Utilizing Immunoinformatics for mRNA Vaccine Design against Influenza D Virus
Abstract
1. Introduction
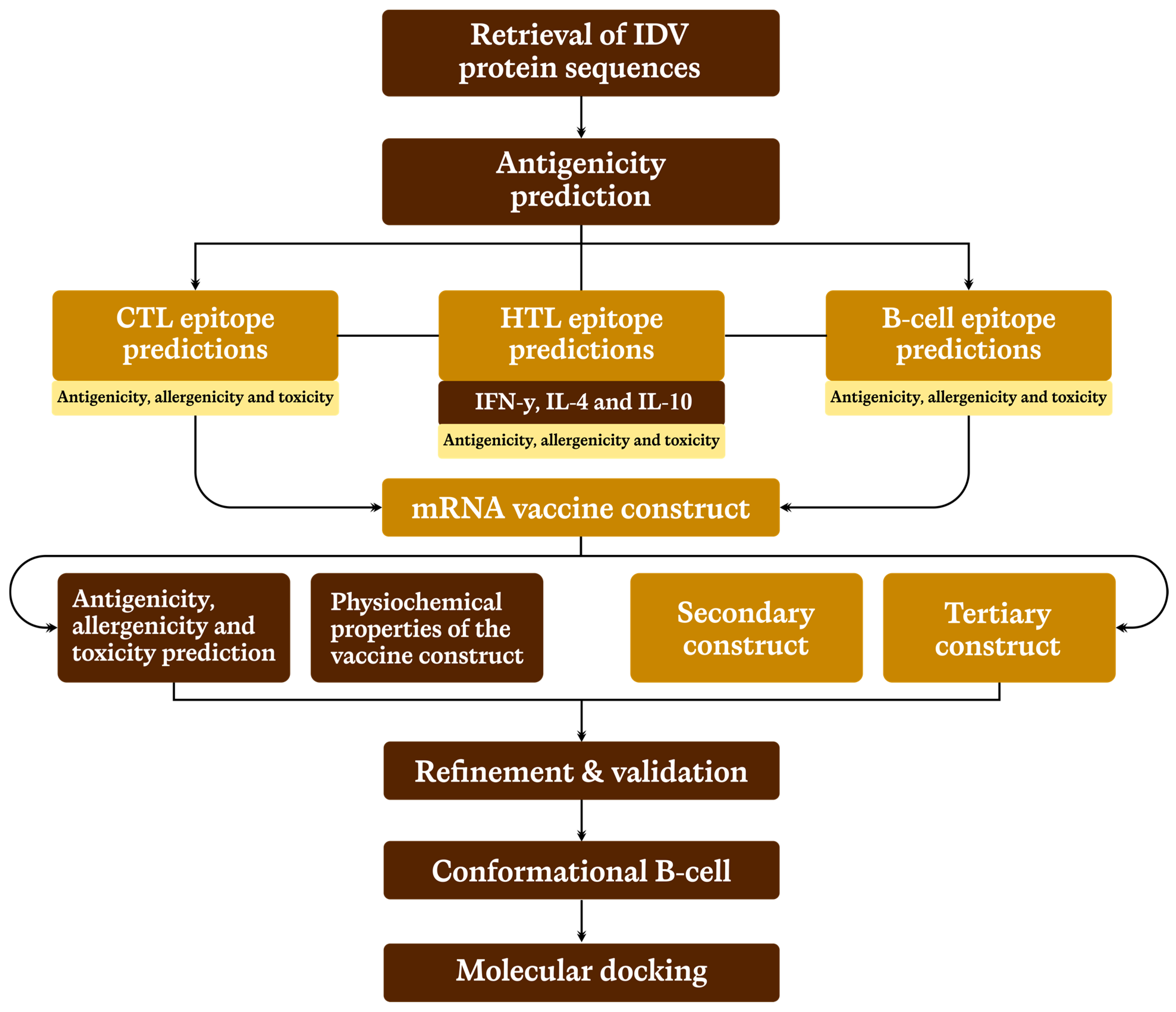
2. Methodology
2.1. Selection and Retrieval of Target Protein Sequences
2.2. Prediction of Cytotoxic T-Cell Lymphocyte (CTL) Epitope
2.3. Prediction of Helper T-Lymphocytes (HTL) Epitope
2.4. Linear B-Lymphocyte (LBL) Epitope Prediction
2.5. Prediction of Conformational B-Cells Epitopes
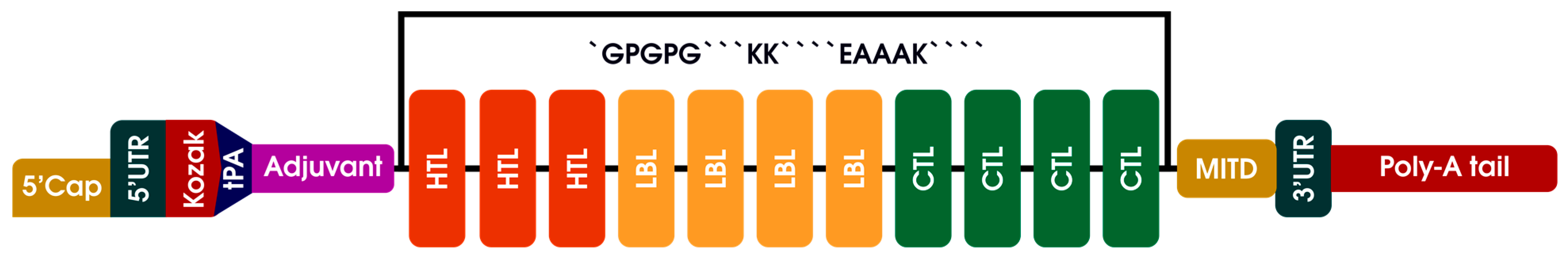
2.6. mRNA Primary Vaccine Construction
2.7. Prediction of Antigenicity, Toxicity and Allergenicity of Vaccine Candidate
2.8. Prediction of Physicochemical Properties of the Vaccine Candidate
2.9. Prediction of the Solubility Properties of the Vaccine Construct
2.10. Prediction of Secondary Vaccine Construct
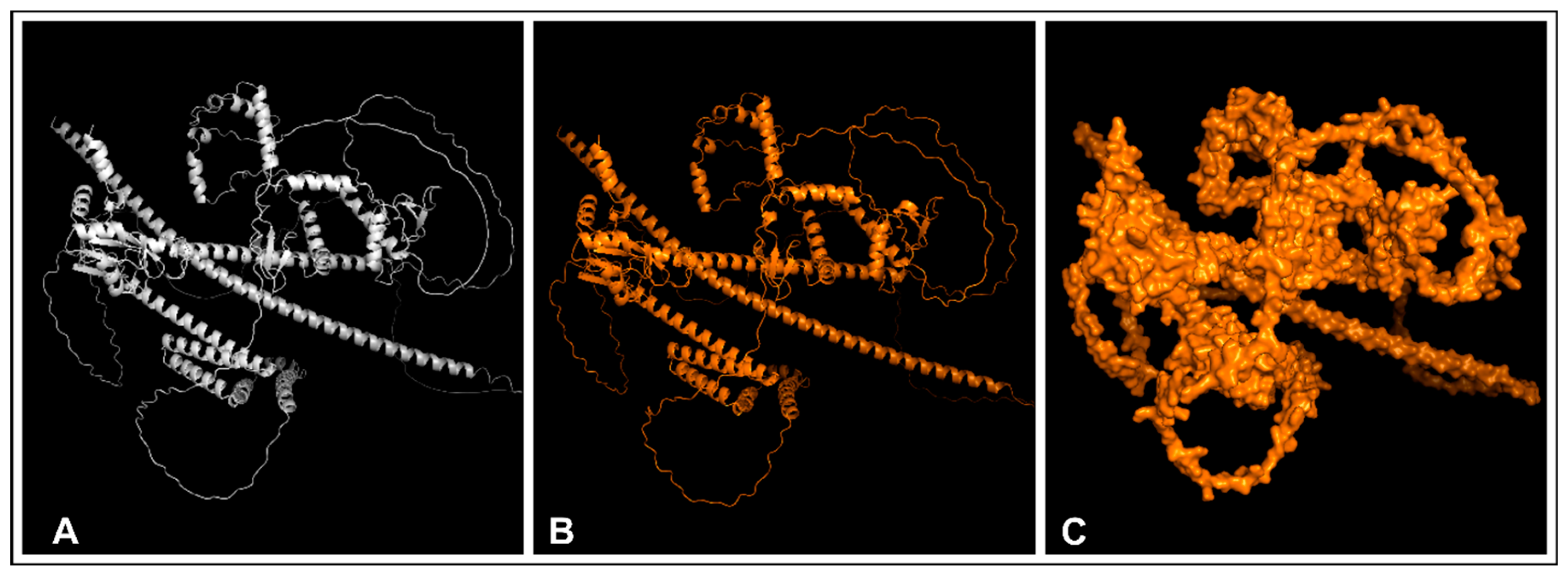
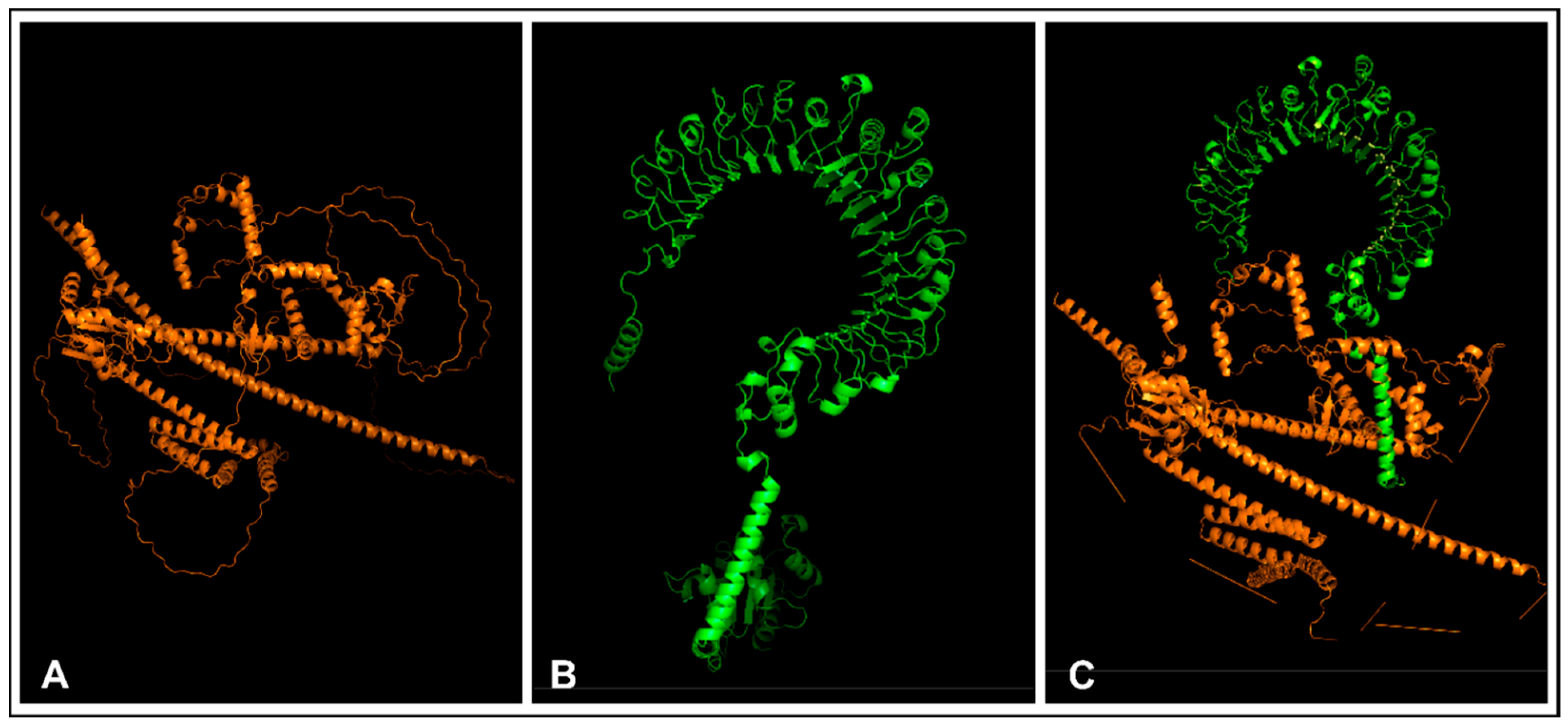
2.11. Tertiary Vaccine Construct
2.12. Refinement and Validation of the Tertiary Vaccine Construct
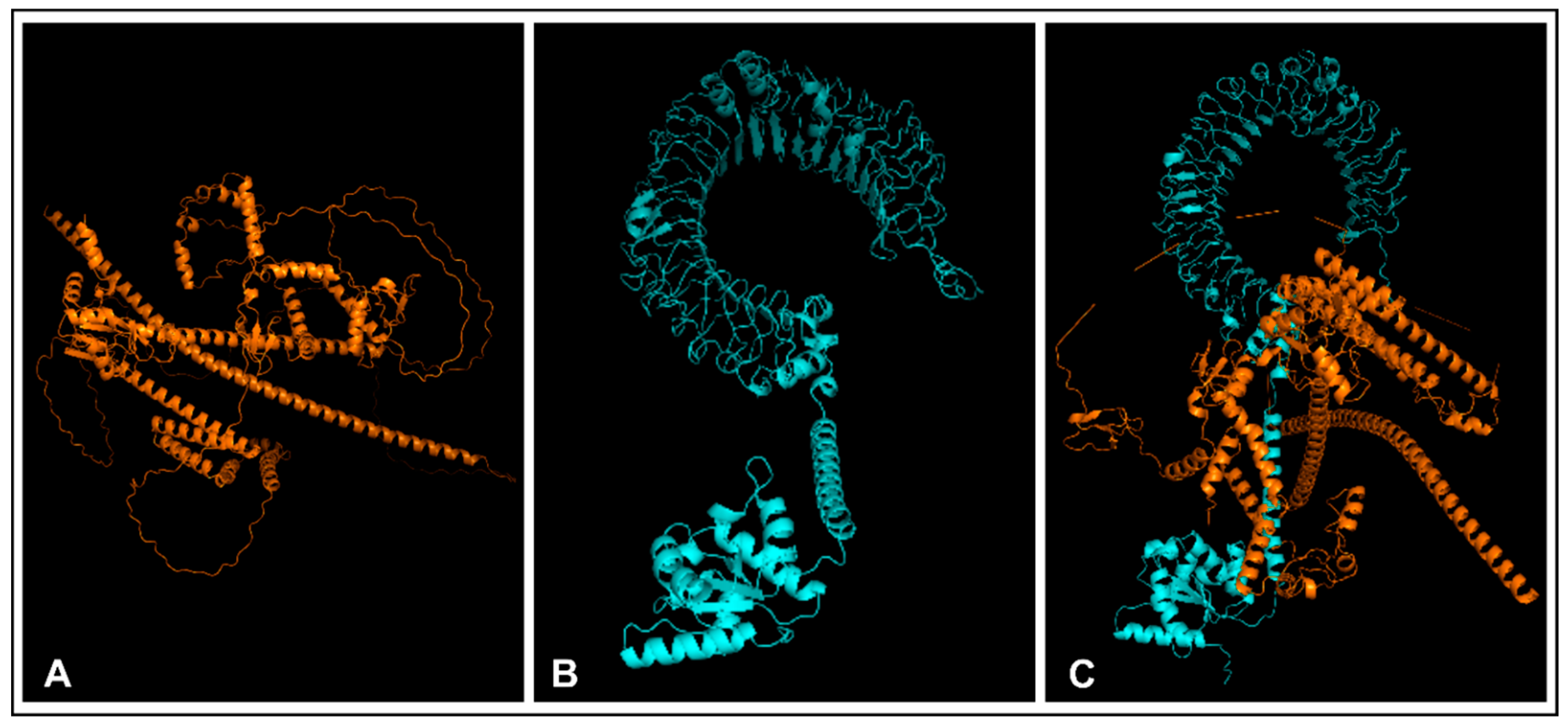
2.13. Molecular Docking
3. Results
3.1. Selection and Retrieval of Target Protein Sequences
3.2. Evaluation of Cytotoxic T-Cell Lymphocyte Epitope
3.3. Evaluation of Helper T-Lymphocytes (HTL) Epitope
3.4. Prediction and Evaluation of Linear B-Cell Epitope
3.5. Prediction and Evaluation of Conformational B-Cells Epitopes
3.6. mRNA Primary Vaccine Construction
3.7. Prediction of Antigenicity, Toxicity and Allergenicity of Vaccine Candidate
3.8. Prediction of Physicochemical Properties of the Vaccine Candidate
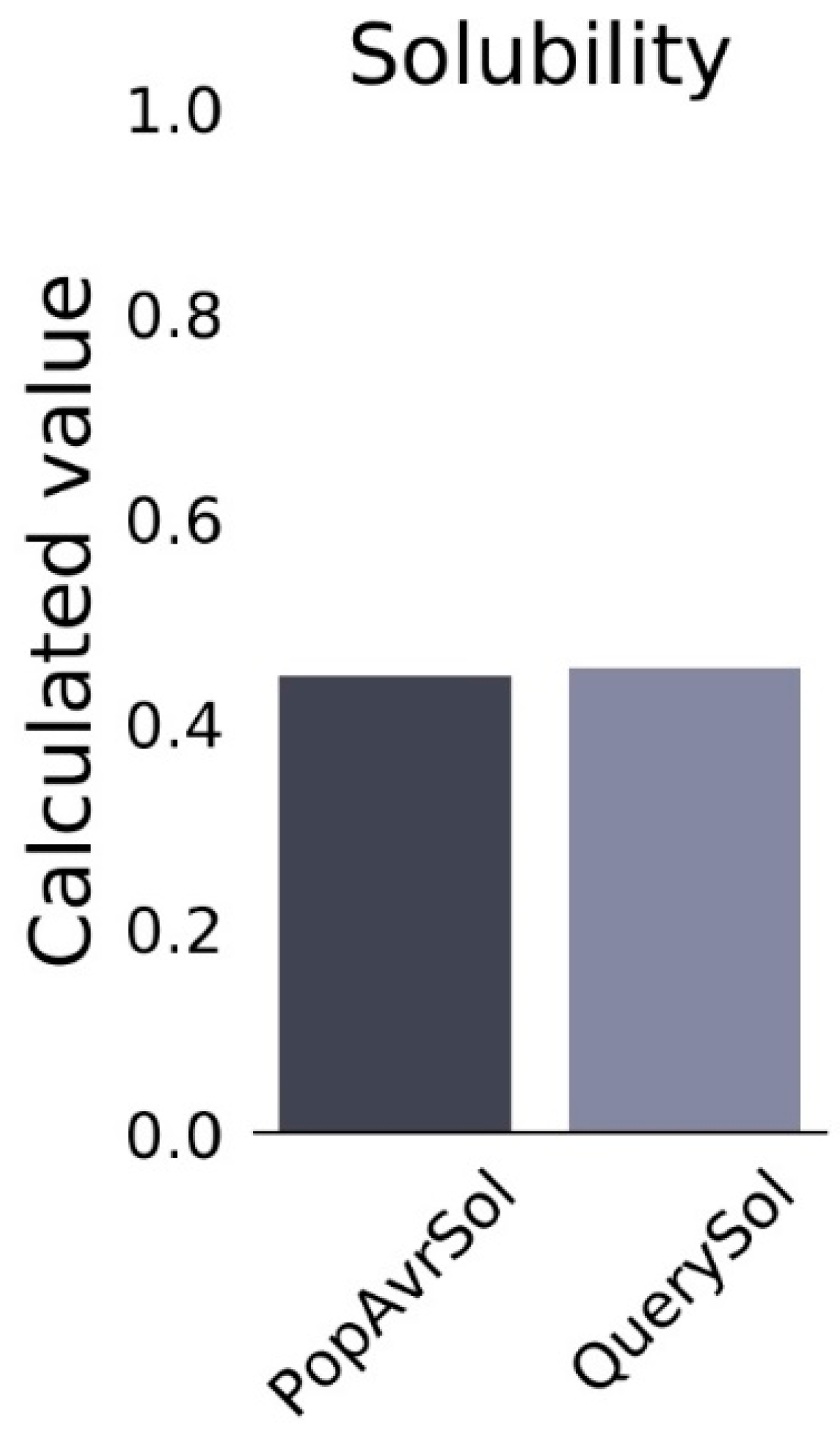
3.9. Prediction of the Solubility Properties of the Vaccine Construct
3.10. Secondary Vaccine Construct
3.11. Tertiary Vaccine Construction, Refinement, and Validation of the Construct
3.12. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. (Eds.) Virus Taxonomy; Family—Orthomyxoviridae; Elsevier: San Diego, CA, USA, 2012; pp. 749–761. [Google Scholar]
