Navigating the Complexity of Psychotic Disorders: A Systematic Review of EEG Microstates and Machine Learning
Abstract
:1. Introduction
1.1. EEG Microstates and Machine Learning
2. Materials and Methods
2.1. Eligibility Criteria
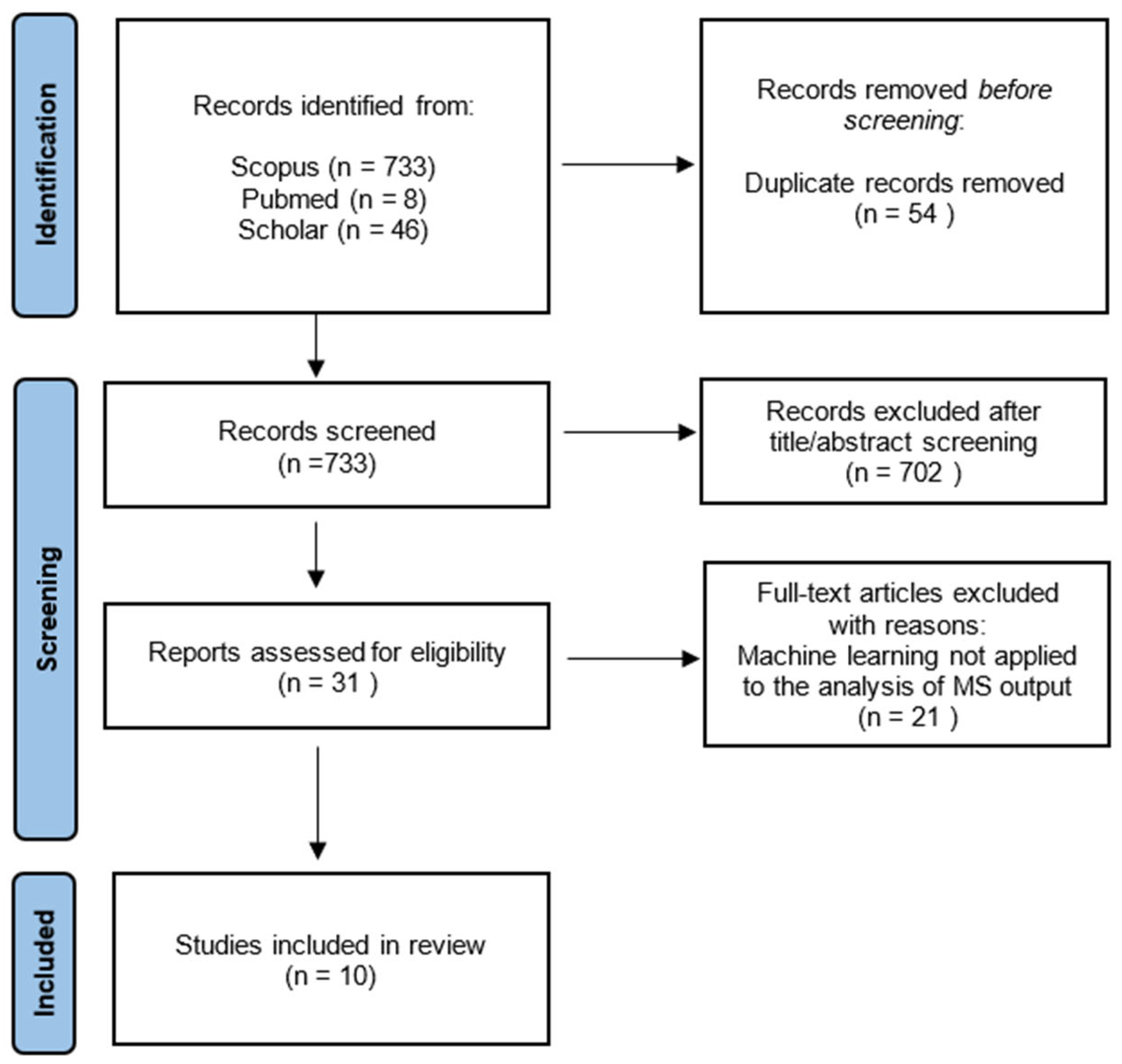
2.2. Information Sources and Search Strategy
2.3. Quality Assessment
3. Results
4. Discussion
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition Text Revision DSM-5-TRTM, 5th ed.; text rev; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, D.B. Psychosis. Contin. Lifelong Learn. Neurol. 2015, 21, 715. [Google Scholar] [CrossRef]
- Awad, P.A.G.; Voruganti, L. The Burden of Schizophrenia on Caregivers. PharmacoEconomics 2012, 26, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Millier, A.; Schmidt, U.; Angermeyer, M.; Chauhan, D.; Murthy, V.; Toumi, M.; Cadi-Soussi, N. Humanistic burden in schizophrenia: A literature review. J. Psychiatr. Res. 2014, 54, 85–93. [Google Scholar] [CrossRef]
- Hasson-Ohayon, I.; Kravetz, S.; Roe, D.; David, A.S.; Weiser, M. Insight into psychosis and quality of life. Compr. Psychiatry 2006, 47, 265–269. [Google Scholar] [CrossRef]
- Malla, A.; Payne, J. First-Episode Psychosis: Psychopathology, Quality of Life, and Functional Outcome. Schizophr. Bull. 2005, 31, 650–671. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “Just the Facts” What we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 2008, 102, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gejman, P.V.; Sanders, A.R.; Duan, J. The Role of Genetics in the Etiology of Schizophrenia. Psychiatr. Clin. N. Am. 2010, 33, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Kestler, L.; Bollini, A.; Hochman, K.M. Schizophrenia: Etiology and Course. Annu. Rev. Psychol. 2004, 55, 401–430. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Schizophrenia and the disconnection hypothesis. Acta Psychiatr. Scand. 1999, 99, 68–79. [Google Scholar] [CrossRef]
