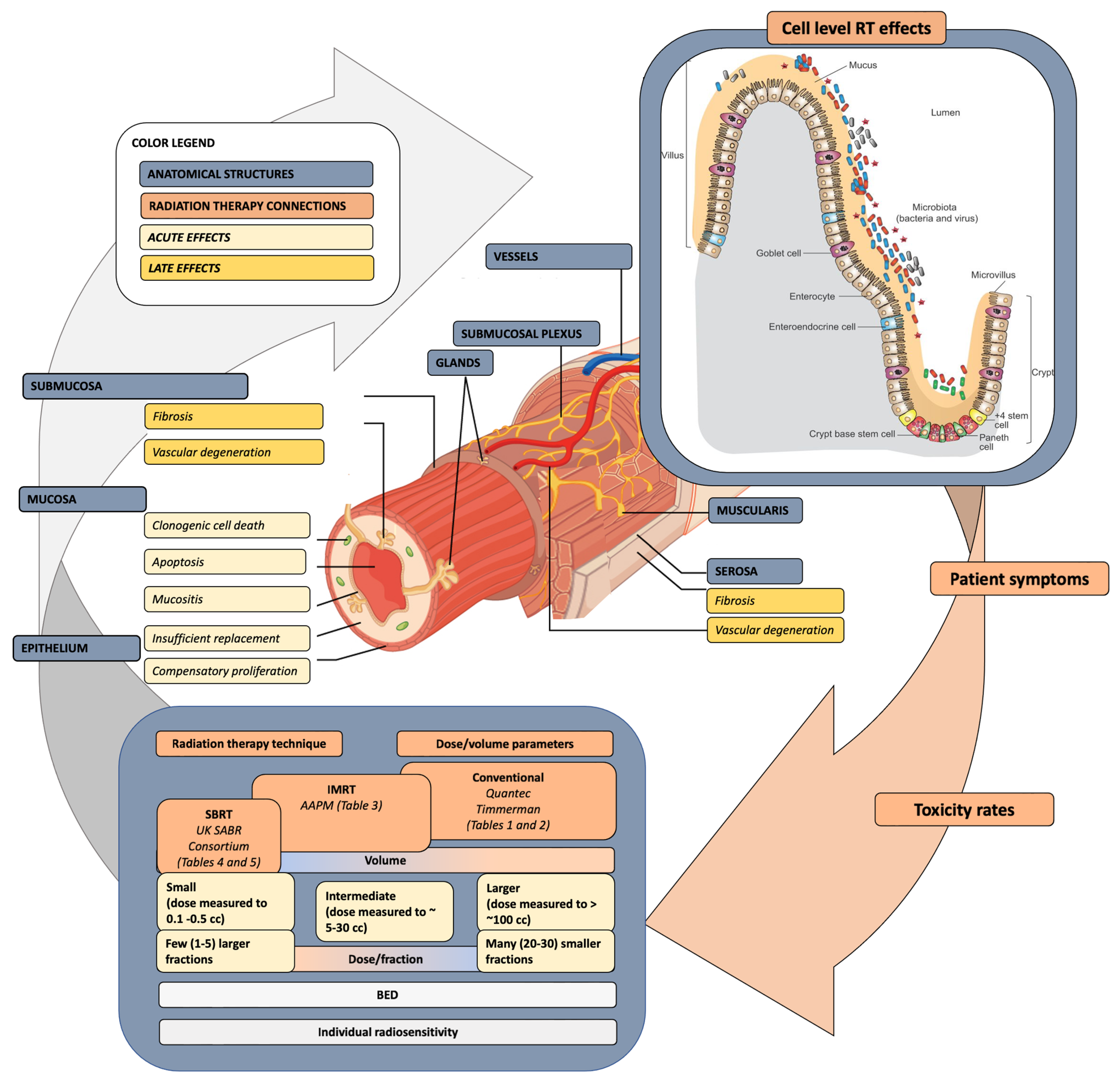
1. Introduction
2. The Pathophysiology and Dose Response of Small Bowel Injury
3. Current SB Constraints—A Walk Back in Time
4. The Biomarker and Bioinformatics Frontier
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAPM-TG | American Association of Physicists in Medicine Task Group |
| BED | Biologically effective dose |
| BMI | Body mass index |
| CRC | Colorectal cancer |
| EQD2 | Equivalent dose at 2 Gy per fraction |
| GI | Gastrointestinal |
| HyTEC | Hypofractionated Treatment Effects in Clinic |
| IMRT | Image-guided intensity-modulated radiation therapy |
| IPA | Ingenuity pathway analysis |
| MM | Multiple myeloma |
| NGS | Next-generation sequencing |
| NSCLC | Non-small-cell lung cancer |
| OARs | Organs at risk |
| PTV | Planning target volume |
| QUANTEC | Quantitative Analysis of Normal Tissue Effects in the Clinic |
| RCC | Renal cell carcinoma |
| RCT | Randomized controlled trial |
| RT | Radiation therapy |
| SABR | Stereotactic ablative radiotherapy |
| SB | Small bowel |
| TNBC | Triple negative breast cancer |
| TD5/5 | Tolerance dose resulting in 5% risk of severe complications within 5 years after irradiation |
| TD50/5 | Tolerance dose resulting in 50% risk of severe complications within 5 years after irradiation |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| GSEA | Gene Set Enrichment Analysis |
References
- Pham, A.; Ballas, L.K. Trimodality therapy for bladder cancer: Modern management and future directions. Curr. Opin. Urol. 2019, 29, 210–215. [Google Scholar] [CrossRef]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Anker, C.J.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Dorth, J.; Garcia-Aguilar, J.; Goff, D.; Jacqmin, D.; et al. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 13–25. [Google Scholar] [CrossRef]
- Kavanagh, B.D.; Pan, C.C.; Dawson, L.A.; Das, S.K.; Li, X.A.; Ten Haken, R.K.; Miften, M. Radiation dose-volume effects in the stomach and small bowel. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S101–S107. [Google Scholar] [CrossRef]