- Hause, B.M.; Collin, E.A.; Liu, R.; Huang, B.; Sheng, Z.; Lu, W.; Wang, D.; Nelson, E.A.; Li, F. Characterization of a Novel Influenza Virus in Cattle and Swine: Proposal for a New Genus in the Orthomyxoviridae Family. mBio 2014, 5, e00031-14. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Kumar, B. Emerging influenza D virus threat: What we know so far! J. Clin. Med. 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Collin, E.A.; Sheng, Z.; Lang, Y.; Ma, W.; Hause, B.M.; Li, F. Cocirculation of two distinct genetic and antigenic lineages of proposed influenza D virus in cattle. J. Virol. 2015, 89, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Nedland, H.; Wollman, J.; Sreenivasan, C.; Quast, M.; Singrey, A.; Fawcett, L.; Christopher-Hennings, J.; Nelson, E.; Kaushik, R.S.; Wang, D. Serological evidence for the co-circulation of two lineages of influenza D viruses in equine populations of the Midwest United States. Zoonoses Public Health 2018, 65, e148–e154. [Google Scholar] [CrossRef] [PubMed]
- Salem, E.; Cook EA, J.; Lbacha, H.A.; Oliva, J.; Awoume, F.; Aplogan, G.L.; Hymann, E.C.; Muloi, D.; Deem, S.L.; Alali, S.; et al. Serologic Evidence for Influenza C and D Virus among Ruminants and Camelids, Africa, 1991–2015. Emerg. Infect. Dis. 2017, 23, 1556–1559. [Google Scholar] [CrossRef]
- Kumar, B.; Asha, K.; Khanna, M.; Ronsard, L.; Meseko, C.A.; Sanicas, M. The emerging influenza virus threat: Status and new prospects for its therapy and control. Arch. Virol. 2018, 163, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013, 9, e1003176. [Google Scholar] [CrossRef] [PubMed]
- Molini, U.; Curini, V.; Jacobs, E.; Tongo, E.; Berjaoui, S.; Hemberger, M.Y.; Puglia, I.; Jago, M.; Khaiseb, S.; Cattoli, G.; et al. First influenza D virus full-genome sequence retrieved from livestock in Namibia, Africa. Acta Trop. 2022, 232, 106482. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hika, B.; Liu, R.; Sheng, Z.; Hause, B.M.; Li, F.; Wang, D. The Hemagglutinin-Esterase Fusion Glycoprotein Is a Primary Determinant of the Exceptional Thermal and Acid Stability of Influenza D Virus. MSphere 2017, 2, e00254-17. [Google Scholar] [CrossRef]
- Katayama, M.; Murakami, S.; Ishida, H.; Matsugo, H.; Sekine, W.; Ohira, K.; Takenaka-Uema, A.; Horimoto, T. Antigenic commonality and divergence of hemagglutinin-esterase-fusion protein among influenza D virus lineages revealed using epitope mapping. J. Virol. 2024, 98, e0190823. [Google Scholar] [CrossRef]
