Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets
Abstract
:1. Introduction
2. Genetic Abnormalities in SCLC
Genomic Characterization of SCLC Using Cell-Free Tumor DNA
3. Gene Expression Studies and Molecular Classification of SCLC
4. DNA Methylation Alterations in SCLC
5. Intratumoral Heterogeneity
6. Targeted Therapy and Immunotherapy for SCLC
6.1. Targeting DNA Damage Response (DDR)
6.2. Cell-Cycle Targeting
6.3. NOTCH Pathway Targeting
6.4. Potential Therapeutic Targets in ASCL1-Driven SCLC: BCL2
6.5. Molecular Targets in MYC-Driven SCLCs
6.6. Molecular Targets in SCLCs with MYCN Amplification
6.7. Insulin Growth Factor Receptor 1 (IGF-1R) Targeting
6.8. BET Inhibitors in SCLC Therapy
6.9. Netrin-3, a Potential Therapeutic Target for SCLC
6.10. Immunotherapy
6.11. Ferroptosis, a New Potential Therapeutic Target in SCLC
6.12. CDK4 and CDK6 Inhibitors
6.13. Fucosyl-GM1
7. Patient Derived Xenografts: A Fundamental Tool for Therapeutic Development in SCLC
8. Other Neuroendocrine Lung Tumors
9. SCLC Transformation from NSCLC
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Fissler-Eckoff, A.; Demes, M. Neuroendocrine tumors of the lung. Cancers 2012, 4, 777–798. [Google Scholar] [CrossRef]
- Rekhtman, N. Lung neuroendocrine neoplasms: Recent progress and persistent challenges. Mod. Pathol. 2021, 35, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Hiddinga, B.I.; Raskin, J.; Janssen, A.; Pauwels, P.; ven Meerbeeck, J.P. Recent developments in the treatment of small cell lung cancer. Eur. Respir. Rev. 2021, 31, 210079. [Google Scholar] [CrossRef] [PubMed]
- Witsuba, I.I.; Gazdar, A.F.; Minna, J.D. Molecular genetics of small cell lung carcinoma. Semin. Oncol. 2001, 28 (Suppl. 4), 3–13. [Google Scholar]
- Pleasance, E.D.; Stephens, R.J.; O’Meara, S.; McBride, D.J.; Meynert, A.; Jones, D.; Lin, M.L.; Beare, D.; Lau, K.W.; Greenman, C.; et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010, 463, 184–190. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibara, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef]
- Peifer, M.; Fernandez-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Sos, M.L.; Dietlain, F.; Peifer, M.; Schöttle, J.; Balke-Want, H.; Müller, C.; Koker, M.; Richters, A.; Heynck, S.; Malchers, F.; et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 17034–17039. [Google Scholar] [CrossRef]
- Rudin, C.; Durinck, S.; Stawiski, E.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive genomic analysis identifies Sox2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef]
- Voigt, E.; Wollenzien, H.; Feiner, J.; Thompson, E.; Vande Kamp, M.; Kareta, M.S. Sox2 in an oncogenic driver of small cell lung cancer. BioRxiv 2019, 10, 657924. [Google Scholar]
- Voigt, E.; Wallenburg, M.; Wollenzien, H.; Thompson, E.; Kumar, K.; Feiner, J.; McNally, M.; Friesen, H.; Mukherjee, M.; Afeworki, Y.; et al. Sox2 is on oncogenic driver of small-cell lung cancer and promotes the classic neuroendocrine subtype. Mol. Cancer Res. 2021, 19, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Tenjin, Y.; Matsuura, K.; Kudoh, S.; Usuki, S.; Yamada, T.; Matsuo, A.; Sato, Y.; Saito, H.; Fujino, K.; Wakimoto, J.; et al. Distinct transcriptional programs of SOX2 in different types of small cell lung cancers. Lab. Investig. 2020, 100, 1575–1588. [Google Scholar] [CrossRef]
- Cui, F.; Hao, Z.; Li, J.; Zhang, Y.; Li, X.; He, J. SOX2 mediates cisplatin resistance in small-cell lung cancer with downregulated expression of hsa-miR-340-5p. Mol. Genet. Genom. Med. 2020, 8, e1195. [Google Scholar] [CrossRef]
- Romero, O.A.; Torres-Diz, M.; Pros, E.; Savola, S.; Gomez, A.; Moran, S.; Saez, C.; Iwakawa, R.; Villanueva, A.; Montuenga, L.M.; et al. MAX inactivation in small cell lung cancer disrupts MYC-SW1/SNF program and is synthetic lethal with BRG1. Cancer Discov. 2014, 4, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Augert, A.; Mathsyaraja, H.; Ibrahim, A.H.; Freie, B.; Geuenich, M.J.; Cheng, P.F.; Alibeckoff, S.; Wu, N.; Hiatt, J.B.; Basom, R.; et al. MAX functions as a tumor suppressor and rewires metabolism in small cell lung cancer. Cancer Cell 2020, 38, 97–114. [Google Scholar] [CrossRef]
- Llabata, P.; Torres-Diz, M.; Gomez, A.; Tomas-Daza, L.; Romero, O.A.; Grego-Bessa, J.; Liams-Arias, P.; Valencia, A.; Esteller, M.; Javierre, B.M.; et al. MAX mutant small-cell lung cancers exhibit impaired activities of MGA-dependent noncanonical polycomb repressive complex. Proc. Natl. Acad. Sci. USA 2021, 118, e2024824118. [Google Scholar] [CrossRef] [PubMed]
- Mollaoglu, G.; Guthrie, M.R.; Bohm, S.; Bragelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC drives progression of small cell lung cancer to variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef]
- Ross, J.S. Next-generation sequencing reveals frequent consistent genomic alterations in small cell undifferentiated lung cancer. J. Clin. Pathol. 2014, 67, 772–776. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretic, L.; Kong, G.; Leenders, F.; Lu, X.; Fernandez-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–52. [Google Scholar] [CrossRef]
- Oser, M.G.; Sabet, A.H.; Gao, W.; Chakraborty, A.A.; Schinzel, A.C.; Jennings, R.B.; Fonseca, R.; Bonal, D.M.; Booker, M.A.; Flaifel, A.; et al. The KDM5A/RBP2 histone demethylase represses NOTCH signaling to sustain neuroendocrine differentiation and promote small cell lung cancer tumorigenesis. Genes Dev. 2019, 33, 1718–1738. [Google Scholar] [CrossRef]
- Augert, A.; Zhang, Q.; Bates, B.; Cui, M.; Wang, X.; Wildey, G.; Dowlati, A.; MacPherson, D. Small cell lung cancer exhibits frequent inactivating mutations in the histone methyltransferase KMTD2/MLL2: CALGB 151111 (Allience). J. Thorac. Oncol. 2016, 12, 704–713. [Google Scholar] [CrossRef]
- Gu, W.; Wang, H.; Li, K.; Wei, G.; Zhang, J.; Zhang, S. KMT2C mutation associated with tumor mutational burden in small cell lung cancer. J. Clin. Oncol. 2019, 37 (Suppl. 1), e20098. [Google Scholar] [CrossRef]
- Alam, H.; Tang, M.; Maitituoheti, M.; Dhar, S.S.; Kumar, M.; Han, C.Y.; Ambati, C.R.; Amin, S.B.; Gu, B.; Chen, T.Y.; et al. KMT2D deficiency impairs super-enhancers to confer a glycolytic vulnerability in lung cancer. Cancer Cell 2020, 37, 599–617. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Augert, A.; Kim, D.W.; Eastwood, E.; Wu, N.; Ibrahim, A.H.; Kim, K.B.; Dunn, C.T.; Pillai, S.; Gazdar, A.F.; et al. Crebbp loss drives small cell lung cancer and increases sensitivity to HDAC inhibition. Cancer Discov. 2018, 8, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Merk, D.J.; Lutz, B.; Schuller, U. Preferential sensitivity to HDAC inhibitors in tumors with CREBBP mutation. Cancer Gene Ther. 2020, 27, 294–300. [Google Scholar] [CrossRef]
- Carazo, F.; Bertolo, C.; Castilla, C.; Cendoya, X.; Campuzano, L.; Serrano, D.; Gimeno, M.; Planes, F.J.; Pio, R.; Montuega, L.M.; et al. DrugSniper, a tool to exploit loss-of-function screens, identifies CREBBP as a predictive biomarker of VOLASERTIB in small cell lung carcinoma (SCLC). Cancers 2020, 12, 1824. [Google Scholar] [CrossRef]
- Dowlati, A.; Lipka, M.B.; McCopll, K.; Dabir, S.; Behtaj, M.; Kresak, A.; Miron, A.; Yang, M.; Sharma, N.; Fu, P.; et al. Clinical correlation of extensive-stage small-cell lung cancer genomics. Ann. Oncol. 2016, 27, 642–647. [Google Scholar] [CrossRef]
- Udagawa, H.; Umemura, S.; Murakami, I.; Mimaki, S.; Makinoshima, H.; Ishii, G.; Miyoshi, T.; Kirita, K.; Matsumoto, S.; Yoh, K.; et al. Genetic profiling-based prognostic prediction of patients with advanced small-cell lung cancer in large scale analysis. Lung Cancer 2018, 126, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Sonkin, D.; Vural, S.; Thomas, A.; Teichjer, B.A. Neuroendocrine negative SCLC is mostly RB1 WT and may be sensitive to CDK4/6 inhibition. BioRxiv 2019, 516351. [Google Scholar] [CrossRef]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Are neuroendocrine negative small cell lung cancer and large cell neuroendocrine carcinoma with WT RB1 two faces of the same entity? Lung Cancer Manag. 2019, 8, LMT13. [Google Scholar] [CrossRef]
- Yokouchi, H.; Nishihara, H.; Harada, T.; Yamazaki, S.; Kikuchi, H.M.; Oizumi, S.; Uramoto, H.; Tanaka, F.; Harada, M.; Akie, K.; et al. Detection of somatic TP53 mutation in surgically resected small-cell lung cancer by targeted exome sequencing: Association with longer relapse-free survival. Heliyon 2020, 6, e04439. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, H.; Teo, A.S.M.; Amer, L.B.; Sherbaf, F.G.; Tan, C.Q.; Santiago Alvarez, J.J.; Lu, B.; Lin, J.Q.; Takano, A.; et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat. Genet. 2020, 52, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Higgs, B.W.; Hu, Z.; Xiao, Z.; Conley, S.; Zhong, H.; Liu, Z.; Brohawn, P.; Shen, D.; et al. Genomic landscape survey identifies SRSF1 as a key oncodriver in small cell lung cancer. PLoS Genet. 2016, 12, e1005895. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Zheng, Y.; Yu, Y.; Chen, H.; Liu, K.; Yao, M.; Wang, K.; Gu, W.; Shou, T. Comprehensive genomic profiling of small cell lung cancer in Chinese patients and the implications for therapeutic potential. Cancer Med. 2019, 8, 4338–4347. [Google Scholar] [CrossRef]
- Chen, D.; Xu, J.; Qian, R.; Zho, Y.; Chu, T.; Han, B.; Zhong, R. Detection of genetic mutations by next-generation sequencing for predicting prognosis of extensive-stage small-cell lung cancer. J. Oncol. 2020, 2020, 8811487. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, X.; Sun, S.; Cao, Z.; Feng, X.; Gao, Y. Profiling of 520 candidate genes in 50 surgically treated chinese small cell lung cancer patients. Front. Oncol. 2021, 11, 644434. [Google Scholar] [CrossRef]