- Shih, K.K.; Hajj, C.; Kollmeier, M.; Frey, M.K.; Sonoda, Y.; Abu-Rustum, N.R.; Alektiar, K.M. Impact of postoperative intensity-modulated radiation therapy (IMRT) on the rate of bowel obstruction in gynecologic malignancy. Gynecol. Oncol. 2016, 143, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Wang, J.; Denham, J.W. Bowel injury: Current and evolving management strategies. Semin. Radiat. Oncol. 2003, 13, 357–371. [Google Scholar]
- Wang, J.; Zheng, H.; Ou, X.; Albertson, C.M.; Fink, L.M.; Herbert, J.M.; Hauer-Jensen, M. Hirudin ameliorates intestinal radiation toxicity in the rat: Support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J. Thromb. Haemost. 2004, 2, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J. Radiation enteropathy—Pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef]
- Robertson, J.M.; Lockman, D.; Yan, D.; Wallace, M. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 413–418. [Google Scholar] [CrossRef]
- Kwak, Y.K.; Lee, S.W.; Kay, C.S.; Park, H.H. Intensity-modulated radiotherapy reduces gastrointestinal toxicity in pelvic radiation therapy with moderate dose. PLoS ONE 2017, 12, e0183339. [Google Scholar] [CrossRef]
- Arbea, L.; Ramos, L.I.; Martínez-Monge, R.; Moreno, M.; Aristu, J. Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): Dosimetric comparison and clinical implications. Radiat. Oncol. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Ishihara, S.; Nozawa, H.; Kawai, K.; Kiyomatsu, T.; Okuma, K.; Abe, O.; Watanabe, T.; Nakagawa, K. Comparison of volumetric-modulated arc therapy using simultaneous integrated boosts (SIB-VMAT) of 45 Gy/55 Gy in 25 fractions with conventional radiotherapy in preoperative chemoradiation for rectal cancers: A propensity score case-matched analysis. Radiat. Oncol. 2017, 12, 156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.M.; Huang, M.Y.; Tsai, H.L.; Huang, C.W.; Ma, C.J.; Lin, C.H.; Huang, C.J.; Wang, J.Y. A retrospective comparison of outcome and toxicity of preoperative image-guided intensity-modulated radiotherapy versus conventional pelvic radiotherapy for locally advanced rectal carcinoma. J. Radiat. Res. 2017, 58, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Simson, D.K.; Mitra, S.; Ahlawat, P.; Saxena, U.; Sharma, M.K.; Rawat, S.; Singh, H.; Bansal, B.; Sripathi, L.K.; Tanwar, A. Prospective study of neoadjuvant chemoradiotherapy using intensity-modulated radiotherapy and 5 fluorouracil for locally advanced rectal