- Gray, G.C.; Robie, E.R.; Studstill, C.J.; Nunn, C.L. Mitigating future respiratory virus pandemics: New threats and approaches to consider. Viruses 2021, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, T.; Hiono, T.; Mekata, H.; Odagiri, T.; Lei, Z.; Kobayashi, T.; Norimine, J.; Inoshima, Y.; Hikono, H.; Murakami, K.; et al. Nationwide Distribution of Bovine Influenza D Virus Infection in Japan. PLoS ONE 2016, 11, e0163828. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.; Cernicchiaro, N.; Torres, S.; Li, F.; Hause, B.M. Metagenomic characterization of the virome associated with bovine respiratory disease in feedlot cattle identified novel viruses and suggests an etiologic role for the influenza D virus. J. Gen. Virol. 2016, 97, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sheng, Z.; Huang, C.; Wang, D.; Li, F. Influenza D virus. Curr. Opin. Virol. 2020, 44, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Leibler, J.H.; Abdelgadir, A.; Seidel, J.; White, R.F.; Johnson, W.E.; Reynolds, S.J.; Gray, G.C.; Schaeffer, J.W. Influenza D virus exposure among US cattle workers: A call for surveillance. Zoonoses Public Health 2023, 70, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef]
- Techakriengkrai, N.; Nedumpun, T.; Golde, W.T.; Suradhat, S. Diversity of the swine leukocyte antigen class I and II in commercial pig populations. Front. Vet. Sci. 2021, 8, 637682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I.A. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemi, O.O.; Oladipo, E.K.; Dairo, E.O.; Ayeni, A.E.; Irewolede, B.A.; Jimah, E.M.; Ogunleye, A.J. Computational construction of a glycoprotein multi-epitope subunit vaccine candidate for old and new South-African SARS-CoV-2 virus strains. Inform. Med. Unlocked 2022, 28, 100845. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Andreatta, M.; Trolle, T.; Yan, Z.; Greenbaum, J.A.; Peters, B.; Nielsen, M. An automated benchmarking platform for MHC class II binding prediction methods. Bioinformatics 2018, 34, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Vir, P.; Raghava, G.P. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct 2013, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Gupta, S.; Vir, P.; Raghava, G.P. Prediction of IL4-inducing peptides. Clin. Dev. Immunol. 2013, 2013, 263952. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, G.; Usmani, S.S.; Dhanda, S.K.; Kaur, H.; Singh, S.; Sharma, M.; Raghava, G.P. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Sci. Rep. 2017, 7, 42851. [Google Scholar] [CrossRef]
- Galanis, K.A.; Nastou, K.C.; Papandreou, N.C.; Petichakis, G.N.; Pigis, D.G.; Iconomidou, V.A. Linear B-Cell Epitope Prediction for In Silico Vaccine Design: A Performance Review of Methods Available via Command-Line Interface. Int. J. Mol. Sci. 2021, 22, 3210. [Google Scholar] [CrossRef]
- Oladipo, E.K.; Adeniyi, M.O.; Ogunlowo, M.T.; Irewolede, B.A.; Adekanola, V.O.; Oluseyi, G.S.; Omilola, J.A.; Udoh, A.F.; Olufemi, S.E.; Adediran, D.A.; et al. Bioinformatics Designing and Molecular Modelling of a Universal mRNA Vaccine for SARS-CoV-2 Infection. Vaccines 2022, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P.S. Prediction of Continuous B-cell Epitopes in an Antigen Using Recurrent Neural Network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Coudert, E.; Gehant, S.; De Castro, E.; Pozzato, M.; Baratin, D.; Neto, T.; Sigrist, C.J.; Redaschi, N.; Bridge, A.; Consortium, T.U.; et al. Annotation of biologically relevant ligands in UniProtKB using ChEBI. Bioinformatics 2023, 39, btac793. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Hebditch, M.; Carballo-Amador, M.A.; Charonis, S.; Curtis, R.; Warwicker, J. Protein–Sol: A web tool for predicting protein solubility from sequence. Bioinformatics 2017, 33, 3098–3100. [Google Scholar] [CrossRef]
- Deléage, G. ALIGNSEC: Viewing protein secondary structure predictions within large multiple sequence alignments. Bioinformatics 2017, 33, 3991–3992. [Google Scholar] [CrossRef] [PubMed]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement is driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Thornton, J.M. PROCHECK: Validation of protein structure coordinates. In International Tables of Crystallography, Volume, F: Crystallography of Biological Macromolecules; Rossmann, M.G., Arnold, E., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2001; pp. 722–725. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Puig, A.; Bassols, M.; Fraile, L.; Armengol, R. Influenza D virus: A review and update of its role in bovine respiratory syndrome. Viruses 2022, 14, 2717. [Google Scholar] [CrossRef] [PubMed]
- Brun, A. Vaccines and Vaccination for Veterinary Viral Diseases: A General Overview. Vaccine Technol. Vet. Viral Dis. 2016, 1349, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Saegerman, C.; Gaudino, M.; Savard, C.; André Broes Ariel, O.; Meyer, G.; Ducatez, M. Influenza D virus in respiratory disease in Canadian, province of Québec, cattle: Relative importance and evidence of new reassortment between different clades. Transbound. Emerg. Dis. 2021, 69, 1227–1245. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, E.K.; Akindiya, O.E.; Oluwasanya, G.J.; Akanbi, G.M.; Olufemi, S.E.; Adediran, D.A.; Babalola, M.O. Bioinformatics analysis of structural protein to approach a vaccine candidate against Vibrio cholerae infection. Immunogenetics 2023, 75, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Oyarzun, P.; Kobe, B. Computer-aided design of T-cell epitope-based vaccines: Addressing population coverage. Int. J. Immunogenet. 2015, 42, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemi, O.O.; Oladipo, E.K.; Kolawole, O.M.; Oloke, J.K.; Adelusi, T.I.; Irewolede, B.A.; Dairo, E.O.; Ayeni, A.E.; Kolapo, K.T.; Akindiya, O.E.; et al. Bioinformatics, Computational Informatics, and Modeling Approaches to the Design of mRNA COVID-19 Vaccine Candidates. Computation 2022, 10, 117. [Google Scholar] [CrossRef]
- Oladipo, E.K.; Ogunmolu, M.D.; Oyelakin, O.D.; Hammed, S.O.; Oluwasanya, G.J.; Hammed, S.O.; Onyeaka, H. Exploring the Potentials of Structural Proteins: Towards a Mrna Vaccine Candidate Against Marburg Virus Disease. Preprints 2023. [Google Scholar] [CrossRef]
- Oladipo, E.K.; Ajayi, A.F.; Ariyo, O.E.; Onile, S.O.; Jimah, E.M.; Ezediuno, L.O.; Adebayo, O.I.; Adebayo, E.T.; Odeyemi, A.N.; Oyeleke, M.O.; et al. Exploration of surface glycoprotein to design multi-epitope vaccine for the prevention of Covid-19. Inform. Med. Unlocked 2019, 21, 100438. [Google Scholar] [CrossRef]