- Wagner, A.H.; Devarakonda, S.; Skidmore, Z.L.; Krysiak, K.; Ramu, A.; Trani, L.; Kunisaki, J.; Masood, A.; Waqar, S.N.; Spies, N.C.; et al. Recurrent WNT pathway alterations are frequent in relapsed small cell lung cancer. Nat. Commun. 2018, 9, 3787. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Mian, I.; Tlesmani, C.; Pongor, L.; Takahashi, N.; Maignan, K.; Snider, J.; Li, G.; Frampton, G.; Ali, S.; et al. Clinical and genomic characteristics of small cell lung cancer in never smokers. Results from a retrospective multicenter cohort study. Chest 2020, 158, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Ogino, A.; Choi, J.; Lin, M.; Wilkens, M.K.; Calles, A.; Xu, M.; Adeni, A.E.; Chambers, E.S.; Capelletti, M.; Butaney, M.; et al. Genomic and pathological heterogeneity in clinically diagnosed small cell lung cancer in never/light smokers identifies therapeutically targetable alterations. Mol. Oncol. 2021, 15, 27–42. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Hu, J.; Song, L.; Ni, J.; He, Y.; Zhou, C. Distinct patterns of somatic genomic alterations and mutational signatures in central and peripheral-type small-cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Almodovar, K.; Iams, W.T.; Maedor, C.R.; Zhao, Z.; York, S.; Horn, L.; Yan, Y.; Hernandez, J.; Chen, H.; Shyr, Y.; et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J. Thorac. Oncol. 2018, 13, 112–123. [Google Scholar] [CrossRef]
- Nong, J.; Gong, Y.; Guan, Y.; Yi, X.; Yi, Y.; Chang, L.; Yang, L.; Lv, J.; Guo, Z.; Jia, H.; et al. Circulating tumor DNA analysis depicts subclonal architecture and genomic evolution of small cell lung cancer. Nat. Commun. 2018, 9, 3114. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Foy, V.; Ayub, M.; Leong, H.S.; Schoffield, P.; Sahoo, S.; Descamps, T.; Kilerci, B.; Smitch, N.K.; Carter, M.; et al. Profiling of circulating free DNA using targeted and genome-wide sequencing in patients with SCLC. J. Thorac. Oncol. 2020, 15, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.; Sankararaman, S.; Herzog, B.H.; Gold, K.A.; Wagar, S.N.; Ward, J.P.; Raymond, V.M.; Lanman, R.B.; Chaudhuri, A.A.; Owonikoko, T.K.; et al. Circulating tumor DNA profiling in small-cell lung cancer identifies potentially targetable alterations. Clin. Cancer Res. 2019, 25, 6119–6126. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.; Dong, G.; Zhang, H.; Zhang, T.; Chang, L.; Xia, X.; Li, L.; Zhu, H.; Zhu, H.; et al. The feasibility of using biomarkers derived from circulating tumor DNA sequencing as predictive classifiers in patients with small-cell lung cancer. Cancer Res. Treat. 2021, 54, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.L.; Morrow, C.J.; Dive, C. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K. Clinical significance and molecular characteristics of circulating tumor cells and circulating microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef]
- Tay, R.Y.; Fernadez-Gutierrez, F.; Foy, V.; Burns, K.; Pierce, J.; Morris, K.; Priest, L.; Tugwood, J.; Ashcroft, L.; Lindsay, C.R.; et al. Prognostic value of circulating tumour cells in limited stage lung cancer: Analysis of the concurtrent once-daily versus twice-daily radiotherapy (CONVERT) randomized controlled trial. Ann. Oncol. 2019, 30, 1114–1120. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Z.; Ni, X.; Duan, J.; Gao, Y.; Zhuo, M.; Li, R.; Zhao, J.; Ma, Q.; Bai, H.; et al. Inferring the evolution and progression of small-cell lung cancer by single-cell sequencing of circulating tumor cells. Clin. Cancer Res. 2019, 25, 5049–5060. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, C.T.; Byers, Z.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Borromeo, M.D.; Savage, T.K.; Kollipara, R.K.; He, M.; Augustyn, A.; Osborne, J.K.; Girard, L.; Minna, J.D.; Gazdar, A.-F.; Cobb, M.H.; et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 2016, 16, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Baine, M.K.; Hsieh, M.S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC subtypes defined by ASCL1, NEUROD1, POUF3, and YAP1: A comprehensive immunohistochemical and histopathologic characterization. J. Thorac. Oncol. 2020, 15, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Klingbeil, O.; Hex, Y.; Arun, G.; Lu, B.; Sommerville, T.; Milazzo, J.P.; Wilkinson, J.E.; Damerdash, D.E.; Spector, D.L. POU2F3 is a master regulator of small cell lung cancer. Genes Dev. 2018, 32, 915–928. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef]
- Simpson, K.L.; Stoney, R.; Frese, K.K.; Simms, N.; Rowe, W.; Pearce, S.P.; Humphrey, S.; Booth, L.; Morgan, D.; Dynowski, M.; et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat. Cancer 2020, 1, 437–451. [Google Scholar] [CrossRef]
- Pearsall, S.M.; Humphrey, S.; Revill, M.; Morgan, D.; Frese, K.K.; Galvin, M.; Kerr, A.; Carter, M.; Priest, L.; Blackhall, F.; et al. The rare YAP1 subtype of SCLC revisited in a biobank of 39 circulating tumor cell patient derived explants models: A brief report. J. Thorac. Oncol. 2020, 15, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.S.; Micinski, A.M.; Kastner, D.W.; Guo, B.; Wait, S.J.; Spainhover, K.B.; Conley, C.-C.; Chen, O.S.; Guthrie, M.R.; Soltero, D.; et al. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell 2020, 38, 60–78. [Google Scholar] [CrossRef]
- Wooten, D.J.; Groves, S.M.; Tyson, D.R.; Liu, Q.; Lim, J.S.; Albert, R.; Lopez, C.F.; Sage, J.; Quaranta, V. Systems-level network modeling of small cell lung cancer subtypes identifies master regulators and destabilizers. PLoS Comput. Biol. 2019, 15, e10007343. [Google Scholar] [CrossRef] [PubMed]
- Ouadah, Y.; Rojas, E.R.; Riordan, D.P.; Capostagno, S.; Kuo, C.S.; Krasnow, M.A. Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and Notch. Cell 2019, 179, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kalari, S.; Jung, M.; Kernstine, K.H.; Takahashi, T.; Pfeifer, G.P. The DNA methylation landscape of small cell lung cancer suggests a differentiation defect of neuroendocrine cells. Oncogene 2013, 32, 3559–3568. [Google Scholar] [CrossRef]
- Poirier, J.T.; Gardner, E.E.; Connis, N.; Moreira, A.L.; de Stanchina, E.; Hann, C.L.; Rudin, C.M. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 2015, 34, 5869–5878. [Google Scholar] [CrossRef]
- Hopkins-Donaldson, S.; Ziegler, A.; Kurtz, S.; Kandioler, D.; Ludwig, C.; Zangemeister-Wittke, U.; Stahel, R. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003, 10, 356–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, N.; Zhou, H.; Song, M.; Li, P.; Guan, Y.F.; Xia, X. Intratumoral heterogeneity and tumor evolution of small cell lung cancer through multi-regional sequencing. J. Clin. Oncol. 2020, 38 (Suppl. 15), e21103. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Luo, R.; Zhao, Y.; Pan, H.; Ji, L.; Zhou, T.; Zhang, L.; Long, H.; Fu, J.; et al. Multi-region sequencing reveals the intratumoral heterogeneity of surgically resected small cell lung cancer. Nat. Commun. 2021, 12, 5431. [Google Scholar] [CrossRef]
- Stewart, C.A.; Gay, C.M.; Xi, Y.; Sivajothi, S.; Sivakamasundari, V.; Fujimoto, J.; Bolisetty, M.; Hartsfield, P.M.; Balasubramaniyanm, V.; Calishabazar, M.D.; et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer 2020, 1, 423–436. [Google Scholar] [CrossRef]
- Gardner, E.E.; Lok, B.H.; Schneeberger, V.E.; Desmeules, P.; Miles, L.A.; Arnold, P.K.; Ni, A.; Khodos, I.; De Stanchina, E.; Nguyen, T.; et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell 2017, 31, 286–299. [Google Scholar] [CrossRef]
- Lok, B.H.; Gardner, E.E.; Schneeberger, V.E.; Ni, A.; Desmeules, P.; Rakhtman, N.; de Stanchina, E.; Teicher, B.A.; Riaz, N.; Powell, S.N.; et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin. Cancer Res. 2016, 23, 523–535. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Waqar, S.N.; Krug, L.M.; Dowlati, A.; Hann, C.L.; Chiappori, A.; Owonikoko, T.K.; Woo, K.M.; Cardnell, R.J.; Fijimoto, N.J.; et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 2018, 36, 2386–2394. [Google Scholar] [CrossRef]
- Farago, A.F.; Yeap, B.Y.; Tanzione, M.; Hung, Y.P.; Heist, R.S.; Marcoux, J.P.; Zhong, J.; Rangachari, D.; Barbie, D.A.; Phat, S.; et al. Combination of Olaparib and Temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019, 9, 13721387. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.E.; Winkler, C.; Roudier, M.P.; Baird, T.; Marco-Casanova, P.; Jones, E.V.; Rowe, P.; Rodriguez-Canales, J.; Angell, H.K.; Ng, F.; et al. Retrospective analysis of Schlafen 11 (SLFN11) to predict the outcomes to therapies affecting the DNA damage response. Br. J. Cancer 2021, 125, 1666–1676. [Google Scholar] [CrossRef]
- Qu, S.; Fetsch, P.; Thomas, A.; Pommier, Y.; Schrump, D.S.; Miettinen, M.M.; Chen, H. Molecular subtypes of primary SCLC tumors and their associations with neuroendocrine and therapeutic markers. J. Thorac. Oncol. 2021, 17, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Stewart, C.A.; Gay, C.M.; Wang, Q.; Cardnell, R.; Fujimoto, J.; Fernandez, L.; Jendrisak, A.; Gilbertson, C.; Schonhoft, J.; et al. Detection of DNA blocker SLFN11 in tumor tissue and circulating tumor cells to predict platinum and PARP inhibitors response in small cell lung cancer. Cancer Res. 2021, 81, 384. [Google Scholar] [CrossRef]
- Krushkal, J.; Silvers, T.; Reinhold, W.C.; Sonkin, D.; Vural, S.; Connelly, J.; Varma, S.; Meltzer, P.S.; Kunkel, M.; Rapisarda, A.; et al. Epigenome-wide DNA methylation analysis of small cell lung cancer cell lines suggests potential chemotherapy targets. Clin. Epigenet. 2020, 12, 93. [Google Scholar] [CrossRef]
- Mao, S.; Chaerkady, R.; Yu, W.; D’Angelo, G.; Garcia, A.; Chen, H.; Barrett, A.M.; Phipps, S.; Fleming, R.; Hess, S.; et al. Resistance to pyrrolobenzodiazepine dimers is associated with SLFN11 downregulation and can be reversed through inhibition of ATR. Mol. Cancer Ther. 2021, 20, 541–552. [Google Scholar] [CrossRef]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; Lopez, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernandez, C.; Alfaro, V.; et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef]
- Kundu, K.; Cardnell, R.J.; Zhang, B.; Shen, L.; Stewart, C.A.; Ramkumar, K.; Cargill, K.R.; Wang, J.; Gay, C.M.; Byers, L.A. SLFN11 biomarker statrus predicts response to lurbinectedin as a single agent and in combination with ATR inhibition in small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 4095–4105. [Google Scholar] [CrossRef]
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012, 2, 798–811. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.; Lee, B.; Lee, S.H.; Sun, J.M.; Park, W.Y.; Ahn, J.S.; Ahn, M.J.; Park, K. DNA damage response and repair pathway alteration and its association with tumor mutation burden and platinum-based chemotherapy in SCLC. J. Thorac. Oncol. 2019, 14, 1640–1650. [Google Scholar] [CrossRef]