- Friston, K.J. The disconnection hypothesis. Schizophr. Res. 1998, 30, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, J.; Bosia, M.; Spangaro, M.; Martini, F.; Agostoni, G.; Cuoco, F.; Cocchi, F.; Cavallaro, R. Schizophrenia and psychedelic state: Dysconnection versus hyper-connection. A perspective on two different models of psychosis stemming from dysfunctional integration processes. Mol. Psychiatry 2023, 28, 59–67. [Google Scholar] [CrossRef]
- Addington, J.; Addington, D. Social and cognitive functioning in psychosis. Schizophr. Res. 2008, 99, 176–181. [Google Scholar] [CrossRef]
- Karbasforoushan, H.; Woodward, N.D. Resting-State Networks in Schizophrenia. Curr. Top. Med. Chem. 2012, 12, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Camchong, J.; MacDonald, A.W., III; Bell, C.; Mueller, B.A.; Lim, K.O. Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophr. Bull. 2011, 37, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Zong, X.-F.; Mann, J.J.; Zheng, J.-J.; Liao, Y.-H.; Li, Z.-C.; He, Y.; Chen, X.-G.; Tang, J.-S. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci. Bull. 2017, 33, 73–84. [Google Scholar] [CrossRef]
- Sapienza, J.; Pacchioni, F.; Spangaro, M.; Bosia, M. Dysconnection in schizophrenia: Filling the dots from old to new evidence. Clin. Neurophysiol. 2024, 162, 226–228. [Google Scholar] [CrossRef]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 2018, 180, 577–593. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D. Multichannel topography of human alpha EEG fields. Electroencephalogr. Clin. Neurophysiol. 1971, 31, 439–449. [Google Scholar] [CrossRef]
- Lehmann, D.; Ozaki, H.; Pal, I. EEG alpha map series: Brain micro-states by space-oriented adaptive segmentation. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 271–288. [Google Scholar] [CrossRef]
- Michel, C.M.; Murray, M.M. Towards the utilization of EEG as a brain imaging tool. NeuroImage 2012, 61, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Biasiucci, A.; Franceschiello, B.; Murray, M.M. Electroencephalography. Curr. Biol. 2019, 29, R80–R85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.S.; Kulkarni, P.; Galatzer-Levy, I.R.; Bigio, B.; Nasca, C.; Zhang, Y. Modern Views of Machine Learning for Precision Psychiatry. Available online: https://www.cell.com/patterns/abstract/S2666-3899(22)00227-6 (accessed on 25 January 2025).