- Dey, J.; Mahapatra, S.R.; Raj, T.K.; Kaur, T.; Jain, P.; Tiwari, A. Designing a novel multi-epitope vaccine to evoke a robust immune response against pathogenic multidrug-resistant Enterococcus faecium bacterium. Gut Pathog. 2022, 14, 21. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Nezafat, N.; Negahdaripour, M.; Eskandari, S.; Zamani, M. In silico design and evaluation of a novel mRNA vaccine against BK virus: A reverse vaccinology approach. Immunol. Res. 2023, 71, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef] [PubMed]
- Althurwi, H.N.; Alharthy, K.M.; Albaqami, F.F.; Altharawi, A.; Javed, M.R.; Muhseen, Z.T.; Tahir ul Qamar, M. mRNA-Based Vaccine Designing against Epstein-Barr Virus to Induce an Immune Response Using Immunoinformatic and Molecular Modelling Approaches. Int. J. Environ. Res. Public Health 2022, 19, 13054. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Chen, Z. Insights into the Molecular Mechanisms of Protein-Ligand Interactions by Molecular Docking and Molecular Dynamics Simulation: A Case of Oligopeptide Binding Protein. Comput. Math. Methods Med. 2018, 2018, 3502514. [Google Scholar] [CrossRef]



| Continent | Isolates |
|---|---|
| Africa | 1 |
| Europe | 25 |
| North America | 43 |
| South America | 4 |
| Asia | 14 |
| Oceania | 1 |
| CTL Epitopes | Antigenicity | Allergenicity | Toxicity | Immunogenicity |
|---|---|---|---|---|
| VANISMNLK | Antigen | Non-allergen | Non-toxic | 1.6606 |
| YSIKSTPRF | Antigen | Non-allergen | Non-toxic | 1.4093 |
| VNPRASPQV | Antigen | Non-allergen | Non-toxic | 1.4867 |
| RGIAGSRVK | Antigen | Non-allergen | Non-toxic | 1.3654 |
| AERELICIV | Antigen | Non-allergen | Non-toxic | 1.3012 |
| AEKELICIV | Antigen | Non-allergen | Non-toxic | 1.2598 |
| GDIEMRQLL | Antigen | Non-allergen | Non-toxic | 1.2738 |
| VNPRASSQV | Antigen | Non-allergen | Non-toxic | 1.2661 |
| Epitopes | Antigenicity | IL-4 | IL-10 | Interferon | Immunogenicity |
|---|---|---|---|---|---|
| GLLFVGFVAGGVAGG | Antigen | Inducer | Inducer | Positive | 0.53738 |
| AGGYFWGRSNERGGG | Antigen | Inducer | Inducer | Positive | 0.4476 |