- Atrafi, F.; Groen, H.J.M.; Byers, L.A.; Garralda, E.; Lolkema, M.P.; Sangha, R.S.; Viteri, S.; Chae, Y.K.; Camidge, D.R.; Gabrail, N.Y.; et al. A phase I dose-escalation study of veliparib combined with carboplatin and etoposide in patients with extensive-stage small cell lung cancer and other solid tumors. Clin. Cancer Res. 2019, 25, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Dahlberg, S.E.; Sica, G.L.; Wagner, L.I.; Wade, J.L., III; Srkalovic, G.; Lash, B.W.; Leach, J.W.; Leal, T.B.; Aggarwal, C.; et al. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small-cell lung cancer: ECOG-ACRIN 2511 study. J. Clin. Oncol. 2018, 37, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Byers, L.A.; Bentsion, D.; Gans, S.; Penkov, K.; Son, C.H.; Sibille, A.; Owonikoko, T.K.; Groen, H.J.M.; Gay, C.M.; Fujimoto, J.; et al. Veliparib in combination with carboplatin and etoposide in patients with treatment-naïve extensive-stage small cell lung cancer: A phase 2 randomized study. Clin. Cancer Res. 2021, 27, 3884–3895. [Google Scholar] [CrossRef]
- Tlemsani, C.; Takahashi, N.; Pongor, L.; Rajapakse, V.; Tyagi, M.; Wen, X.; Fasaye, G.A.; Schmidt, K.T.; Desai, P.; Kim, C.; et al. Whole-exome sequencing reveals germline-mutated small cell lung cancer subtype with favorable response to DNA repair-targeted therapies. Sci. Transl. Med. 2021, 13, eabc7488. [Google Scholar] [CrossRef]
- Knelson, E.H.; Patel, S.A.; Sands, J.M. PARP inhibitors in small-cell lung cancer: Rational combinations to improve responses. Cancers 2021, 13, 727. [Google Scholar] [CrossRef]
- Thomas, A.; Takahashi, N.; Rajapakse, V.N.; Zhang, X.; Sun, Y.; Ceribelli, M.; Wilson, K.M.; Zhang, Y.; Beck, E.; Sciuto, L.; et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell 2021, 39, 566–579. [Google Scholar] [CrossRef]
- Nagel, R.; Avelar, A.T.; Aben, A.; Proost, N.; ven de Ven, M.; van der Vliet, J.; Cozijnsen, M.; de Vries, H.; Wessels, L.; Berns, A. Inhibition of the replication stress response is a synthetic sulverability in SCLC that acts synergistically in combination with cisplatin. Mol. Cancer Ther. 2019, 18, 762–770. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Tang, H.; Hubert, S.M.; Li, Q.; Hu, X.; Su, D.; Xu, H.; Fan, Y.; Yu, X.; et al. Activation of PI3K/AKT pathway is a potential mechanism of treatment resistance in small cell lung cancer. Clin. Cancer Res. 2021, 28, 526–539. [Google Scholar] [CrossRef]
- Quintanal-Villalonga, A.; Taniguchi, H.; Hao, Y.; Chow, A.; Zhan, Y.A.; Chavan, S.S.; Uddin, F.; Allaj, V.; Manoj, P.; Shah, N.S.; et al. Inhibition of XPO1 sensitizes small cell lung lung cancer to first- and second-line chemotherapy. Cancer Res. 2021, 82, 472–483. [Google Scholar] [CrossRef]
- Schenk, M.W.; Humprey, S.; Hossain, M.; Revill, M.; Pearsall, S.; Lallo, A.; Brown, S.; Bratt, S.; Galvin, M.; Descampos, T.; et al. Soluble guanylate cyclase signaling mediates etoposide resistance in progressing small cell lung cancer. Nat. Commun. 2021, 12, 6652. [Google Scholar] [CrossRef]
- Sen, T.; Tong, P.; Stewart, A.; Cristea, S.; Valliani, A.; Shames, D.S.; Redwood, A.; Fan, Y.H.; Li, L.; Glisson, B.S. CHK1 inhibition in small-cell lung cancer produces single-agent activity in biomarker-defined disease subsets and combination activity with cisplatin or olaparib. Cancer Res. 2017, 77, 3870–3884. [Google Scholar] [CrossRef] [PubMed]
- Doerr, F.; George, J.; Schmitt, A.; Beleggia, F.; Rehkamper, T.; Hermann, S.; Walter, V.; Weber, J.P.; Thomas, R.K.; Wittersheim, M.; et al. Targeting a non-oncogene addiction to the ATR/CHK1 axis for the treatment of small cell lung cancer. Sci. Transl. 2017, 7, 15511. [Google Scholar] [CrossRef]
- Hsu, W.H.; Zhao, X.; Zhu, J.; Kim, I.K.; Rao, G.; McCutcheon, J.; Hsu, S.T.; Teicher, B.; Kallakury, B.; Dowlati, A.; et al. Checkpoint kinase 1 inhibition enhances cisplatin cytotoxicity and overcomes cisplatin resistance in SCLC promoting mitotic cell death. J. Thorac. Oncol. 2019, 4, 1032–1045. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Della Corte, C.M.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Della Corte, C.M.; Milutinovic, S.; Cardnell, R.J.; Diao, L.; Ramkumar, K.; Gay, C.M.; Stewart, A.; Fan, Y.; Shen, L.; et al. Combination treatment of the oral CHK1 inhibitor, SRA737, and low-dose gemcitabine enhances the effect of programmed death ligand 1 blockade by modulating the immune microenvironment in SCLC. J. Thorac. Oncol. 2019, 12, 2152–2163. [Google Scholar] [CrossRef]
- Byers, L.A.; Navarro, A.; Schaefer, E.; Johnson, M.; Ozguroglu, M.; Han, J.Y.; Bondarenko, I.; Cicin, I.; Dragnev, K.; Abel, A.; et al. A phase II trial of prexasertib (LY2606368) in patients with extensive-stage small-cell lung cancer. Clin. Lung Cancer 2021, 22, 531–540. [Google Scholar] [CrossRef]
- Sen, T.; Tong, P.; Diao, L.; Li, L.; Fan, Y.; Hoff, J.; Heymach, J.V.; Wang, J.; Byers, L.A. Targeting AXL and mTOR pathway overcomes primary and acquired resistance to WEE1 inhibition in small-cell lung cancer. Clin. Cancer Res. 2017, 23, 6239–6253. [Google Scholar] [CrossRef]
- Park, S.; Shim, J.; Jung, H.A.; Sun, J.M.; Lee, S.H.; Park, W.Y. Biomarker driven phase II umbrella trial study of AZD1775, AZD2014, AZD2811 monotherapy in relapsed small cell lung cancer. J. Clin. Oncol. 2017, 37 (Suppl. 5), abst. 8514. [Google Scholar] [CrossRef]
- Lallo, A.; Frese, K.K.; Morrow, C.J.; Sloane, R.; Gulati, S.; Schenk, M.W.; Trapani, F.; Simms, N.; Galvin, M.; Brown, S.; et al. The combination of the PARP inhibitor olaparib and the WEE1 inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin. Cancer Res. 2018, 24, 5153–5164. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, I.K.; Kallakury, B.; Chahine, J.J.; Iwama, E.; Pierobon, M.; Petricoin, E.; McCucheon, J.N.; Zhang, Y.W.; Umemura, S.; et al. Acquired small cell lung cancer resistance to Chk1 inhibitors involves Wee1 up-regulation. Mol. Oncol. 2021, 15, 1130–1145. [Google Scholar] [CrossRef]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef] [PubMed]
- Alì, G.; Di Stefano, I.; Poma, A.M.; Ricci, S.; Proietti, A.; Davini, F.; Lucchi, M.; Melfi, F.; Fontanini, G. Prevalence of delta-like protein 3 in a consecutive series of surgically resected lung neuroendocrine neoplasms. Front. Oncol. 2021, 11, 729765. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Isse, K.; Fujihira, T.; Takenoyama, M.; Saunders, L.; Cheddah, S.; Nakanishi, Y.; Okamoto, I. Prevalence of Delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer 2018, 115, 116–120. [Google Scholar] [CrossRef]
- Rojo, F.; Corassa, M.; Mavroudis, D.; Oz, A.B.; Biesma, B.; Brcic, L.; Pauwels, P.; Sailer, V.; Gosney, J.; Miljkovic, D.; et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer 2020, 147, 237–243. [Google Scholar] [CrossRef]
- Hu, C.; Dong, J.; Liu, L.; Liu, J.; Sun, X.; Teng, F.; Wang, X.; Ying, J.; Li, J.; Xing, P.; et al. ASCL1 and DLL3 expressions and their clinicopathological implications in surgically resected pure small cell lung cancer: A study of 247 cases from the National Cancer Center of China. Thorac. Cancer 2021, 13, 338–345. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A.; Robert, F.; et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef]
- Sharma, S.K.; Pourat, J.; Abdel-Atti, D.; Carlin, S.D.; Piersigilli, A.; Bankovich, A.J.; Gardner, E.E.; Hamdy, O.; Isse, K.; Bheddah, S.; et al. Noninvasive interrogation of DLL3 expression in metastatic small cell lung cancer. Cancer Res. 2017, 77, 3931–3941. [Google Scholar] [CrossRef]
- Komarnitsky, P.B.; Lee, H.J.; ShaH, M.; Wong, S.; Gauthier, S.; Dziubinski, J. A phase III study of ravailpituzumab teserine maintenance therapy following first-line platinum-based chemotherapy in patients with extensive disease small cell lung cancer (ED SCLC). J. Clin. Oncol. 2017, 35, abst. TPS8583. [Google Scholar] [CrossRef]
- Blackhall, F.; Jao, K.; Greillier, L.; Cho, B.C.; Penkov, K.; Reguart, N.; Majem, M.; Nackaerts, K.; Syrigos, K.; Hansen, K.; et al. Efficacy and safety oif rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCXLC: Results from the phase 3 TAHOE study. J. Thorac. Oncol. 2021, 16, 1547–1558. [Google Scholar] [CrossRef]
- Johnsson, M.L.; Zvirbule, Z.; Laktionov, K.; Helland, A.; Cho, B.C.; Gutierrez, V.; Colinet, B.; Lena, H.; Wolf, M.; Gottfried, M.; et al. Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-satge-SCLC: Results from the phase 3 MERU study. J. Thorac. Oncol. 2021, 16, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Besse, B.; Greillier, L. Efficacy and safety of Rovalpituzumab Tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: Results from the phase II TRINITY study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef] [PubMed]
- Hipp, S.; Voynov, V.; Drobits-Dandl, B.; Giragossian, C.; Trapani, F.; Nixon, A.E.; Scheer, J.M.; Adam, P.J. A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin. Cancer Res. 2020, 26, 5258–5268. [Google Scholar] [CrossRef] [PubMed]
- Giffin, M.J.; Cooke, K.; Lobenhofer, E.K.; Estrada, J.; Zhan, J.; Deegen, P.; Thomas, M.; Murawsky, C.M.; Werner, J.; Liu, S.; et al. AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin. Cancer Res. 2021, 27, 1526–1537. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Champiat, S.; Johnson, M.L.; Govindan, R.; Tzumi, H.; Lai, V.V. Updated results from a phase I study of AMG 757, a half-life extended bispecific T-cell engager (BiTE) immune-oncology therapy against delta-like ligand 3 (DLL3), in small cell lung cancer (SCLC). J. Clin. Oncol. 2021, 39 (Suppl. 5), abst. 8510. [Google Scholar] [CrossRef]
- Chen, X.; Amar, N.; Zhu, Y.; Wang, C.; Xia, C.; Yang, X.; Wu, D.; Feng, M. Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth. J. Immunother. Cancer 2020, 8, e000785. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Sato, K.; Nishinaga, Y.; Takahashi, K.; Taki, S.; Yasui, H.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine 2020, 52, 102632. [Google Scholar] [CrossRef] [PubMed]
- Ardeshir-Larijani, F.; Widley, G.; Fu, P.; Bhateya, P.; Dowlati, A. Frequency of NOTCH pathway mutation in primary tumor of SCLC compared to metastatic biopsies and association with better survival. J. Clin. Oncol. 2018, 36 (Suppl. 1), abst. E20574. [Google Scholar] [CrossRef]