cancer-toxicities and response assessment. Cancer Manag. Res. 2018, 10, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L., 3rd; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Krishnatry, R.; Dora, T.; Kannan, S.; Thomas, B.; Sonawone, S.; Engineer, R.; Paul, S.; Phurailatpam, R.; Mahantshetty, U.; et al. Predictors of late bowel toxicity using three different methods of contouring in patients undergoing post-operative radiation for cervical cancer. Br. J. Radiol. 2015, 88, 20150054. [Google Scholar] [CrossRef]
- Jadon, R.; Higgins, E.; Hanna, L.; Evans, M.; Coles, B.; Staffurth, J. A systematic review of dose-volume predictors and constraints for late bowel toxicity following pelvic radiotherapy. Radiat. Oncol. 2019, 14, 57. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.; Marks, L.B.; Jackson, A.; Kavanagh, B.D.; Xue, J.; Yorke, E. High Dose per Fraction, Hypofractionated Treatment Effects in the Clinic (HyTEC): An Overview. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.D. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin. Radiat. Oncol. 2008, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.N.; Medin, P.M.; Timmerman, R.D. Emphasis on Repair, Not Just Avoidance of Injury, Facilitates Prudent Stereotactic Ablative Radiotherapy. Semin. Radiat. Oncol. 2017, 27, 378–392. [Google Scholar] [CrossRef]
- Hanna, G.G.; Murray, L.; Patel, R.; Jain, S.; Aitken, K.L.; Franks, K.N.; van As, N.; Tree, A.; Hatfield, P.; Harrow, S.; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin. Oncol. 2018, 30, 5–14. [Google Scholar] [CrossRef]
- Gerhard, S.G.; Palma, D.A.; Arifin, A.J.; Louie, A.V.; Li, G.J.; Al-Shafa, F.; Cheung, P.; Rodrigues, G.B.; Bassim, C.W.; Corkum, M.T. Organ at Risk Dose Constraints in SABR: A Systematic Review of Active Clinical Trials. Pract. Radiat. Oncol. 2021, 11, e355–e365. [Google Scholar] [CrossRef]
- Irritable Bowel Syndrome (IBS). Slidesgo. Available online: https://slidesgo.com/theme/irritable-bowel-syndrome-ibs (accessed on 1 November 2023).
- Teken, G. Layers of the Alimentary Canal. The Wall of the Alimentary Canal Has Four Basic Tissue Layers: The Mucosa, Submucosa, Muscularis, and Serosa. 2014. Available online: https://images.app.goo.gl/a9jhhoBrFzocLRAR8 (accessed on 1 November 2023).