- de Bardeci, M.; Ip, C.T.; Olbrich, S. Deep learning applied to electroencephalogram data in mental disorders: A systematic review. Biol. Psychol. 2021, 162, 108117. [Google Scholar] [CrossRef] [PubMed]
- von Wegner, F.; Knaut, P.; Laufs, H. EEG Microstate Sequences from Different Clustering Algorithms Are Information-Theoretically Invariant. Front. Comput. Neurosci. 2018, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, G. Machine Learning Algorithms: Popular Algorithms for Data Science and Machine Learning, 2nd ed.; Packt Publishing: Birmingham, UK, 2018; 514p. [Google Scholar]
- What Is a Machine Learning Algorithm? Available online: https://www.ibm.com/think/topics/machine-learning-algorithms (accessed on 25 January 2025).
- Tugwell, P.; Tovey, D. PRISMA 2020. J. Clin. Epidemiol. 2021, 134, A5–A6. [Google Scholar] [CrossRef] [PubMed]
- CASP—Critical Appraisal Skills Programme. CASP Checklists—Critical Appraisal Skills Programme. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 1 November 2024).
- Baradits, M.; Bitter, I.; Czobor, P. Multivariate patterns of EEG microstate parameters and their role in the discrimination of patients with schizophrenia from healthy controls. Psychiatry Res. 2020, 288, 112938. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Li, C.; Zhang, J.; Wang, C. Dynamic brain functional network based on EEG microstate during sensory gating in schizophrenia. J. Neural Eng. 2022, 19, 026007. [Google Scholar] [CrossRef]
- Giuliani, L.; Koutsouleris, N.; Giordano, G.M.; Koenig, T.; Mucci, A.; Perrottelli, A.; Reuf, A.; Altamura, M.; Bellomo, A.; Brugnoli, R.; et al. A multivariate approach to investigate the associations of electrophysiological indices with schizophrenia clinical and functional outcome. Eur. Psychiatry 2023, 66, e46. [Google Scholar] [CrossRef]
- Keihani, A.; Sajadi, S.S.; Hasani, M.; Ferrarelli, F. Bayesian Optimization of Machine Learning Classification of Resting-State EEG Microstates in Schizophrenia: A Proof-of-Concept Preliminary Study Based on Secondary Analysis. Brain Sci. 2022, 12, 1497. [Google Scholar] [CrossRef]
- Kim, K.; Duc, N.T.; Choi, M.; Lee, B. EEG microstate features for schizophrenia classification. PLoS ONE 2021, 16, e0251842. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Ma, L.; Xu, C.; Li, H. EEG analysis in patients with schizophrenia based on microstate semantic modeling method. Front. Hum. Neurosci. 2024, 18, 1372985. [Google Scholar] [CrossRef]
- Lillo, E.; Mora, M.; Lucero, B. Automated diagnosis of schizophrenia using EEG microstates and Deep Convolutional Neural Network. Expert Syst. Appl. 2022, 209, 118236. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, Q.; Wang, C.; Zhang, K.; Wang, C.; Zhang, J. Biomarkers for prediction of schizophrenia: Insights from resting-state EEG microstates. IEEE Access 2020, 8, 213078–213093. [Google Scholar] [CrossRef]
- Yan, T.; Wang, G.; Liu, T.; Li, G.; Wang, C.; Funahashi, S.; Suo, D.; Pei, G. Effects of Microstate Dynamic Brain Network Disruption in Different Stages of Schizophrenia. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Li, H.-Z.; Wang, W.; Kuang, L. Changes in oscillatory patterns of microstate sequence in patients with first-episode psychosis. Sci. Data 2024, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.; Hernandez, L.D.; Baenninger, A.; Koenig, T. 15 Years of microstate research in schizophrenia—Where are we? A meta-analysis. Front. Psychiatry 2016, 7, 22. [Google Scholar] [CrossRef]
- Saeidi, M.; Karwowski, W.; Farahani, F.V.; Fiok, K.; Taiar, R.; Hancock, P.A.; Al-Juaid, A. Neural Decoding of EEG Signals with Machine Learning: A Systematic Review. Brain Sci. 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Acharya, U.R. Automated detection of schizophrenia using optimal wavelet-based norm features extracted from single-channel EEG. Cogn. Neurodyn. 2021, 15, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, J.R.; Favrod, O.; Roinishvili, M.; Chkonia, E.; Brand, A.; Mohr, C.; Figueiredo, P.; Herzog, M.H. EEG microstates are a candidate endophenotype for schizophrenia. Nat. Commun. 2020, 11, 3089. [Google Scholar] [CrossRef]