- Tendler, S.; Kamper, L.; Lewensohn, R.; Ortiz-Villala, C.; Viktorsson, K.; De Petris, L. The prognostic implications of Notch1, Hes1, Ascl1, and DLL3 protein expression in SCLC patients receiving platinum-based chemotherapy. PLoS ONE 2020, 15, e0240973. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Liu, Y.W.; Jahchan, N.S.; Hamard, C.; et al. Intratumoral heterogeneity generated by Notch signaling promotes small-cell lung cancer. Nature 2017, 545, 361–366. [Google Scholar] [CrossRef]
- Daniel, D.B.; Rudin, C.M.; Hart, L.; Faoro, L.; Chaing, A.C. Results of a randomized, placebo-controlled, phase 2 study of tarextumab (TRTX, anti Notch2/3) in combination with etoposide and platinum (EP) in patients (pts) with untreated extensive-stage small-cell lung cancer (ED-SCLC). Ann. Oncol. 2017, 28 (Suppl. 5), V540. [Google Scholar] [CrossRef]
- Yan, W.; Chung, C.Y.; Xia, T.; Ozeck, M.; Nichols, T.C.; Frey, J.; Udyavar, A.R.; Sharma, S.; Paul, T.A. Intrinsic and acquired drug resistance o LSD1 inhibitors in small cell lung cancer occurs through a TEAD4-driven transcriptional state. Mol. Oncol. 2021, 16, 1309–1328. [Google Scholar] [CrossRef]
- Ponce Aix, S.; Juan-Vidal, O.; Carcereny, E.; Trigo, J.M.; Provencio, M.; Greillier, L.; Navarro, A.; Bennouna, J.; Santoro, A.; Berardi, R.; et al. A phase Ib study of CC-90011, a potent, reversible, oral LSD1 inhibitor, plus etoposide and cisplatin (EP) or carboplatin (EC) in patients (pts) with first-line (1L) extensive-stage (ES) small cell lung cancer (SCLC): Updated results. J. Thorac. Oncol. 2021, 16 (Suppl. 4), S720–S728. [Google Scholar]
- Augert, A.; Eastwood, E.; Ibrahim, A.H.; Wu, N.; Grunblatt, E.; Basom, R.; Liggitt, D.; Eaton, K.D.; Martins, R.; Poirier, J.T.; et al. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci. Signal. 2019, 12, eauu2922. [Google Scholar] [CrossRef] [PubMed]
- Pozo, K.; Kollipara, R.K.; Rodarte, K.; Kelenis, D.P.; Zhang, X.; Minna, J.D.; Johnson, J.E. Lineage transcription factors ASCL1, NKX2-1 and PROX1 are enriched at superenhancers and co-regulate subtype-specific genes in small cell lung cancer. BioRxiv 2020, 24, 102953. [Google Scholar] [CrossRef]
- Pozo, K.; Kollipara, R.K.; Kelenis, D.P.; Rodarte, K.E.; Ullrich, M.S.; Zhang, X.; Minna, J.D.; Johnson, J.E. ASCL1, NKX2-1, and PROX1 co-regulate subtype-specific genes in small-cell lung cancer. iScience 2021, 24, 102953. [Google Scholar] [CrossRef]
- Tlemsani, C.; Pongor, L.; Elloumi, F.; Girard, L.; Huffman, K.E.; Raper, N.; Varma, S.; Luna, A.; Rajapakse, V.N.; Sebastian, R.; et al. SCLC-cell miner: A resource for small cell lung cancer cell line genomics and pharmacology based on genomic signatures. Cell Rep. 2020, 33, 108296. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, L.; Guo, Y.; Zhang, J.; Wang, X.; Dong, J.; Xing, P.; Ying, Y.; Yang, L.; Li, J. Clinicopathological features and prognostic implications of ASCL1 expression in surgically resected small cell lung cancer. Thorac. Cancer 2021, 12, 40–47. [Google Scholar] [CrossRef]
- Augustyn, A.; Borromeo, M.; Wang, T.; Fujimoto, J.; Shao, C.; Dospoy, P.D.; Lee, V.; Tan, C.; Sullivan, J.P.; Larsen, J.E.; et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 14788–14793. [Google Scholar] [CrossRef]
- He, M.; Liu, S.; GalloluKankamalage, S.; Borromeo, M.D.; Girard, L.; Gazdar, A.F.; Minna, J.D.; Johnson, J.E.; Cobb, M.H. The epithelial sodium channel (αENaC) is a downstream therapeutic target of ASCL1 in pulmonary neuroendocrine tumors. Transl. Oncol. 2018, 11, 292–299. [Google Scholar] [CrossRef]
- Alam, S.R.; Wang, L.; Ren, Y.; Hernandez, C.E.; Kosari, F.; Roden, A.C.; Yang, R.; Hoeppner, L.H. ASCL1-regulated DARPP-32 and t-DARPP stimulate small cell lung cancer growth and neuroendocrine tumor cell proliferation. Br. J. Cancer 2020, 123, 819–832. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; Ribeiro de Oliveira, M.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II study of the single agent Navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Lochmann, T.L.; Floros, K.; Naseri, M.; Powell, K.M.; Cook, W.; March, R.J.; Stein, G.T.; Greninger, P.; Maves, Y.K.; Saunders, L.R.; et al. Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin. Cancer Res. 2017, 24, 360–369. [Google Scholar] [CrossRef]
- Yasuda, Y.; Ozasa, H.; Kim, Y.H.; Yamazoe, M.; Ajimizu, H.; Funazo, T.Y.; Nomizo, T.; Tsuji, T.; Yoshida, H.; Sakamori, Y.; et al. MCL1 inhibition is effective against a subset of small-cell lung cancer with high MCL1 and low BCL-XL expression. Cell Death Dis. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Kalemkerian, G.P.; Patel, J.D.; Mohindra, N.A.; Carlisle, J.W.; Sands, J.; He, Z.; Winkler, R.; Liang, Z.; Hammock, V.; et al. Trial in progress: A multicenternphase Ib/II study of pelcitoclax (APG-1252) in combination with paclitaxel in patients with relapsed/refractory small-cell lung cancer (R/R SCLC). J. Clin. Oncol. 2021, 39 (Suppl. 15), TP58589. [Google Scholar] [CrossRef]
- Caesar, R.; Hulton, C.; Costa, E.; Durani, V.; Little, M.; Chen, X.; Tischfield, S.E.; Asher, M.; Kombak, F.E.; Chavan, S.S.; et al. MAPK pathway activation selectively inhibits ASCL1-driven small cell lung cancer. iScience 2021, 24, 103224. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Yoo, S.; Kong, R.; Sato, T.; Sinha, A.; Bao, L.; Fridrikh, M.; Emoto, K.; Nudelman, G.; Powell, C.A.; et al. Prototypical oncogene family Myc defines unappreciated distinct lineage states of small cell lung cancer. Sci. Adv. 2021, 7, eabc2578. [Google Scholar] [CrossRef]
- Melichar, B.; Adenis, A.; Lochart, A.C. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: A five-arm phase 2 study. Lancet Oncol. 2015, 16, 395–405. [Google Scholar]
- Owonikoko, T.K.; Niu, H.; Nackaerts, K.; Csoski, T.; Ostoros, G.; Mark, Z.; Balk, C.; Joy, A.A.; Chouaid, C.; Jaime, J.C.; et al. Randomized phase II study of paclitaxel plus alisertib versus paclitaxel plus placebo as second-line therapy for SCLC: Primary and correlative biomarker analyses. J. Thorac. Oncol. 2020, 15, 274–287. [Google Scholar] [CrossRef]
- Oser, M.G.; Fonseca, R.; Chakraborty, A.A.; Brough, R.; Spektor, A.; Jenning, R.B.; Flaifel, A.; Novak, J.S.; Gulati, A.; Buss, E.; et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on Aurora B kinase for survival. Cancer Discov. 2019, 9, 230–247. [Google Scholar] [CrossRef]
- Kolla, B.C.; Racila, E.; Patel, M.R. Deep and prolonged response to Aurora A kinase inhibitor and subsequently to nivolumab in MYCL1-driven small-cell lung cancer: Case report and literature review. Case Rep. Oncol. Med. 2020, 2020, 8026849. [Google Scholar] [CrossRef]
- Vural, S.; Palmiosano, A.; Reshold, W.C.; éommier, Y.; Teichjer, B.A.; Krushkal, J. Association of expression of epigenetic molecular factors with DNA methylation and sensitivity to chemotherapeutic agents in cancer cell lines. Clin. Epigenet. 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Dammert, M.A.; Bragelmann, J.; Olsen, R.R.; Bohm, S.; Monhasery, N.; Whitney, C.P.; Calhisazar, M.D.; Tumbrink, H.L.; Guthrie, M.R.; Klein, S.; et al. MYC paralog-dependent apoptoic priming orchestrates a spectrum of vulnerabilities in small cell lung cancer. Nat. Commun. 2019, 10, 3485. [Google Scholar] [CrossRef] [PubMed]
- Chalishazar, M.D.; Wait, S.J.; Huang, F.; Ireland, A.S.; Mukhopadhyay, A.; Lee, Y.; Schuman, S.S.; Guthrie, M.R.; Berrett, K.C.; Vahrenkamp, J.M.; et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin. Cancer Res. 2019, 25, 5107–5121. [Google Scholar] [CrossRef]
- Hall, P.E.; Ready, N.; Johnston, A.; Bomalaski, J.S.; Venhaus, R.R.; Sheaff, M.; Krug, L.; Szlosarek, P.W. Phase II study of arginine deprivation therapy with pegargiminase in patients with relapsed sensitive or refractory small-cell lung cancer. Clin. Lung Cancer 2020, 21, 527–533. [Google Scholar] [CrossRef]
- Cargill, K.R.; Stewart, C.A.; Park, E.M.; Ramkumar, K.; Gay, C.M.; Cardnell, R.J.; Wang, Q.; Diao, L.; Shen, L.; Fan, Y.H.; et al. Targeting MYC-enhnaced glycolysis for the treatment of small cell lung cancer. Cancer Metab. 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Cargill, K.R.; Gay, C.M.; Cardnell, R.J.; Fan, Y.H.; Wang, Q.; Diao, L.; Wang, J.; Byers, L.A. Comprehenesive metabolic profiling and vulnerabilities to metabolic inhibitors among small cell lung cancer subtypes. Cancer Res. 2020, 80, 232. [Google Scholar] [CrossRef]
- Cristea, S.; Coles, G.L.; Hornburg, D.; Gershkovitz, M.; Arand, J.; Cao, S.; Sen, T.; Williamson, S.C.; Kim, J.W.; Drainas, A.P.; et al. The MEK-ERK kinase axis controls lipid metabolism in small-cell lung camcer. Cancer Res. 2020, 80, 1293–1303. [Google Scholar] [CrossRef]
- Huang, F.; Ni, M.; Chalisazhar, M.D.; Huffman, K.E.; Kim, J.; Cai, L.; Shi, X.; Cai, F.; Zacharias, L.G.; Ireland, A.S.; et al. Inosine monophosphate dehydrogenase dependence in a subset of small cell lung cancers. Cell Metab. 2018, 28, 369–382. [Google Scholar] [CrossRef]
- Huang, F.; Huffman, K.E.; Wang, Z.; Wang, X.; Li, K.; Cai, F.; Yang, C.; Cai, L.; Shih, T.S.; Zacharias, L.G.; et al. Guanosine triphosphate links MYC-dependent metabolic and ribosome programs in small-cell lung cancer. J. Clin. Investig. 2021, 131, e139929. [Google Scholar] [CrossRef]
- Kim, Y.H.; Girard, L.; Giacomini, C.P.; Wang, P.; Hernandez-Boussard, T.; Tibshirani, R.; Minna, J.D.; Pollack, J.R. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene 2006, 25, 130–138. [Google Scholar] [CrossRef]
- Iwakawa, R.; Takenaka, M.; Kohno, T.; Shimada, Y.; Totoki, Y.; Shibata, T.; Tsuta, K.; Nishikawa, R.; Noguchi, M.; Sato-Otsubo, A.; et al. Genome-wide identification of genes with amplification and/or fusion in small cell lung cancer. Genes Chromosom. Cancer 2013, 52, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Grunblatt, E.; Wu, N.; Zhang, H.; Liu, X.; Norton, J.P.; Ohol, Y.; Leger, P.; Hiatt, J.B.; Eastwood, E.C.; Thomas, R.; et al. MYCN drives chemoresistance in small cell lung cancer while USP7 inhibition can restore chemosensitivity. Genes Dev. 2020, 34, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Ouyang, S.; Chen, R.; Huang, J.; Guo, L. MYCN-mediated regulation of the HES1 promoter enhances the chemoresistance of small-cell lung cancer by modulating apoptosis. Am. J. Cancer Res. 2019, 9, 1938–1956. [Google Scholar] [PubMed]