- Wong, A.C.; Vanhove, A.S.; Watnick, P.I. The interplay between intestinal bacteria and host metabolism in health and disease: Lessons from Drosophila melanogaster. Dis. Models Mech. 2016, 9, 271–281. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Ten Haken, R.K.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Baglan, K.L.; Frazier, R.C.; Yan, D.; Huang, R.R.; Martinez, A.A.; Robertson, J.M. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 176–183. [Google Scholar] [CrossRef]
- Roeske, J.C.; Bonta, D.; Mell, L.K.; Lujan, A.E.; Mundt, A.J. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother. Oncol. 2003, 69, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tho, L.M.; Glegg, M.; Paterson, J.; Yap, C.; MacLeod, A.; McCabe, M.; McDonald, A.C. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: Investigating dose-volume relationships and role for inverse planning. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Sung, C.C.; Ko, S.F.; Wang, C.J.; Yang, K.D. The different volume effects of small-bowel toxicity during pelvic irradiation between gynecologic patients with and without abdominal surgery: A prospective study with computed tomography-based dosimetry. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 732–739. [Google Scholar] [CrossRef]
- Gunnlaugsson, A.; Kjellén, E.; Nilsson, P.; Bendahl, P.O.; Willner, J.; Johnsson, A. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007, 46, 937–944. [Google Scholar] [CrossRef]
- Folkert, M.R.; Timmerman, R.D. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv. Drug Deliv. Rev. 2017, 109, 3–14. [Google Scholar] [CrossRef]
- Diez, P.; Hanna, G.G.; Aitken, K.L.; van As, N.; Carver, A.; Colaco, R.J.; Conibear, J.; Dunne, E.M.; Eaton, D.J.; Franks, K.N.; et al. UK 2022 Consensus on Normal Tissue Dose-Volume Constraints for Oligometastatic, Primary Lung and Hepatocellular Carcinoma Stereotactic Ablative Radiotherapy. Clin. Oncol. 2022, 34, 288–300. [Google Scholar] [CrossRef]
- Peeters, K.C.; van de Velde, C.J.; Leer, J.W.; Martijn, H.; Junggeburt, J.M.; Kranenbarg, E.K.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Marijnen, C.A. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch colorectal cancer group study. J. Clin. Oncol. 2005, 23, 6199–6206. [Google Scholar] [CrossRef]
- Marijnen, C. A, Kapiteijn, E.; van de Velde, C.J.; Martijn, H.; Steup, W.H.; Wiggers, T.; Kranenbarg, E.K.; Leer, J.W.; Cooperative Investigators of the Dutch Colorectal Cancer Group. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: Report of a multicenter randomized trial. J. Clin. Oncol. 2002, 20, 817–825. [Google Scholar] [PubMed]
- Cai, Y.; Rattray, N.J.W.; Zhang, Q.; Mironova, V.; Santos-Neto, A.; Muca, E.; Vollmar, A.K.R.; Hsu, K.S.; Rattray, Z.; Cross, J.R.; et al. Tumor Tissue-Specific Biomarkers of Colorectal Cancer by Anatomic Location and Stage. Metabolites 2020, 10, 257. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Eade, T.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; Hruby, G.; Stevens, M.; Boyle, F.; Clarke, S.; et al. The Gut Microbiome and Gastrointestinal Toxicities in Pelvic Radiation Therapy: A Clinical Review. Cancers 2021, 13, 2353. [Google Scholar] [CrossRef] [PubMed]
- Bennike, T.; Birkelund, S.; Stensballe, A.; Andersen, V. Biomarkers in inflammatory bowel diseases: Current status and proteomics identification strategies. World J. Gastroenterol. 2014, 20, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chance, M.R.; Nicholas, C.; Ahmed, N.; Guilmeau, S.; Flandez, M.; Wang, D.; Byun, D.S.; Nasser, S.; Albanese, J.M.; et al. Proteomic changes during intestinal cell maturation in vivo. J. Proteom. 2008, 71, 530–546. [Google Scholar] [CrossRef]