- Brugger, S.P.; Howes, O.D. Heterogeneity and Homogeneity of Regional Brain Structure in Schizophrenia: A Meta-analysis. JAMA Psychiatry 2017, 74, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Kremen, W.S.; Seidman, L.J.; Faraone, S.V.; Toomey, R.; Tsuang, M.T. Heterogeneity of schizophrenia: A study of individual neuropsychological profiles. Schizophr. Res. 2004, 71, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, D.; da Cruz, J.R.; Chkonia, E.; Lin, W.H.; Favrod, O.; Brand, A.; Figueiredo, P.; Roinishvili, M.; Herzog, M.H. The EEG multiverse of schizophrenia. Cereb Cortex 2023, 33, 3816–3826. [Google Scholar] [CrossRef]
- Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Castelvecchi, D. Can we open the black box of AI? Nat. News 2016, 538, 20. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Swerdlow, N.R. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann. N. Y. Acad. Sci. 2015, 1344, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, T.; Oribe, N.; Nakamura, I.; Kanba, S. Review of neurophysiological findings in patients with schizophrenia. Psychiatry Clin. Neurosci. 2013, 67, 461–470. [Google Scholar] [CrossRef]
- Rissling, A.J.; Makeig, S.; Braff, D.L.; Light, G.A. Neurophysiologic Markers of Abnormal Brain Activity in Schizophrenia. Curr. Psychiatry Rep. 2010, 12, 572–578. [Google Scholar] [CrossRef] [PubMed]
- de Miras, J.R.; Ibáñez-Molina, A.; Soriano, M.; Iglesias-Parro, S. Schizophrenia classification using machine learning on resting state EEG signal. Biomed. Signal Process. Control. 2023, 79, 104233. [Google Scholar] [CrossRef]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef]
| Model | Description | Characteristics |
|---|---|---|
| Linear Regression (Simple and Multiple) | A supervised model that predicts a target variable based on one (simple) or multiple (multiple) input features with a linear relationship. | Simple, interpretable, and effective for linear relationships, but struggles with non-linear patterns and multicollinearity in multiple regression. |
| Logistic Regression | A supervised model for binary classification tasks that estimates probabilities using a sigmoid function. | Simple, interpretable, and effective for binary classification, but assumes a linear relationship between features and the log-odds. |
| K-Means Clustering | An unsupervised method that partitions data into K clusters based on similarity. | Efficient and easy to implement, but sensitive to the choice of K and initial cluster centroids. |
| Hierarchical clustering | Groups data into a hierarchy or tree of clusters based on similarity measures. | Provides a visual hierarchy (dendrogram); computationally expensive for large datasets. |
| Decision Tree | A supervised model that uses a tree-like structure to make decisions based on feature splits. | Interpretable and easy to visualize; prone to overfitting without pruning. |
| Random Forest | An ensemble method that combines multiple Decision Trees to improve performance. | Reduces overfitting compared to a single tree; more computationally expensive. |
| Support Vector Machines (SVMs) | A supervised method that finds the optimal hyperplane to classify data into distinct categories. | Effective for high-dimensional spaces; computationally expensive with large datasets. |
| K-Nearest Neighbors (KNN) | A simple algorithm that classifies data based on the majority class of its nearest neighbors. | Easy to implement and small number of hyperparameters; computationally intensive for large datasets and less effective for high-dimensional data. |