- Wang, H.; Hong, B.; Li, X.; Lin, W. JQ1 synergizes with the Bcl-2 inhibitor ABT-263 against MYCN-amplified small cell lung cancer. Oncotarget 2017, 8, 21146. [Google Scholar] [CrossRef]
- Wang, X.D.; Hu, R.; Ding, Q.; Savage, T.K.; Huffman, K.E.; Williams, N.; Cobb, M.H.; Minna, J.D.; Johnson, J.E.; Yu, Y. Subtype-specific secretome characterization of pulmonary neuroendocrine tumors. Nat. Commun. 2019, 10, 3201. [Google Scholar] [CrossRef]
- Ding, M.; Bruick, R.Y.; Yu, Y. Secreted IGFBP5 mediates mTORC1-dependent feedback inhibition of IGF-1 signalling. Nat. Cell Biol. 2016, 18, 319–327. [Google Scholar] [CrossRef]
- Ferté, C.; Loriot, Y.; Clémenson, C.; Commo, F.; Gombos, A.; Bibbault, J.E.; Fumagalli, I.; Hamama, S.; Auger, N.; Lahot, B.; et al. IGF-1R targeting increases the antitumor effects of DNA-damaging agents in SCLC model: An opportunity to increase the efficacy of standard therapy. Mol. Cancer Ther. 2013, 12, 1213–1222. [Google Scholar] [CrossRef]
- Lkshamanan, I.; Chaudhary, S.; Perumal, S.; Batra, S.; Ganti, A.K. IGF-1R inhibition in small cell lung cancer: Role of brigatinib. Ann. Oncol. 2021, 16, S221. [Google Scholar] [CrossRef]
- Antonia, S.J.; Lopez-Martin, J.A.; Bandell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jager, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumb alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (Checkmate 032): A multicenter, open label, phase1-2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Lee, M.S.; Jung, K.; Song, J.Y.; Sung, M.J.; Ahn, S.B.; Lee, B.; Oh, D.Y. IRS2 amplification as a predictive biomarker in response to ceritinib in small cell lung cancer. Mol. Ther. Oncolytics 2020, 16, 188–196. [Google Scholar] [CrossRef]
- Lenhart, R.; Kirov, S.; Desilva, H.; Cao, J.; Lei, M.; Johnston, K.; Peterson, R.; Schweizer, L.; Purandare, A.; Ross-Macdonald, P.; et al. Sensitivity of small cell lung cancer to BET inhibition is mediated by regulation of ASCL1 gene expression. Mol. Cancer Ther. 2015, 14, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Fiorentino, F.P.; Alibés, A.; Perucho, M.; Sanchez-Céespedes, M.; Kohno, T.; Yokota, J. MYCL is a target of a Bet bromodomain inhibitor, JQ1, on growth suppression efficacy in small cell lung cancer cells. Oncotarget 2016, 7, 77378–77387. [Google Scholar] [CrossRef] [PubMed]
- Shorstova, T.; Foulkes, W.D.; Witcher, M. Achieving clinical success with BET inhibitors as anti-cancer agents. Br. J. Cancer 2021, 124, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Hann, C.L.; French, C.A.; Cousin, S.; Brana, I.; Cassier, P.A.; Moreno, V.; de Bono, J.S.; Duckworth Harward, S.; Ferron-Brady, G.; et al. Phase 1 study of molibresib (GSK52525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other sold tumors. JNCI Cancer Spectr. 2019, 4, pkz093. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.T.; Lin, X.; Faivre, E.J.; Yang, Z.; Huang, X.; Wilcox, D.M.; Bellin, R.J.; Jin, S.; Tahir, S.K.; Mitten, M.; et al. Vulnerability of small-cell lung cancer to apoptosis induced by the combination of BET bromodomain proteins and BCL2 inhibitors. Mol. Cancer Ther. 2017, 16, 1511–1520. [Google Scholar] [CrossRef]
- Bian, X.; Wang, X.; Zhang, Q.; Ma, L.; Cao, G.; Xu, A.; Han, J.; Huang, J.; Lin, W. The MYC paralog-PARP1 axis as a potential therapeutic target in MYC paralog-activated small cell lung cancer. Front. Oncol. 2020, 10, 565820. [Google Scholar] [CrossRef]
- Fiorentino, F.P.; Marchesi, I.; Schroeder, C.; Schmidt, R.; Yokota, J.; Bagella, L. BET-inhibitor I-BET762 and PARP-inhibitor talzoparib synergy in small cell lung cancer cells. Int. J. Mol. Sci. 2020, 21, 9595. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Rao, M.; Zhang, H.; Beers, J.; Wangsa, D.; Wangsa, D.; Buishand, F.O.; Wang, Y.; Yu, Z.; Stevenson, H.S.; et al. ASXL3 is a novel pluripotency factor in human respiratory epithelial cells and a potential therapeutic target in small cell lung cancer. Cancer Res. 2017, 77, 6267–6281. [Google Scholar] [CrossRef]
- Szczepanski, A.P.; Zhao, Z.; Sosnowski, T.; Goo, Y.A.; Barton, E.T.; Wang, L. ASXL3 bridges BRD4 to BAP1 complex and governs enhancer activity in small cell lung cancer. Genome Med. 2020, 12, 63. [Google Scholar] [CrossRef]
- Sun, D.; Nikonova, A.S.; Zhang, P.; Deneka, A.Y.; Fitzgerald, M.E.; Michael, R.E.; Lee, L.; Lilly, A.C.; Fisher, S.L.; Phillips, A.J.; et al. Evaluation of the small-molecule BRD4-degrader CFT-2718 in small cell lung cancer and pancreatic cancer models. Mol. Cancer Ther. 2021, 20, 1367–1377. [Google Scholar] [CrossRef]
- Jiang, S.; Richaud, M.; Vieugué, P.; Rama, N.; Delcros, J.G.; Siouda, M.; Sanada, M.; Redavid, A.R.; Ducarouge, B.; Hervieu, M.; et al. Targeting netrin-3 in small cell lung cancer and neuroblastoma. EMBO Mol. Med. 2021, 13, e12878. [Google Scholar] [CrossRef]
- Reck, M.; Bondarenko, I.; Luft, A.; Sterwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Lu, H.; Cuillerot, J.M.; Lynch, T.J. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase 2 trial. Ann. Oncol. 2013, 24, 75–83. [Google Scholar] [CrossRef]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.W.; Akerley, W.; Pietanza, M.C.; Wu, Y.L.; Zielinski, C.; Thomas, M.; et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive -stage small-cell lung cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, C.; Johson, M.L.; Nishioi, M. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated opverallk survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (Impower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (cisplatin): A randomized, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y. Dervalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC (ES-SCLC): Updated results from the phase III CASPIAN study. J. Clin. Oncol. 2020, 15 (Suppl. 9002), 378. [Google Scholar]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomized, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, K.; Hotta, D.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; Voitko, F.; et al. Durvalumb ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC (ES-SCLS): 3-year overall survival update from the phase III CASPIAN study. Ann. Oncol. 2021, 32 (Suppl. 5), S1283–S1346. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csoszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized double-blind, phase III Keynote-604 trial. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Leal, T.; Wang, Y.; Dowlati, A.; Chen, Y.; Ramesh, A.; Razaq, M.; Liu, J.; Joseph, C.; King, D.M.; Ahuja, H.G.; et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with EA5161. J. Clin. Oncol. 2020, 38, 9000. [Google Scholar] [CrossRef]
- Ott, P.A.; Elez, E.; Hiret, S.; Kim, D.W.; Morosky, A.; Saraf, S. Pembrolizumab in patients with extensive-stage small-cell lung cancer: Results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2017, 35, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Lopez-Martin, J.A.; Kao, S.C.; Miller, W.H.; Ros, W.; Gao, B. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 2018, 36, 8506. [Google Scholar] [CrossRef]
- Owonikoko, T.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodriguiez-Cid, J.; Schenker, M.; et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef]
- Hong, L.; Negrao, M.V.; Dibaj, S.S.; Chen, R.; Reuben, A.; Bohac, J.M.; Liu, X.; Skoulidis, F.; Gay, C.M.; Cascone, T.; et al. Programmed death-ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in NSCLC. J. Thorac. Oncol. 2020, 15, 1449–1459. [Google Scholar] [CrossRef]
- Yu, H.; Batenchuk, C.; Badzio, A.; Boyle, T.A.; Czapiewski, P.; Chan, D.C.; Lu, X.; Gao, D.; Ellison, K.; Kowalewski, A.A.; et al. PD-L1 expression by two complementary diagnostic assays and mRNA in situ hybridization in small cell lung cancer. J. Thorac. Oncol. 2016, 12, 110–120. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rivzi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018, 33, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Kravers, S.; Dahlberg, S.E.; Umeton, R.; Albayrak, A.; Subegdjo, S.J.; Johnson, B.E.; Nishino, M.; Sholl, L.M.; Awad, M.M. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J. Immun. Cancer 2019, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Dwivedi, B.; Chen, Z.; Zhang, C.; Barwick, B.; Ernani, V.; Zhang, G.; Gilbert-Ross, M.; Carlisle, J.; Zhuri, F.R.; et al. YAP1 expression in SCLC defines a distinct subtype with T-cell-inflamed phenotype. J. Thorac. Oncol. 2021, 16, 464–476. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.J.; Piha-Paul, S.A.; Razak, A.; Bennouna, J.; Soria, J.C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-cell-inflamed gene-expression profile, programmed detah ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2018, 37, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, R.; Jin, J.; Hu, X.; Zhang, J.; Fujimoto, J.; Hubert, S.M.; Gay, C.M.; Zhu, B.; Tian, Y.; et al. Cold and heterogeneous T cell repertoire is associated with copy number aberrations and loss of immune genes in small-cell lung cancer. Nat. Commun. 2021, 12, 6655. [Google Scholar] [CrossRef] [PubMed]
- Davoli, T.; Uno, H.; Wooten, E.C.; Elledge, S.J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017, 355, eeaf8399. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, X.; Wang, J.; Tang, X.R.; Wu, D.H.; Du, S.S.; Du, X.J.; Zhang, Y.W.; Zhu, H.B.; Fang, Y.; et al. Combination of TMB and CAN stratifies prognostic and predictive responses to immunotherapy across metastatic cancer. Clin. Cancer Res. 2019, 25, 7413–7423. [Google Scholar] [CrossRef]
- Chan, J.M.; Quintanal-Villalonga, A.; Gao, V.R.; Xie, Y.; Allaj, V.; Choudhary, O.; Masilionis, I.; Egger, J.; Chow, A.; Walle, T.; et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of humn small cell lung cancer. Cancer Cell 2021, 39, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Roper, N.; Velez, M.J.; Chiappori, A.; Kim, Y.S.; Wei, J.S.; Sindiri, S.; Takahashi, N.; Mulford, D.; Kumar, S.; Vlaya, K.; et al. Notch signaling and efficacy of PD-1/PD-L1 blockade in relapsed small cell lung cancer. Nat. Commun. 2021, 2, 3880. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Knelson, E.H.; Wolff, J.O.; Vajdi, A.; Saigì, M.; Campisi, M.; Hong, D.; Thai, T.C.; Piel, B.; Han, S.; et al. Inrtinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov. 2021, 11, 1952–1969. [Google Scholar] [CrossRef]