- Broin, P.Ó.; Vaitheesvaran, B.; Saha, S.; Hartil, K.; Chen, E.I.; Goldman, D.; Fleming, W.H.; Kurland, I.J.; Guha, C.; Golden, A. Intestinal microbiota-derived metabolomic blood plasma markers for prior radiation injury. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 360–367. [Google Scholar] [CrossRef]
- Stidham, R.W.; Wu, J.; Shi, J.; Lubman, D.M.; Higgins, P.D. Serum Glycoproteome Profiles for Distinguishing Intestinal Fibrosis from Inflammation in Crohn’s Disease. PLoS ONE 2017, 12, e0170506. [Google Scholar] [CrossRef]
- Sproull, M.; Kawai, T.; Krauze, A.; Shankavaram, U.; Camphausen, K. Prediction of Total-Body and Partial-Body Exposures to Radiation Using Plasma Proteomic Expression Profiles. Radiat. Res. 2022, 198, 573–581. [Google Scholar] [CrossRef]
- Tsuboi, A.; Urabe, Y.; Oka, S.; Sumioka, A.; Iio, S.; Yuge, R.; Hayashi, R.; Kuwai, T.; Kitadai, Y.; Kuraoka, K.; et al. Genomic analysis for the prediction of prognosis in small-bowel cancer. PLoS ONE 2021, 16, e0241454. [Google Scholar] [CrossRef]
- West, C.M.; Barnett, G.C. Genetics and genomics of radiotherapy toxicity: Towards prediction. Genome Med. 2011, 3, 52. [Google Scholar] [CrossRef]
- Amundson, S.A. The transcriptomic revolution and radiation biology. Int. J. Radiat. Biol. 2022, 98, 428–438. [Google Scholar] [CrossRef]
- Jiang, W.; Jones, J.C.; Shankavaram, U.; Sproull, M.; Camphausen, K.; Krauze, A.V. Analytical Considerations of Large-Scale Aptamer-Based Datasets for Translational Applications. Cancers 2022, 14, 2227. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, N.; Ji, N.; Chen, Z.S. Proteomics technologies for cancer liquid biopsies. Mol. Cancer 2022, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Pietrowska, M.; Widlak, P. Systemic effects of ionizing radiation at the proteome and metabolome levels in the blood of cancer patients treated with radiotherapy: The influence of inflammation and radiation toxicity. Int. J. Radiat. Biol. 2017, 93, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Albulescu, R.; Neagu, M. Proteomic Approaches for Biomarker Panels in Cancer. J. Immunoass. Immunochem. 2016, 37, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Walker, J.J.; Wilcox, S.K.; Williams, S. Advances in human proteomics at high scale with the SOMAscan proteomics platform. New Biotechnol. 2012, 29, 543–549. [Google Scholar] [CrossRef]
- Leite, G.G.S.; Weitsman, S.; Parodi, G.; Celly, S.; Sedighi, R.; Sanchez, M.; Morales, W.; Villanueva-Millan, M.J.; Barlow, G.M.; Mathur, R.; et al. Mapping the Segmental Microbiomes in the Human Small Bowel in Comparison with Stool: A REIMAGINE Study. Dig. Dis. Sci. 2020, 65, 2595–2604. [Google Scholar] [CrossRef]
- Flemer, B.; Lynch, D.B.; Brown, J.M.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Iadsee, N.; Chuaypen, N.; Techaawiwattanaboon, T.; Jinato, T.; Malakorn, S.; Petchlorlian, A.; Praditpornsilpa, K.; Patarakul, K. Identification of a novel gut microbiota signature associated with colorectal cancer in Thai population. Sci. Rep. 2023, 13, 6702. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?cond=microbiome&term=radiation&aggFilters=status:rec&viewType=Table&page=2 (accessed on 1 November 2023).