| Multi-Layer Perceptron (MLP) | A neural network model with one or more hidden layers for learning non-linear mappings. | Suitable for a variety of tasks; requires significant data and computational resources. |
| Long Short-Term Memory (LSTM) | A type of recurrent neural network (RNN) designed for sequence prediction and temporal data. | Effective for long-term dependencies in sequential data; computationally intensive. |
| Convolutional neural network (CNN) | A deep learning model optimized for image and spatial data processing. | Highly effective for image-related tasks; automatic feature learning; requires large datasets and resources. |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baradits et al. (2020) [33] |  |  |  |  |  |  |  |  |  |  |  | 11/11 |
| Chang et al. (2022) [34] |  |  |  |  |  |  |  |  |  |  |  | 11/11 |
| Giuliani et al. (2023) [35] |  |  |  |  |  |  |  |  |  |  |  | 11/11 |
| Keihani et al. (2022) [36] |  |  |  |  |  |  |  |  |  |  |  | 8/11 |
| Kim et al. (2021) [37] |  |  |  |  |  |  |  |  |  |  |  | 8/11 |
| Li et al. (2024) [38] |  |  |  |  |  |  |  |  |  |  |  | 8/11 |
| Lillo et al. (2022) [39] |  |  |  |  |  |  |  |  |  |  |  | 8/11 |
| Luo et al. (2020) [40] |  |  |  |  |  |  |  |  |  |  |  | 9/11 |
| Yan et al. (2023) [41] |  |  |  |  |  |  |  |  |  |  |  | 9/11 |
| Zhou et al. (2024) [42] |  |  |  |  |  |  |  |  |  |  |  | 10/11 |
 : Yes : Yes  : No/Cannot tell : No/Cannot tell | ||||||||||||
| Study | Objective | Design, Sample, and Eeg Recording | Ml Methods and Features | Results | Interpretation and Research Implications |
| Baradits et al. (2020) [33] | To classify SZ vs. HC | Cross–sectional; 70 SZ, 75 HC; Resting–state EEG (256 channels). | SVM; hyperparameter tuning; factor analysis with varimax rotation; 10–fold CV (100 reps). MS features (4 clusters—classical). | AUC 0.84; Acc 82.7%; Sens 82.67%; Spec 81.43%. | Confirms the diagnostic potential of EEG microstate combined with machine learning fine-tuned models. |
| Chang et al. (2022) [34] | To classify FESZ vs. UHR vs. HC; To compare the model using demographic data, MCCB, and ERP with or without MS. | Cross–sectional; 35 FESZ, 30 UHR, 40 HC; EEG during sensory gating task (P50 response to S1–S2 stimuli, 128 channels). | Decision Tree; 5–fold CV; dimensionality reduction (factor analysis). Demographic data; MCCB; ERP P50 (S1/S2 amplitudes, S1–S2 difference, S2/S1 ratio); MS features (7 clusters—classical). | Acc 76.92%. | Combining microstate features with clinical (cognitive) and demographic data enhances diagnostic accuracy. Highlights the value of MS7 and MS8 alterations in P50 responses. |
| Giuliani et al. (2023) [35] | To classify SZ vs. HC; To correlate EEG features with clinical and functional outcome; To compare the model using MS vs. frequency bands vs. N100–P300 vs. MMN–P3a. | Cross–sectional and longitudinal (4–year follow–up); 148 SZ, 70 HC; EEG only at baseline (resting–state and ERP, 29 channels). | One SVM model for each EEG feature (freq bands, MS, N100–P300, MMN–P3a). Combined stacked Global classifier; double cycle nested CV. EEG features (freq bands, MS, N100–P300, MMN–P3a). | Acc up to 75.4% (global classifier). | Combining microstates with other EEG metrics highlights correlations with clinical outcomes (depression, negative symptoms, cognitive deficits and functioning). EEG microstates had limited predictive value compared to other EEG futures. |
| Keihani et al. (2022) [36] | To classify SZ vs. HC. | Cross–sectional; 14 SZ, 14 HC; Resting–state EEG (19 channels). | Bayesian optimized model and hyperparameter tuning; 5–fold CV; MS features (4 clusters—classical). | Acc 90.93%; AUC 0.90; Sens 91.37%; Spec 90.48%. | Microstate C is a key predictive feature; robust classification performance, even with only six parameters, demonstrates potential for minimal diagnostic tools. |