- Muppa, P.; Terra, S.B.; Sharma, A.; Mansfield, A.S.; Aubry, M.C.; Bhinge, K.; Asiedu, M.K.; de Andrade, M.; Janaki, N.; Murphy, S.J.; et al. Immune cell infiltration may be a key determinant of long-term survival in small cell lung cancer. J. Thorac. Oncol. 2019, 14, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kallakury, B.; Chahine, J.J.; Hartmann, D.; Zhang, Y.W.; Chen, Y.; Zhang, H.; Zhang, B.; Wang, C.; Giaccone, G. Surgical resection of SCLC: Prognostic factors and the tumor microenvironment. J. Thorac. Oncol. 2019, 14, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Best, S.A.; Hess, J.B.; Souza-Fonseca-Guimares, F.; Cursons, J.; Kersbergen, A.; Dong, X.; Rautela, J.; Hyslop, S.R.; Ritchie, M.E.; Davis, M.J.; et al. Harnessing natural killer immunity in metastatic SCLC. J. Thorac. Oncol. 2020, 15, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, Y.; Bender, M.E.; Girard, L.; Kollipara, R.; Eglenen-Polat, B.; Naito, Y.; Savage, T.K.; Huffman, K.E.; Koyama, S.; et al. Evasion of innate immunity contributes to small cell lung cancer progression and metastasis. Cancer Res. 2021, 81, 1813–1826. [Google Scholar] [CrossRef]
- Thomas, P.L.; Groves, S.M.; Zhang, Y.K.; Zhang, Y.K.; Li, J.; Gonzalez-Ericsson, P.; Sivagnam, S.; Betts, C.B.; Chen, H.C.; Liu, Q.; et al. Beyond programmed death-ligand 1: B7-H6 emerges as a potential immunotherapy target in SCLC. J. Thorac. Oncol. 2021, 16, 1211–1223. [Google Scholar] [CrossRef]
- Yu, H.; Coczara, C.; Badzio, A.; Gao, D.; Rivard, C.J.; Ellison, K.; Suda, K.; Ren, S.; Caldwell, C.; Brosky, K.A.; et al. Expression of an immune checkpoint-Poliovirus receptor (PVR) in small cell liugn cancer. Cancer Res. 2018, 78, 3637. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, G.; Jiang, Z.; Li, N.; Zhang, X. Survival analysis with regard to PD-L1 and CD155 expression in human small cell luing canecr and a comparison with associatyed receptors. Oncol. Lett. 2019, 17, 2960–2968. [Google Scholar]
- Dora, D.; Rivard, C.; Yu, H.; Bunn, P.; Suda, K.; Ren, S.; Pickard, S.L.; Laszlo, V.; Harko, T.; Majesfalvi, Z.; et al. Neuroendocrine subtypes of small cell lung cancer differ in terms of immune microenvironment and checkpoint nolecule distribution. Mol. Oncol. 2020, 14, 1947–1965. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, S.S.; Hong, M.J.; Choi, J.E.; Kim, S.; Kang, H.G.; Do, S.K.; Kim, J.H.; Baek, S.A.; Lee, W.K.; et al. Impact of immune checkpoint gene CD155 Ala67Thr and CD226 Gly307Ser polymorphisms on small cell lung cancer clinical outcome. Sci. Rep. 2021, 11, 1794. [Google Scholar] [CrossRef]
- Remon, J.; Aldea, M.; Besse, B.; Planchard, D.; Reck, M.; Giaccone, G.; Soria, J.C. Small cell lung cancer: A slightly less orphan disease after immunotherapy. Ann. Oncol. 2021, 32, 698–709. [Google Scholar] [CrossRef]
- Bebber, C.M.; Thomas, E.S.; Stroh, J.; Chen, Z.; Androulidaki, A.; Schmitt, A.; Hohne, M.N.; Stuker, L.; de Paula Alves, C.; Khonsari, A.; et al. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. Nat. Commun. 2021, 12, 2048. [Google Scholar] [CrossRef]
- Simbolo, M.; Centonze, G.; Alì, G.; Garzone, G.; Taormina, S.; Sabella, G.; Ciaparrone, G.; Mafficini, A.; Grillo, F.; Mangogna, A.; et al. Integrative molecular analysis of combined small-cell lung carcinomas identifies major subtyeps with different therapeutic opportunities. ESMO Open 2021, 21, 100308. [Google Scholar]
- Iida, Y.; Okamoto-Katsuyama, M.; Maruoka, S.; Takahashi, M.; Tsuya, K.; Shikano, S.; Hikichi, M.; Takahashi, M.; Tsuya, K.; Okamoto, S.; et al. Effective ferroptic small-cell lung cancer cell death from SLC7A11 inhibition by sulphoraphane. Oncol. Lett. 2021, 21, 71. [Google Scholar] [CrossRef]
- Lai, A.Y.; Sorrentino, J.A.; Dragnev, K.H.; Weiss, J.M.; Owonikoko, T.K.; Rytlewski, J.A.; Hood, J.; Yang, Z.; Malik, R.K.; Strum, J.C.; et al. CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J. Immunother. Cancer 2020, 8, e000847. [Google Scholar] [CrossRef]
- Weiss, J.M.; Csoszi, T.; Maglakekelidze, M.; Hoyer, R.J.; Beck, J.T.; Gomez, M.D.; Aljumaily, R.; Rocha Lima, C.M.; Boccia, R.V.; Hanna, W.; et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: A phase Ib/randomized phase II trial. Ann. Oncol. 2019, 30, 1613–1621. [Google Scholar] [CrossRef]
- Weiss, J.; Goldschmidt, J.; Andric, Z.; Dragnev, K.H.; Gwaltney, C.; Slatsa, K.; Pritchett, Y.; Antal, J.M.; Morris, S.R.; Daniel, D. Effects of trilaciclib on chemotherapy-induced myelosuppression and patient-erported outcomes in patients with extensive-stage small cell lung cancer: Pooled results from three phase II randomized, double-blind, placebo-controlled studies. Clin. Lung Cancer 2021, 22, 449–460. [Google Scholar] [CrossRef]
- Daniel, D.; Kuchava, V.; Bondarenko, I.; Ivashchuk, O.; Reddy, S.; Jaal, J.; Kudaba, I.; Hart, L.; Matitashvili, A.; Pritchett, Y.; et al. Tricaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: A multicenter, randomized, double-blind, placebo-controlled phase II trial. Int. J. Cancer 2020, 148, 2557–2570. [Google Scholar] [CrossRef]
- Nilsson, O.; Brezicka, F.T.; Holmgren, J.; Sorenson, S.; Svennerholm, L.; Yngvason, F. Detection of a ganglioside antigen associated with small cell lung carcinoma using monoclonal antibodies directed against fucosyl GM1. Cancer Res. 1986, 46, 1403–1407. [Google Scholar]
- Tokuda, N.; Zhang, Q.; Yoshida, S.; Kusunoki, S.; Urano, T.; Furukawa, K.; Furukawa, K. Genetic mechanisms for the synthesis of fucosyl GM1 in small cell lung cancer cell lines. Glycobiology 2006, 16, 916–925. [Google Scholar] [CrossRef]
- Ponath, P.; Menezes, D.; Pan, C.; Chen, B.; Oyasu, M.; Strachan, D.; LeBlanc, H.; Sun, H.; Wang, X.T.; Rangan, V.S.; et al. A novel, fully human anti-fucosyl-GM1 antibody demonstrates potent in vitro and in vivo antitumor activity in preclinical models of small cell lung cancer. Clin. Cancer Res. 2018, 24, 5178–5189. [Google Scholar] [CrossRef]
- Matsuo, T.; Iguchi-Manaka, A.; Shibuya, A.; Shibuya, K. CD155 Mutation (Ala67Thr) Increases the Binding Affinity for and the Signaling via an Inhibitory Immunoreceptor TIGIT. Cancer Sci. 2022; in press. [Google Scholar]
- Chu, Q.S.C.; Markman, B.; Leighl, N.; Krug, L.; Rudin, C.; Lathers, D.; Basciano, P.; Fracasso, P.M.; Kollia, G.; Phillips, P.; et al. A phase I/II trial of a transcriptional targeting fucoyl GM1 in relapsed/refractory small cell lung lung cancer /SCLC): Safety and preliminary efficacy. Ann. Oncol. 2016, 27 (Suppl. 6), 1427PD. [Google Scholar] [CrossRef]
- Chu, Q.; Leighl, N.; Surmont, V.; Van Herpen, C.; Sibille, A.; Markman, B.; Calrke, S.; Juergens, R.; Acosta Rivera, M.; Adelkovic, V.; et al. Clinical activity of BMS-986012, an anti-fucosyl-GM1 monoclonal antibody, plus nivolumab in small cell lung cancer. J. Thorac. Oncol. 2021, 16, S195. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Provencio, M.; Trigo, J.M.; Tannenbuam-Dvir, S.; Basciano, P.; Lathers, D.; Urbanska, K.; Kollia, G.; He, C.; Dipiero, A.; et al. Safety of BMS-986012, an anti-fucosyl-GM1 monoclonal antibody plus platinum/etposide in untreated extensive-stage SCLC. J. Thorac. Oncol. 2021, 16, S345–S346. [Google Scholar] [CrossRef]
- Vidhyasagar, V.; Haq, S.U.; Lok, B.H. Patient-derived xenograft models of small cell lung cancer for therapeutic development. Clin. Oncol. 2020, 32, 619–625. [Google Scholar] [CrossRef]
- Drapkin, B.J.; George, J.; Christensen, C.L.; Mino-Kenudson, M.; Dries, R.; Sundaresan, T.; Phat, S.; Myers, D.T.; Zhong, J.; Igo, P.; et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 2018, 8, 600–615. [Google Scholar] [CrossRef]
- Vickers, A.J.; Frise, K.; Galvin, M.; Carter, M.; Franklin, L.; Morris, K.; Pierce, J.; Descamps, T.; Blackhall, F.; Dive, C.; et al. Brief report on the clinical characteristics of patients whose samples generate small cell lung cancer circulating tumour cell derived explants. Lung Cancer 2020, 150, 216–220. [Google Scholar] [CrossRef]
- Swarts, D.; Ramaekers, F.C.; Speel, E.J. Molecular and cellular biology of neuroendocrine lung tumors: Evidence for separate biological entities. Biochim. Biophys. Acta 2012, 1826, 255–271. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart, 4th ed.; World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2015. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G. The 2015 World Health Organization Classification of lung tumours: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Metovic, J.; Barella, M.; Bianchi, F.; Hofman, P.; Hofman, V.; Remmelink, M.; Kern, I.; Carvalho, L.; Pattini, L.; Sonzogni, A.; et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021, 478, 5–19. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editoprial Board. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Fernandez-Cuesta, M.; Peifer, M.; Lu, X.; Sun, R.; Ozretić, L.; Seidal, D.; Zander, T.; Leenders, F.; George, J.; Müller, C.; et al. Frequent mutations in chromatin-remodeling genes in pulmonary carcinoids. Nat. Commun. 2014, 3, 3518. [Google Scholar] [CrossRef]
- Qiu, H.; Jin, B.M.; Wang, Z.F.; Xu, B.; Zheng, Q.F.; Zhang, L.; Zhu, L.Y.; Shi, S.; Yuan, J.B.; Lin, X.; et al. MEN1 deficiency leads to neuroendocrine differentiation of lung cancer and disrupts the DNA damage response. Nat. Commun. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Simbolo, M.; Mafficini, A.; Sikora, K.O.; Fassan, M.; Barbi, S.; Corbo, V.; Mastracci, L.; Rusev, B.; Grillo, F.; Vicentini, C.; et al. Lung neuroendocrine tumours: Deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling gene as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J. Pathol. 2017, 241, 488–500. [Google Scholar] [CrossRef]
- Centonze, G.; Biganzoli, D.; Prinzi, N.; Pusceddu, S.; Mangogna, A.; Tamborini, E.; Perrone, F.; Busico, A.; Lagano, V.; Cattaneo, L.; et al. Beyond traditional morphological characterization of lung neuroendocrine neoplasms: In silico study of next-generation sequencing mutations analysis across the four world health organization defined groups. Cancers 2020, 12, 2753. [Google Scholar] [CrossRef]
- Laddha, S.V.; DaSilva, E.M.; Robzyk, K.; Untch, B.R.; Ke, H.; Rekhtman, N.; Poirier, J.T.; Travis, W.D.; Tang, L.H.; Chan, C.S. Integrative genomic characterization identifies molecular subtypes of lung carcinoids. Cancer Res. 2019, 79, 4339–4347. [Google Scholar] [CrossRef]