- Qian, X.; Zhang, H.Y.; Li, Q.L.; Ma, G.J.; Chen, Z.; Ji, X.M.; Li, C.Y.; Zhang, A.Q. Integrated microbiome, metabolome, and proteome analysis identifies a novel interplay among commensal bacteria, metabolites and candidate targets in non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e947. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dai, X.; Zhou, C.C.; Li, K.X.; Zhang, Y.J.; Lou, X.Y.; Zhu, Y.M.; Sun, Y.L.; Peng, B.X.; Cui, W. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut 2022, 71, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Dublineau, I.; Dudoignon, N.; Monti, P.; Combes, O.; Wysocki, J.; Grison, S.; Baudelin, C.; Griffiths, N.M.; Scanff, P. Screening of a large panel of gastrointestinal peptide plasma levels is not adapted for the evaluation of digestive damage following irradiation. Can. J. Physiol. Pharmacol. 2004, 82, 103–113. [Google Scholar] [CrossRef]
- Onal, C.; Kotek, A.; Unal, B.; Arslan, G.; Yavuz, A.; Topkan, E.; Yavuz, M. Plasma citrulline levels predict intestinal toxicity in patients treated with pelvic radiotherapy. Acta Oncol. 2011, 50, 1167–1174. [Google Scholar] [CrossRef]

| Number of Fractions | Volume (cm3) | Volume Max (Gy) | Max Point Dose (Gy) * | Endpoint (Grade ≥ 3) |
|---|---|---|---|---|
| 1 | <5 | 17.4 | 22 | Ulceration |
| 2 | <5 | 20 | 26 | |
| 3 | <5 | 22.5 | 30 | |
| 4 | <5 | 25 | 33.2 | |
| 5 | <5 | 26.5 | 35 | |
| 8 | <5 | 31.2 | 42 | |
| 10 | <5 | 33.9 | 45 | |
| 15 | <5 | 39 | 51 | |
| 20 | <5 | 42 | 54 | |
| 30 | <5 | 45 | 60 |
| Number of Fractions | Volume (cm3) | Volume Max (Gy) | Max Point Dose (Gy) * | Endpoint (Grade ≥ 3) |
|---|---|---|---|---|
| 1 | <30 | 17.6 | 20 | Enteritis/obstruction |
| 2 | <30 | 19.2 | 24 | |
| 3 | <30 | 20.7 | 28.5 | |
| 4 | <30 | 22.4 | 31.6 | |
| 5 | <30 | 24 | 34.5 | |
| 8 | <30 | 28.8 | 40 | |
| 10 | <120 | 33.9 | 41 | |
| 15 | <120 | 39 | 46.5 | |
| 20 | <120 | 42 | 50 | |
| 30 | <120 | 45 | 54 |
| Max Critical Volume above Threshold | One Fraction | Three Fractions | Five Fractions | ||||
|---|---|---|---|---|---|---|---|
| Threshold Dose (Gy) | Max Point Dose (Gy) | Threshold Dose (Gy) | Max Point Dose (Gy) | Threshold Dose (Gy) | Max Point Dose (Gy) | ||
| Duodenum | <5 cc | 11.2 | 12.4 | 16.5 (5.5 Gy/fx) | 22.2 (7.4 Gy/fx) | 18 (3.6 Gy/fx) | 32 (6.4 Gy/fx) |
| <10 cc | 9 | 11.4 (3.8 Gy/fx) | 12.5 (2.5 Gy/fx) | - | |||
| Jejunum/Ileum | <5 cc | 11.9 | 15.4 | 17.7 (5.9 Gy/fx) | 25.2 (8.4 Gy/fx) | 19.5 (3.9 Gy/fx) | 35 (7 Gy/fx) |
| 2017 | 2022 | |||||
|---|---|---|---|---|---|---|
| Constraint | Optimal | Mandatory | Constraint | Optimal | Mandatory | |
| 1 fx | - | - | - | D0.1 cc | - | 12.4 Gy |
| - | - | - | D10 cc | - | 9 Gy | |
| 3 fx | DMax (0.5 cm3) | - | <22.2 Gy | D0.1 cc | - | 22.2 Gy |
| D5 cm3 | - | <16.5 Gy | D10 cc | - | 11.4 Gy | |
| D10 cm3 | - | <11.4 Gy | ||||
| 5 fx | DMax (0.5 cm3) | - | <35 Gy | D0.1 cc | 33 Gy | 35 Gy |
| D1 cm3 | <33 Gy | - | D10 cc | 25 Gy | - | |
| D5 cm3 | <25 Gy | - | ||||
| D9 cm3 | <15 Gy | - | ||||
| D10 cm3 | - | <25 Gy | ||||
| 2017 | 2022 | |||||
|---|---|---|---|---|---|---|
| Constraint | Optimal | Mandatory | Constraint | Optimal | Mandatory | |
| 1 fx | - | - | - | D0.1 cc | - | 15.4 Gy |
| D5 cc | - | 11.9 Gy | ||||
| 3 fx | DMax (0.5 cm3) | - | <25.2 Gy | D0.1 cc | - | 25.2 Gy |
| D5 cm3 | - | <17.7 Gy | D5 cc | - | 17.7 Gy | |
| D10 cm3 | - | <11.4 Gy | ||||
| 5 fx | DMax (0.5 cm3) | <30 Gy | <35 Gy | D0.1 cc | 30 Gy | 35 Gy |
| D1 cm3 | - | - | D10 cc | 25 Gy | - | |
| D5 cm3 | <25 Gy | - | ||||
| D9 cm3 | - | - | ||||
| D10 cm3 | <25 Gy | - | ||||
| Study Name | Intervention | Microbiome Analysis |
|---|---|---|