| Kim et al. (2021) [37] | To classify SZ vs. HC; To compare the model using MS vs. conventional EEG features. | Cross–sectional; 14 SZ, 14 HC; Resting–state EEG (19 channels). | SVM; 10–fold CV; MS features (4 clusters—classical), conventional EEG features. | Acc 75.64% (MS); combined features: Acc 76.85%. | Microstate features account for most diagnostic information, outperforming traditional EEG metrics. |
| Li et al. (2024) [38] | To classify SZ vs. HC. | Cross–sectional; 14 SZ, 14 HC; Resting–state EEG (19 channels). | SVM, KNN, and MLP; dual–template strategy; leave–one–out CV. MS features (4 clusters—classical, semantic and quality). | Acc 97.2% (KNN); cross–subject Acc 96.4%. | Integrating semantic and traditional features into classification models enhances diagnostic accuracy and suggests novel approaches for microstate analysis. |
| Lillo et al. (2022) [39] | To classify SZ vs. HC. | Cross–sectional; 14 SZ, 14 HC; Resting–state EEG (19 channels). | CNN; leave–one–out CV; MS features (4 clusters–random walk model for temporal and spatial dynamics), EEG features. | Acc 93%. | CNN and random walk models effectively capture temporal dynamics; potential for portable and automated diagnostic applications. |
| Luo et al. (2020) [40] | To classify FESZ vs. UHR vs. HR vs. HC; To compare the model using PANSS, CDSS, and MCCB with or without MS features. | Cross–sectional; 20 FESZ, 19 UHR, 12 HR, 14 HC; Resting–state EEG (128 channels). | RF, SVM, LSTM networks; 5–fold CV. MS features (6 clusters—classical), PANSS, CDSS, MCCB. | Global Acc 92%; Sens 91.8%; Spec 90.8% (RF); MS–D predictive. | Combining microstate and clinical data offers high diagnostic accuracy, which is particularly useful for classifying early stages of schizophrenia. |
| Yan et al. (2023) [41] | To classify FESZ vs. UHR vs. HR vs. HC; To correlate MS features with clinical and functional outcome. | Cross–sectional; 30 FESZ, 21 UHR, 17 HR, 31 HC; Resting–state EEG (128 channels). | Autoregressive modeling ((AR(1) model); 10–fold CV. MS features (4 clusters–classical, FC, global efficiency and clustering coefficient). | AUC: HC vs. FES 92.50%, UHR 97.22%, or HR 88.63%. Altered MS–A/C. | Microstate–based functional connectivity (reduced global efficiency) correlates with cognitive deficits and symptom severity. |
| Zhou et al. (2024) [42] | To classify FEP vs. HC; To compare the model using classical MS features vs. CGR features. | Cross–sectional; 81 FEP, 61 HC; Resting–state EEG (60 channels). | SVM; 5–fold CV. MS features (4 clusters–classical, frequency matrix CGR, temporal series analysis D and Z). | AUC: 0.46 (classical MS features). AUC: 0.49 (FCGR). AUC: 0.61 (CGR derived oscillatory features). Metrics. | CGR–derived oscillatory features outperform traditional microstate metrics, suggesting a novel and more sensitive approach to microstate analysis for early detection. |
| Study | Main Microstate Findings | |
|---|---|---|
| Baradits et al. (2020) [33] | Increased occurrence and coverage of microstate A; reduced duration, occurrence, and coverage of microstate B; increased occurrence and coverage of microstate D; no significant changes in microstate C; altered transition probabilities: reduced to B, increased to A and D. | A ↑ (cov, occ); B ↓ (cov, occ, dur); D (occ, cov) ↑; C no change; transitions: B ↓, A/D ↑. |
| Chang et al. (2022) [34] | In FESZ, MS7 (S1-P50): increased duration and coverage, reduced occurrence; MS8 (S1-S2-P50): increased coverage. FESZ lacked MS6→MS7 transitions and showed restricted MS5. Connectivity changes in temporal and frontal regions (Brodmann areas 21 and 11). | FESZ: MS7 (dur/cov ↑, occ ↓); MS8 (cover ↑); MS6→MS7 transitions absent; MS5 restricted; connectivity: BA21, BA11. |
| Giuliani et al. (2023) [35] | Highest-weighted microstate parameters (GFP peaks, sum of average occurrences, mean duration, delta between MSB and MSC, and between MSB and MSD) nonetheless showed a weak correlation with clinical and functional scores over 4 years. | GFP peaks, sum of occurrence, mean duration, Δ(MSB-MSC), Δ(MSB-MSD) → weak correlations with depression, cognition, functional outcomes (4 years). |