- Alcala, N.; Leblay, N.; Gabriel, A.A.G.; Mangiante, L.; Hervas, D.; Giffon, T.; Sertier, A.S.; Ferrari, A.; Derks, J.; Ganthous, A.; et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat. Commun. 2019, 10, 3407. [Google Scholar] [CrossRef]
- Miyoshi, T.; Umemura, S.; Matsumura, Y.; Mimaki, S.; Tada, S.; Makinoshima, H.; Ishii, G.; Udagawa, H.; Matsumoto, S.; Yoh, K.; et al. Genomic profiling of large-cell neuroendocrine carcinoma of the lung. Clin. Cancer Res. 2016, 23, 757–765. [Google Scholar] [CrossRef]
- Rekhtman, N.; Pietanza, M.C.; Hellmann, M.D.; Naidoo, J.; Arora, A.; Won, H.; Halpenny, D.F.; Wang, H.; Tian, S.K.; Litvak, A.M.; et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin. Cancer Res. 2016, 22, 3618–3629. [Google Scholar] [CrossRef]
- Esfahani, H.S.; Vela, C.M.; Chauhan, A. Prevalence of TP-53/Rb-1 co-mutation in large cell neuroendocriene carcinoma. Front. Oncol. 2021, 11, 653153. [Google Scholar] [CrossRef]
- Rekhtman, N.; Pietanza, C.M.; Sabari, J.; Montecalvo, J.; Wang, H.; Habeeb, O.; Kadota, K.; Adusumilli, P.; Ruding, C.M.; Ladanyi, M.; et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: Napsin A expression and genomic alterations. Mod. Pathol. 2018, 31, 111–121. [Google Scholar] [CrossRef]
- Derks, J.L.; Leblay, N.; Thunissen, E.; van Suylen, R.J.; den Bakker, M.; Groen, H.; Smit, E.F.; Damhuis, R.; den Broek, E.; Charbier, A.; et al. Molecular subtypes of pulmonary large-cell neuroendocrine carcinoma predict chemotherapy treatment outcome. Clin. Cancer Res. 2017, 24, 33–42. [Google Scholar] [CrossRef]
- George, J.; Walter, W.; Peifer, M.; Alexandrov, L.B.; Seidel, D.; Leenders, F.; Maas, L.; Muller, C.; Dahmen, I.; Delhomme, T.M.; et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat. Commun. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Simbolo, M.; Barbi, S.; Fassan, M.; Mafficini, A.; Ali, G.; Vicentini, C.; Sperandio, N.; Corbo, V.; Rusev, B.; Mastracci, L.; et al. Gene expression profiling of lung atypical carcinoids and large neuroendocrine carcinomas identifies three transcriptomic subtypes with specific genomic alterations. J. Thorac. Oncol. 2019, 14, 1651–1661. [Google Scholar] [CrossRef]
- Zhuo, M.; Guan, Y.; Yang, X.; Hong, L.; Wang, Y.; Li, Z.; Chen, R.; Abbas, H.A.; Chang, L.; Gong, Y.; et al. The prognostic and therapeutic role of genomic subtyping by sequencing tumor or cell-free DNA in pulmonary large-cell neuroendocrine cancer. Clin. Cancer Res. 2020, 26, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Yoshida, J.; Ishii, G.; Aokage, K.; Hishida, T.; Nagai, K. The differences of biological behavior based on the clinicopathological data between resectable large-cell neuroendocrine carcinoma and small-cell luing carcinoma. Clin. Lung Cancer 2013, 14, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Li, Y.; Yan, L.X.; Zhu, Z.F.; Dong, X.R.; Chu, Q.; Wu, L.; Zhang, H.M.; Xu, C.W.; Lin, G.; et al. Disparity in clinical outcomes between pure and combined pulmonary large-cell neuroendocrine carcinoma: A multi-center retrospective study. Lung Cancer 2020, 139, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Maisonnuieve, P.; Grillo, F.; Mangogna, A.; Centonze, G.; Prinzi, N.; Pusceddu, S.; Garzone, G.; Cattaneo, L.; Busico, A.; et al. Ki-67 index of 55% distinguishes ywo groups of bronchopulmonary pure and composite large cell neuroendocrine carcinomas with distinct prognosis. Neuroendocrinology 2021, 111, 475–489. [Google Scholar] [CrossRef]
- Quinn, A.M.; Chaturvedi, A.; Nonaka, D. High-grade neuroendocrine carcinoma of the lung with carcinoid morphology: A study of 12 cases. Am. J. Surg. Pathol. 2017, 41, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Rekhtman, N.; Desmeules, P.; Litvak, A.M.; Pietanza, M.C.; Santos-Zabala, M.L.; Ni, A.; Montecalvo, J.; Chang, J.C.; Beras, A.; Preeshagul, I.R.; et al. Stage IV carcinoids: Spectrum and evolution of proliferation rate, focusibng on variants with elevated proliferation indices. Mod. Patol. 2019, 32, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Rubino, M.; Scoazec, J.Y.; Pisa, E.; Faron, M.; Spaggioari, L.; Hadoux, J.; Spada, F.; Planchjard, D.; Cella, C.A.; Leboullex, S.; et al. Lung carcinoids with high proliferative activity: Firther suppport for the identification of a new tumor category in the classification of lung neuroendocrine neoplasms. Lung Cancer 2020, 148, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.C.M.; Derks, J.L.; Moonen, L.; Habraken, C.H.J.; von der Thusen, J.; Hillen, L.M.; Speel, E.J.M.; Dingemans, A.M.C. Pulmonary neuroendocrine neoplasms with well differentiated morphology and high proliferative activity: Illustrated by a case series and review of the literature. Lung Cancer 2020, 150, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Bianchi, F.; Dama, E.; Simbolo, M.; Mafficini, A.; Sonzogni, A.; Pilotto, S.; Harari, S.; Papotti, M.; Volante, M.; et al. Most high-grade neuroendocrine tumours of the lung are likely to secondarily develop from pre-existing carcinoids: Innovative findings skipping the current pathogenesis paradigm. Virchows Arch. 2018, 472, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Théou-Anton, N.; Gounant, V.; Nicolle, R.; Reyes, C.; Humez, S.; Hescot, S.; Thomas de Montpréville, V.; Guyétant, S.; Scoazec, J.Y.; et al. Specific genomic alterations in high-grade pulmonary neuroendocrine tumours with carcinoid morphology. Neuroendocrinology 2021, 111, 158–169. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Uccella, S. Classification of neuroendocrine neoplasms: Lights and shadows. Rev. Endocr. Metab. Disord. 2021, 22, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, S.; Pérez-Crespo, M.; Lorz, C.; Bernardini, A.; Oteo, M.; Enguita, A.B.; Romero, E.; Hernández, P.; Tomás, L.; Morcillo, M.Á.; et al. Differential development of large-cell neuroendocrine or small-cell lung carcinoma upon inactivation of 4 tumor suppressor genes. Proc. Natl. Acad. Sci. USA 2019, 116, 22300–22306. [Google Scholar] [CrossRef]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelamn, J.A. Transformation from non-small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef]
- Marcoux, N.; Gettinger, S.N.; O’Kanne, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikansky, A.; Bonomi, P.D.; et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: Clinical outcomes. J. Clin. Oncol. 2018, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; Levra, M.G.; Brevet, M.; Antoine, M.; Mazieres, J.; Rossi, G.; Chiari, R.; Westeel, V.; Poudenx, M.; Letreut, J.; et al. A brief report of transformation from NSCLC to SCLC: Molecular and therapeutic characteristics. J. Thorac. Oncol. 2018, 14, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Niederst, M.J.; Sequist, L.V.; Porier, J.T.; Mermel, C.H.; Lockerman, E.L.; Garcia, A.R.; Katayama, R.; Costa, C.; Ross, K.N.; Miron, T.; et al. RB loss in resistant EGFR mutant lung adenocarcinoma that transform to small-cell lung cancer. Nat. Commun. 2015, 6, 6377. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Tomashfskiu, J.F.; Snow, N.J.; Horrigan, T.P.; Hines, J.D. Mixed small cell and non-small cell lung cancer. Chest 1986, 8, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Mangum, M.D.; Greco, F.A.; Hainsworth, J.D.; Hande, K.R.; Johnson, D.H. Combined small-cell -lung cancer. J. Clin. Oncol. 1989, 7, 607–612. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Tatematsu, A.; Shimizu, J.; Murakami, Y. Epidermal growth factor receptor mutations in small cell lung cancer. Clin. Cancer Res. 2008, 14, 6092–6096. [Google Scholar] [CrossRef]
- Shiao, T.H.; Chang, Y.L.; Yu, C.J. Epidermal growth factor receptor mutations in small cell lung cancer. J. Thorac. Oncol. 2011, 6, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Luo, J.; Ge, T.; Fan, P.; Sun, L.; Hou, L.; Li, J.; Yu, H.; Wu, C. Comprehensive genomic profiling of combined small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, J.; Kim, S.; Kim, S.; Youk, J.; Park, S.; An, Y.; Keam, B.; Kim, D.W.; Heo, D.S.; et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 2017, 35, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Hwang, S.H.; Choi, Y.L.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J.; Park, W.Y. Transformation to small cell lung cancer of pulmonary adenocarcinoma. Clinicopathologic analysis of six cases. J. Pathol. Transl. Med. 2016, 50, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; McCutcheon, J.N.; Kallakury, B.; Chahine, J.; Pratt, D.; Raffeld, M.; Chen, Y.; Wang, C.; Giaccone, G. Combined small cell carcinoma of the lung: Is it a single entity? J. Thorac. Oncol. 2017, 13, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Feng, H.; Qiang, H.; Shang, Z.; Chang, C.; Qian, J.; Zhang, Y.; Zhong, R.; Fan, X.; Chu, T. Clinical characteristics and prognostic factors of surgically resected combined small cell lung cancer: A retrospective study. Lung Cancer 2020, 146, 244–251. [Google Scholar] [CrossRef]
- Offin, M.; Chen, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbord, Y.; Quintal-Villalonga, A.; Penson, A.; et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, Y.; Yimng, J.; Cai, W.; Li, J.; Lee, K.Y.; Ricciuti, B.; Pacheco, J.; Xing, P. Whole exome sequencing (WES) analysis of transformed small cell lung cancer (SCLC) from lung adenocarcinoma (LUAD). Transl. Lung Cancer Res. 2020, 9, 2428–2439. [Google Scholar] [CrossRef]
- Quintanal-Villalonga, A.; Taniguchi, H.; Zhan, Y.A.; Hasan, M.M.; Chavan, S.S.; Meng, F.; Uddin, F.; Manoj, P.; Donoghue, M.; Won, H.H.; et al. Multi-omic analysis of lung tumors defines pathways activated in neuroendocrine transformation. Cancer Discov. 2021, 11, 3028–3047. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Minami, Y.; Iijima, T.; Kameya, T.; Asamura, H.; Noguchi, M. Characteristics of loss of heterozygosity in large cell neuroendocrine carcinomas of the lung and small cell lung carcinomas. Pathol. Int. 2006, 56, 434–439. [Google Scholar] [CrossRef]
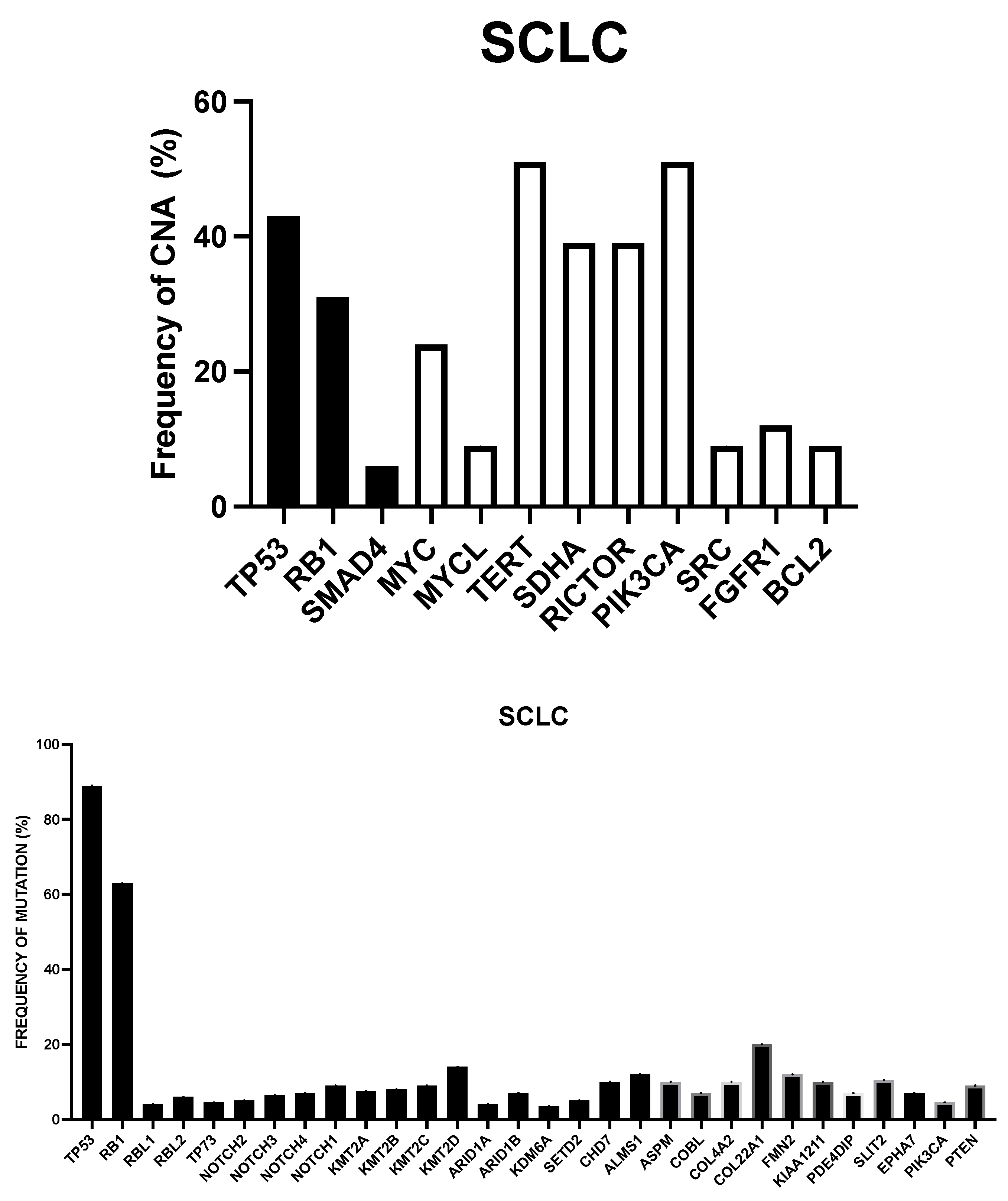

| Copy Number Alterations | Recurrent Mutations | Gene Fusions |
|---|---|---|