| VAGGYFWGRSNERGG | Antigen | Inducer | Inducer | Positive | 0.44493 |
| LTTITAITACQAERE | Antigen | Inducer | Inducer | Positive | 0.41419 |
| NRVAAYRGIASAEVK | Antigen | Inducer | Inducer | Positive | 0.32272 |
| VAGGYFWGRSNERGG | Antigen | Inducer | Inducer | Positive | 0.44493 |
| NRVAAYRGIASAEVK | Antigen | Inducer | Inducer | Positive | 0.32272 |
| AGGYFWGRSSERGGG | Antigen | Inducer | Inducer | Positive | 0.2928 |
| SYCFDTDGGYPIQVV | Antigen | Inducer | Inducer | Positive | 0.28454 |
| RSYCFDTDGGYPIQV | Antigen | Inducer | Inducer | Positive | 0.23615 |
| B-Cell Epitopes | Allergenicity | Toxicity | Immunogenicity |
|---|---|---|---|
| FGLLFIGFVAGGVAGGYF | Non-allergen | Non-toxic | 0.65678 |
| PEAGIDCFGLNWTQTK | Non-allergen | Non-toxic | 0.45256 |
| GGIAQEAGPGCWYIDS | Non-allergen | Non-toxic | 0.44557 |
| TAITACQAERELICIVQR | Non-allergen | Non-toxic | 0.3973 |
| GGIAQEAGPGCWYVDS | Non-allergen | Non-toxic | 0.36809 |
| LGSTIALCLLGLVAIAAF | Non-allergen | Non-toxic | 0.34982 |
| KTTPYAGEADDNHGDI | Non-allergen | Non-toxic | 0.31345 |
| GYFWGRSNGRGGGASV | Non-allergen | Non-toxic | 0.29455 |
| ASVINQKDWIGFGDSR | Non-allergen | Non-toxic | 0.28608 |
| KRGGGIAQEAGP | Non-allergen | Non-toxic | 0.28007 |
| TKRGGGIAQEA | Non-allergen | Non-toxic | 0.24407 |
| TKRGGGIAQE | Non-allergen | Non-toxic | 0.20752 |
| KRGGGIAQEA | Non-allergen | Non-toxic | 0.20527 |
| ASVINQKDWVGFGDSR | Non-allergen | Non-toxic | 0.19668 |
| TKVVITSDPYYWGSTI | Non-allergen | Non-toxic | 0.1955 |
| GYRGIAPGTYSIRSTP | Non-allergen | Non-toxic | 0.17528 |
| SPLWYAESSVNPGARP | Non-allergen | Non-toxic | 0.14735 |
| Property | Value |
|---|---|
| Molecular weight | 110,576.04 Da |
| Number of Amino acids | 1058 |
| Theoretical pI | 8.47 |
| Total number of negatively charged residues (Asp and Glu) | 101 |
| Total number of positively charged residues (Arg and Lys) | 118 |
| Formula | C4822H2727N1347O1475S75 |
| Total number of atoms | 15,446 |
| Estimated half-life [The N-terminal of the sequence considered is A (Ala)] | 30 h (mammalian reticulocytes, in vitro) >20 h (yeast, in vivo) >10 h (Escherichia coli, in vivo) |
| Instability index | 35.40 (<40) |