| Pelvic Primaries | ||
| Study to Detect Changes in Urinary and Gut Microbiome During Androgen Deprivation Therapy and Radiation Therapy in Patients with Prostate Cancer | Androgen deprivation therapy and Radiation Therapy | Stool and urine |
| L. Plantarum 299v and Gut Microbiome, Diarrhea, and Clostridioides Difficile Infection in Colorectal Cancer Patients | Dietary Supplement: Sanprobi IBS®/chemotherapy and radiation | Stool |
| The Gut Microbiome and Immune Checkpoint Inhibitor Therapy in Solid Tumors (NSCLC, MM, TNBC or RCC, Stage 1–4) | Checkpoint inhibitor, immune | Stool |
| Non-Pelvic primaries | ||
| The Association Between Radiation Dermatitis and Skin Microbiome in Breast Cancer Patients | Post-operative radiotherapy | Skin |
| Assessing the Impact of the Microbiome on Breast Cancer Radiotherapy Toxicity | Stool sample and skin swab sample | Skin and stool |
| Correlation of Fecal Microbiome and Its Metabolites with Outcome of Radiotherapy in Head and Neck Carcinoma | Radiotherapy | Stool and serum |
| THERApeutic Outcomes Related to Gut microBIOME in Glioblastoma (GBM) Patients Receiving Chemo-radiation (THERABIOME-GBM) | Chemoradiation | Stool |
| Study/Author (Year) | Study Design | Biomarker | Outcome |
|---|---|---|---|
| Dublineau [67] (2004) | Pre-clinical | Gastrointestinal peptide plasma levels | Changes in gastrin and neurotensin plasma levels were associated with structural alterations in the stomach and ileum, respectively. |
| Onal [68] (2011) | Prospective | Plasma citrulline levels | Citrulline concentration changes significantly differed during treatment according to RTOG intestinal toxicity grades. |
| West [54] (2011) | Review | Genetic variation (SNPs) | It is impossible to say with certainty whether any genetic variations predispose patients to toxicity. |
| Guo [46] (2020) | Pre-clinical | Gut microbiome | Gut microbiome contributes substantially to radioprotection. |
| Liu [45] (2021) | Review | Gut microbiome | Underlying mechanisms are still obscure, and more research is needed to clarify the links between the gut microbiome and variations in RT response. |
| Oh [47] (2021) | Review | Gut microbiome | Toxicity of RT was related to dysbiosis of the gut microbiome. |
| Sproull [52] (2022) | Analysis | Plasma proteomic expression profiles | Identified novel panels of radiation-responsive proteins useful for predicting radiation exposure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yariv, O.; Camphausen, K.; Krauze, A.V. Small Bowel Dose Constraints in Radiation Therapy—Where Omics-Driven Biomarkers and Bioinformatics Can Take Us in the Future. BioMedInformatics 2024, 4, 158-172. https://doi.org/10.3390/biomedinformatics4010011
Yariv O, Camphausen K, Krauze AV. Small Bowel Dose Constraints in Radiation Therapy—Where Omics-Driven Biomarkers and Bioinformatics Can Take Us in the Future. BioMedInformatics. 2024; 4(1):158-172. https://doi.org/10.3390/biomedinformatics4010011
Chicago/Turabian StyleYariv, Orly, Kevin Camphausen, and Andra V. Krauze. 2024. "Small Bowel Dose Constraints in Radiation Therapy—Where Omics-Driven Biomarkers and Bioinformatics Can Take Us in the Future" BioMedInformatics 4, no. 1: 158-172. https://doi.org/10.3390/biomedinformatics4010011
APA StyleYariv, O., Camphausen, K., & Krauze, A. V. (2024). Small Bowel Dose Constraints in Radiation Therapy—Where Omics-Driven Biomarkers and Bioinformatics Can Take Us in the Future. BioMedInformatics, 4(1), 158-172. https://doi.org/10.3390/biomedinformatics4010011