| Gene Deletions CDKN2A, FHIT, RASSF1, RB1 TP53 Gene Amplifications CCNET, FGFR1. IRS2, MET, MYC, MYCL, MYCN, NFIB, SOX2, SOX4 | Cell Cycle and Apoptosis RB1, RBL1, RBL2, TP53, TP73 Epigenetic Regulators ARID1A, ARID1B, CHD7, CREBBP, EP300, KDM6A, KMT2A, KMT2B, KMT2C, KMT2D, PBMR1, SETD2 NOTCH Pathway NOTCH1, NOTCH2, NOTCH3, NOTCH4 Receptor Kinase Signaling EPHa7, PIK3CA, PTEN Cell Adhesion-Cytoskeleton ALMS1, ASPM, COBL, COL4A2, COL22A1, FMN2, KIAA1211, PDE4DIP, SLIT2 | KIAA432-JAK2 PLEKHM2-ALK PVT1-CHD7 PVT1-CCNB1IPI RFL-MYCL1 RFL-FAM132A RFL-SMAP2 |
| Pathway | Target | Clinical Context and Biomarker | Drug and Clinical Studies |
|---|---|---|---|
| DNA Damage Repair (DDR) | PARP1 | Relapsed SLCL. SLFN11 is a biomarker correlated with response. Low inflammatory signature is a biomarker of resistance. | Veripalib or Oripalib with Temozolomide: Improved PFS in SLFN11-positive patients |
| DNA Damage Repair (DDR) | PARP1 | First-line untreated SCLC | Veripalib with standard chemotherapy (platinum + etoposide): improved PFS but not OS. |
| DNA Damage Repair (DDR) | ATR | Relapsed SCLC. CNAs in genes driving replication stress (CCNE1 gain, ARID1A loss) in responding patients | Berzosertib (ATR inhibitor) with Topotecan 1: 36% of responding patients. |
| Cell Cycle | CHK1 | Relapsed SCLC. CHK1 inhibitors synergize with cisplatin. PARP inhibitors and ICIs. CHK1 and MYC overexpression are biomarkers of response to CHK1 inhibitors | Prexaserib, a CHK1 inhibitor alone showed very limited antitumor activity in relapsed SCLC patients. |
| Cell Cycle | WEE1 | Relapsed SCLC | AZD17765, a WEE1 inhibitor, used in monotherapy showed very limited antitumor activity in relapsed SCLC patients. |
| Cell Cycle | CDK4 CDK6 | CDK4/CDK6 inhibitors cannot decrease the proliferations of SCLCs that are RB1-defective, but can decrease the cytotoxic effects of chemotherapy on the hematopoietic system reducing the cycling activity of HSCs and stimulate the anti-tumor immune response | Trilaciclib, a CDK4/CDK6 inhibitor when associated with various chemotherapy regimens in first- or second-line improved chemotherapy-induced myelosuppression but did not modify anti-tumor efficacy outcomes. |
| NOTCH Pathway | DLL3 | Maintenance therapy or second-line therapy. DLL3 tumor positivity is a biomarker of sensitivity. | Rovalpituzumab tesirine as maintenance therapy or as second-line therapy: no effect on OS. |
| NOTCH Pathway | NOTCH2 NOTCH3 | First-line therapy in association with standard chemotherapy (platinum plus etoposide) | Tarextumab in association with platinum plus etoposide in first-line, compared to placebo plus chemotherapy. |
| Apoptosis | BCL-2 | First-line therapy in association with chemotherapy; second-line therapy, relapsed patients. High BCL-2 level is a biomarker of in vitro sensitivity to Venetoclax | Nivotoclax in monotherapy, in relapsed SCLC patients: very low responsding rate. |
| Mitotic pathway | AURORA A KINASE | Relapsed/refractory SCLC in association with chemotherapy c-MYC expression is a biomarker of sensitivity | Alisertib plus plataxel in second line therapy: no benefit in the overall population; improvement of PFS in patients with c-MYC expression. |
| Epigenetic Regulation | BET Proteins | Second-line therapy in association with PARPis or Ventoclax. | No clinical response using BET inhibitors in monotherapy in relapsed/refractory patients. |
| Trial Number | Study Population | Trial Design | Medication | Phase | Peculiar Features of The Trial |
|---|---|---|---|---|---|
| NCT02511795 | Relapsed/refractory ES-SCLC | Phase Ib including a dose-escalation and a dose-expansion phases | AZD1775 (WEE1 TK inhibitor) + Olaparib | Ib | |
| NCT03532880 | ES-SCLC | Phase I including patients with E-SCLC who have completed induction chemotherapy and have no evidence of tumor growth | Olaparib + low-dose radiotherapy | I | Patients with ES-SCLC with stable disease after chemotherapy |
| NCT04939662 | Relapsed/refractory ES-SCLC | Single-arm phase II study of Olaparib and Bevacizumab combination therapy in SCLC as second/third line therapy. Enrolled patients must display ATM deficiency, SLFN11 positivity, POU2F3+; HR pathway gene mutation | Olaparib + Bevacizumab (VEGF inhibitor) | II | Samples from primary and metastatic tumors in progression are investigated for analysis of the mechanisms of resistance |
| NCT02498613 | Relapsed/refractory ES-SCLC | Single-arm phase II study of Olaparib and Cediranib combination in metastatic SCLC patients who received one prior line of platinum chemotherapy | Olaparib + Cediranib (VEGF inhibitor) | II | Correlation with DNA repair gene expression. Promising clinical activity in biomarker unselected patients with ORR of 28%. |
| NCT02769962 | Relapsed/refractory ES-SCLC | Single-arm phase I/II study of Olaparib with CLRX101 | Olaparib + CLRX101 (nanoparticle Campothecin) | I | |
| NCT02484404 | Patients previously treated with chemotherapy | Single-arm phase I/II study of Olaparib with MEDI4736, an anti-PD-L1 Ab | Olaparib + MEDI4736 (PD-L1 Ab) | I/II | |
| NCT02734004 | Relapsed/refractory ES-SCLC | Single-arm phase I/II study of Olaparib with MEDI4736, an anti-PD-L1 Ab | Olaparib + MEDI4736 (PD-L1 Ab) | I/II | |
| NCT04538378 | Lung cancers with EGFR mutations developing resistance to EGFR inhibitors through transformation to SCLC | Single-arm phase II study of Olaparib with Durvalumab, an anti-PD-L1 Ab | Olaparib + Durvalumab (PD-L1 Ab) | II | |
| NCT04170946 | Patients with ES-SCLC with stable disease after standard induction chemotherapy | Single-arm phase I study of Talazoparib in combination with consolidative thoracic radiotherapy | Talazoparib + Consolidative radiotherapy | I | Maintenance therapy for stable disease after induction chemotherapy |
| NCT04334941 | Patients with SLFN11 positive ES-SCLC | Randomized phase II study of maintenance versus Atezolizumab with Talazoparib | Talazoparib + Atezolizumab | II | |
| NCT03672773 | Patients with ES-SCLC with stable disease after standard induction chemotherapy | Single-arm phase II study of Talozapir in combination with Temozolomide | Talazoparib + Temozolomide | II | Intermittent low-dose Temozolomide in association with continuous talazoparib |
| NCT03958045 | ES-SCLC | Single-arm phase II study of Rucapirib in combination with Nivolumab | Rucaparib + Nivolumab (PD-1 Ab) | II | Maintenance therapy for stable disease after induction chemotherapy |
| NCT04209595 | ES-SCLC | Single-arm phase I/II study of Rucapirib in combination with PLX038 | Rucaparib + PLX038 (PEGylated conjugate of irinotecan) | I/II | Improvement in DNA damage induced by PLX038 |
| NCT03830918 | Patients with ES-SCLC with stable disease after standard induction chemotherapy | Niraparib + Temozolomide + Atezolizumab vs Atezolizumab as maintenance therapy in ES-SCLC | Niraparib + Atezolizumab + Temozolomide | Maintenance therapy for stable disease after induction chemotherapy | |
| NCT04701307 | Patients with ES-SCLC with stable disease after standard induction chemotherapy | Single-arm phase II study of Nirapirib in combination with Dosratlimab | Niraparib + Dostarlimab (anti-PDCD1 Ab) | II |
| Trial Number | Study Population | Trial Design | Medication | Phase |
|---|---|---|---|---|
| NCT02157792 | ES-SCLC | Phase I study involving treatment with the ATR inhibitor Berzosertib and chemotehrapy | Berzosertib (VX-970 M6620) + Cisplatin/ Etoposide or Cisplatin | I |
| NCT02487095 | Refractory/relapsed ES-SCLC | Phase I/II study involving treatment with ATR inhibitor Berzosertib and Topotecan | Berzosertib + Topotecan | I/II |
| NCT04768296 | Refractory/relapsed platinum-resistant ES-SCLC | Single-arm phase II study involving treatment with ATR inhibitor Berzosertib and Topotecan | Berzosertib + Topotecan | II |
| NCT03896503 | Refractory/relapsed ES-SCLC | Randomized trial involing treatment with Topotecan alone or in combination with Berzosertib | Berzosertib + Topotecan | II |
| NCT0328607 | Refractory/relapsed ES-SCLC | Two-arms phase II study involving the treatment of refractory/relapsed ES-SCLC with either Olaparib alone (patients with HR pathway gene mutations) or in association with Ceralasertib (unselected patients) | Olaparib + Ceralasertib (AZD6738) | II |
| NTC04361825 | Refractory/relapsed ES-SCLC | Single-arm phase II study involving treatment with ATR inhibitor Cerlasertib and the anti anti-PD-L1 Ab Durvalumab | Cerlasertib + Durvalumab | II |
| Tumor | % of Lung Tumors | Main Biologic Properties | Recurrent Genetic Alterations | Molecular Subgroups | Prognosis Survival |
|---|---|---|---|---|---|
| Typical Carcinoid | 1–2 | Cell size variable Low mitotic index Ki-67: low | Mutation/loss: EIF1AX, ARID1A, LRP1B, NF1, DST | Good 5-yr: high | |
| Atypical Carcinoid | 1–2 | Cell size variable Low/intermediate mitotic index Ki-67: intermediate | Mutation/loss: MEN1, ATP1A2, EIF1AX, ARID1A, SMARCA4, PKD1, AMER1, RAD51C | Cluster A1: immune infiltration, ASCL1+, DLL3+ Cluster A2: EIF1AXmutation SLIT1, ROBO1 downregulation Cluster B: MEN1 mutations, monocytes, poor prognosis. | Variable 5-yr: middle |
| Large-cell NE cancer (LCNEC) | 3 | Cell size large, with abundant cytoplasm High mitotic index Ki-67: high | Mutation/loss: TP53, LRP1B, RB1, SYNE1, ADAMTS12, USH2A, KEAP1, STK11, PTEN, NOTCH1, KMT2A Amplification: MYC, MYCN, MYCL1 | Type I (TP53, STK11, KEAP1) Type II (TP53, RB1) | Poor 5-yr: low |
| Small-cell lung cancer (SCLC) | 15–20 | Cell size small, with scarce cytoplasm High mitotic index Ki-67: very high | Mutation/loss: TP53, RB1, LRP1B, CSMD3, ZFHX4, SYNE1, USH2A, KMT2D, PTEN, NOTCH1, KMT2A Amplification: MYC, MYCN, MYCL1 | SCLC-A SCLC-N SCLC-P SCLC-Y | Poor 5-yr: very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, U.; Pelosi, E.; Castelli, G. Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets. Onco 2022, 2, 186-244. https://doi.org/10.3390/onco2030013
Testa U, Pelosi E, Castelli G. Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets. Onco. 2022; 2(3):186-244. https://doi.org/10.3390/onco2030013
Chicago/Turabian StyleTesta, Ugo, Elvira Pelosi, and Germana Castelli. 2022. "Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets" Onco 2, no. 3: 186-244. https://doi.org/10.3390/onco2030013
APA StyleTesta, U., Pelosi, E., & Castelli, G. (2022). Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets. Onco, 2(3), 186-244. https://doi.org/10.3390/onco2030013