| Aliphatic index | 79.04 (>50) |
| Grand average of hydropathicity (GRAVY) | 0.058 |
| Predicted Scaled Solubility | 0.454 |
| Name of the Examined Unit | Number of Residues | Percentages |
|---|---|---|
| Alpha-helix | 501 | 47.35% |
| 310 helixes | 0 | 0.00% |
| Beta bridge | 0 | 0.00% |
| Extended strand | 151 | 14.27% |
| Beta turn | 0 | 0.00% |
| Random coil | 406 | 38.37% |
| Other states | 0 | 0.00% |
| Docking Complexes | Docking Score | Confidence Score | Ligand RMSD (Å) |
|---|---|---|---|
| Construct and TLR-2 | −359.19 | 0.9850 | 115.12 |
| Construct and TLR-4 | −379.08 | 0.9899 | 67.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladipo, E.K.; Adeyemo, S.F.; Akinboade, M.W.; Akinleye, T.M.; Siyanbola, K.F.; Adeogun, P.A.; Ogunfidodo, V.M.; Adekunle, C.A.; Elutade, O.A.; Omoathebu, E.E.; et al. Utilizing Immunoinformatics for mRNA Vaccine Design against Influenza D Virus. BioMedInformatics 2024, 4, 1572-1588. https://doi.org/10.3390/biomedinformatics4020086
Oladipo EK, Adeyemo SF, Akinboade MW, Akinleye TM, Siyanbola KF, Adeogun PA, Ogunfidodo VM, Adekunle CA, Elutade OA, Omoathebu EE, et al. Utilizing Immunoinformatics for mRNA Vaccine Design against Influenza D Virus. BioMedInformatics. 2024; 4(2):1572-1588. https://doi.org/10.3390/biomedinformatics4020086
Chicago/Turabian StyleOladipo, Elijah Kolawole, Stephen Feranmi Adeyemo, Modinat Wuraola Akinboade, Temitope Michael Akinleye, Kehinde Favour Siyanbola, Precious Ayomide Adeogun, Victor Michael Ogunfidodo, Christiana Adewumi Adekunle, Olubunmi Ayobami Elutade, Esther Eghogho Omoathebu, and et al. 2024. "Utilizing Immunoinformatics for mRNA Vaccine Design against Influenza D Virus" BioMedInformatics 4, no. 2: 1572-1588. https://doi.org/10.3390/biomedinformatics4020086
APA StyleOladipo, E. K., Adeyemo, S. F., Akinboade, M. W., Akinleye, T. M., Siyanbola, K. F., Adeogun, P. A., Ogunfidodo, V. M., Adekunle, C. A., Elutade, O. A., Omoathebu, E. E., Taiwo, B. O., Akindiya, E. O., Ochola, L., & Onyeaka, H. (2024). Utilizing Immunoinformatics for mRNA Vaccine Design against Influenza D Virus. BioMedInformatics, 4(2), 1572-1588. https://doi.org/10.3390/biomedinformatics4020086