| Keihani et al. (2022) [36] | Occurrence and coverage of C reduced; D showed increased occurrence, coverage, and duration in SZ compared to HC. Microstate C the most predictive, followed by D and B. | C ↓; D ↑ (dur/occ/cov); predictive: C > D > B. |
| Kim et al. (2021) [37] | Increased occurrence, duration, and coverage of microstates B and C in SZ; microstate B also showed increased GFP. Reduced occurrence, duration, and coverage of microstate D in SZ; no differences in GFP. | B ↑, C ↑ (dur/occ/cov); D ↓; A no change; GFP: B ↑. |
| Li et al. (2024) [38] | Temporal, semantic, and quality-based microstate features extracted; microstate C increased frequency; D decreased in SCZ. SCZ-specific subsequences (e.g., BA, BC, ABA). Higher GEV in SCZ (88.5%) | C ↑, D ↓ (freq/GEV); SCZ subsequences: BA, BC, ABA. |
| Lillo et al. (2022) [39] | Microstates A and C increased in frequency and durations in SZ, while B and D had reduced durations and occurrence. Random walk analysis showed stable transitions in SZ. The trend component was the most informative for classification, with microstates B and D being critical for differentiation. | A, C ↑ (freq/dur); B, D ↓ (freq/dur); transitions stable, B/D important for classification. |
| Luo et al. (2020) [40] | Focus on microstate D; shorter duration and less frequent occurrence in FESZ and UHR compared to HC, with reduced coverage in FESZ, UHR, and HR groups. Alterations correlated with schizophrenia stage, showing the strongest effect in FESZ. | D ↓ (dur/freq/cov) in FESZ/UHR; correlation: SZ progression. |
| Yan et al. (2023) [41] | Reduced coverage, duration, and occurrence of microstate A in FES, UHR, and HR; increased coverage and duration of microstate C in FES; altered functional connectivity: reduced global efficiency in intra- and inter-msFCs; increased clustering coefficient in FES and UHR. | A ↓ (dur/cov/occ); C ↑ (dur/cov); B/D no change; connectivity: efficiency ↓, clustering ↑. |
| Zhou et al. (2024) [42] | Microstates A and D longer; more transitions A-D in HC; B-C occurred more frequently with increased transitions in FEP. Oscillatory features (CF, RMSF) higher in FEP. Microstate D’s duration and coverage negatively correlated with BPRS scores. | A, D ↑ (dur/trans); B, C ↑ (freq/trans) in FEP; D (dur/cov) correlated with BPRS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacchioni, F.; Germagnoli, G.; Calbi, M.; Agostoni, G.; Sapienza, J.; Repaci, F.; D’Incalci, M.; Spangaro, M.; Cavallaro, R.; Bosia, M. Navigating the Complexity of Psychotic Disorders: A Systematic Review of EEG Microstates and Machine Learning. BioMedInformatics 2025, 5, 8. https://doi.org/10.3390/biomedinformatics5010008
Pacchioni F, Germagnoli G, Calbi M, Agostoni G, Sapienza J, Repaci F, D’Incalci M, Spangaro M, Cavallaro R, Bosia M. Navigating the Complexity of Psychotic Disorders: A Systematic Review of EEG Microstates and Machine Learning. BioMedInformatics. 2025; 5(1):8. https://doi.org/10.3390/biomedinformatics5010008
Chicago/Turabian StylePacchioni, Federico, Giacomo Germagnoli, Marta Calbi, Giulia Agostoni, Jacopo Sapienza, Federica Repaci, Michele D’Incalci, Marco Spangaro, Roberto Cavallaro, and Marta Bosia. 2025. "Navigating the Complexity of Psychotic Disorders: A Systematic Review of EEG Microstates and Machine Learning" BioMedInformatics 5, no. 1: 8. https://doi.org/10.3390/biomedinformatics5010008
APA StylePacchioni, F., Germagnoli, G., Calbi, M., Agostoni, G., Sapienza, J., Repaci, F., D’Incalci, M., Spangaro, M., Cavallaro, R., & Bosia, M. (2025). Navigating the Complexity of Psychotic Disorders: A Systematic Review of EEG Microstates and Machine Learning. BioMedInformatics, 5(1), 8. https://doi.org/10.3390/biomedinformatics5010008






