Journal Description
Onco
Onco
is an international, peer-reviewed, open access journal on the whole field of oncotargets and cancer therapies research published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 20.7 days after submission; acceptance to publication is undertaken in 4.8 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Onco is a companion journal of Cancers.
- Journal Clusters of Oncology: Cancers, Current Oncology, Onco and Targets.
Latest Articles
Targeting of Tumor Dormancy Pathways: An Editorial to the Special Issue
Onco 2025, 5(4), 48; https://doi.org/10.3390/onco5040048 - 3 Nov 2025
Abstract
A central obstacle in contemporary oncology is tumor relapse and metastatic recurrence [...]
Full article
(This article belongs to the Special Issue Targeting of Tumor Dormancy Pathway)
Open AccessReview
The Role of Metabolic Inflammation and Insulin Resistance in Obesity-Associated Carcinogenesis–A Narrative Review
by
Ademar Dantas da Cunha Junior, Larissa Ariel Oliveira Carrilho, Paulo Ricardo Santos Nunes Filho, Luca Cantini, Laura Vidal, Maria Carolina Santos Mendes, José Barreto Campello Carvalheira and Kamal S. Saini
Onco 2025, 5(4), 47; https://doi.org/10.3390/onco5040047 - 27 Oct 2025
Abstract
►▼
Show Figures
The inflammatory milieu surrounding tumors plays a pivotal yet paradoxical role in promoting carcinogenesis. Rather than simply acting as a host defense mechanism, chronic low-grade inflammation actively nurtures tumor development and supports hallmarks such as sustained proliferative signaling, apoptosis resistance, angiogenesis, and metastasis.
[...] Read more.
The inflammatory milieu surrounding tumors plays a pivotal yet paradoxical role in promoting carcinogenesis. Rather than simply acting as a host defense mechanism, chronic low-grade inflammation actively nurtures tumor development and supports hallmarks such as sustained proliferative signaling, apoptosis resistance, angiogenesis, and metastasis. Obesity, characterized by a chronic inflammatory state, exacerbates this tumor-promoting environment through metabolic imbalances like insulin resistance, hyperglycemia, and dyslipidemia. These conditions stimulate oncogenic signaling pathways and reshape the tumor microenvironment. Obesity-associated cytokines, altered adipokines, and insulin-related growth signals synergistically enhance processes such as epithelial-to-mesenchymal transition (EMT) and matrix remodeling. This review explores the mechanistic interplay between obesity-induced inflammation and insulin resistance in cancer progression, discusses the molecular pathways involved, and highlights emerging therapeutic approaches targeting these intersecting tumor promotion axes.
Full article

Figure 1
Open AccessReview
Preoperative Assessment of Surgical Resectability in Ovarian Cancer Using Ultrasound: A Narrative Review Based on the ISAAC Trial
by
Juan Luis Alcázar, Cristian Morales, Carolina Venturo, Florencia de la Maza, Laura Lucio, Manuel Lozano, José Carlos Vilches, Rodrigo Orozco and Manuela Ludovisi
Onco 2025, 5(4), 46; https://doi.org/10.3390/onco5040046 - 16 Oct 2025
Abstract
►▼
Show Figures
Background: Ovarian cancer remains a major contributor to cancer-related morbidity and mortality worldwide. Primary cytoreductive surgery is the cornerstone of treatment, and accurate preoperative assessment of tumor resectability is critical to guiding optimal therapeutic strategies in patients with advanced tubo-ovarian cancer. Methods:
[...] Read more.
Background: Ovarian cancer remains a major contributor to cancer-related morbidity and mortality worldwide. Primary cytoreductive surgery is the cornerstone of treatment, and accurate preoperative assessment of tumor resectability is critical to guiding optimal therapeutic strategies in patients with advanced tubo-ovarian cancer. Methods: A narrative review about the role of ultrasound for assessing tumor spread and prediction of tumor resectability was performed. Results: The ISAAC study represents the largest prospective multicenter trial to date comparing the diagnostic performance of ultrasound (US), computed tomography (CT), and whole-body diffusion-weighted magnetic resonance imaging (WB-DWI/MRI) in predicting non-resectability, using surgical and histopathological findings as the reference standard. Key strengths of the study include the use of standardized imaging and intraoperative reporting protocols across ESGO-accredited high-volume oncologic centers. All three imaging modalities were performed within four weeks prior to surgery by independent, blinded expert operators. US demonstrated diagnostic accuracy comparable to that of CT and WB-DWI/MRI. The study also defined modality-specific thresholds for the Peritoneal Cancer Index (PCI) and Predictive Index Value (PIV), offering quantitative tools to support surgical decision-making. A noteworthy secondary finding was patient preference: in a cohort of 144 participants who underwent all three imaging modalities, nearly half preferred US, while WB-DWI/MRI was the least favored due to discomfort and examination duration. Conclusions: The ISAAC study represents a significant advancement in imaging-based prediction of surgical non-resectability in tubo-ovarian cancer. Its findings suggest that, in expert hands, ultrasound can match or even surpass cross-sectional imaging for preoperative staging, supporting its integration into routine clinical practice, particularly in resource-constrained settings.
Full article

Figure 1
Open AccessReview
Integration of Radical Intent Treatment in Colorectal Liver Metastases
by
Francisco J. Pelegrín-Mateo and Javier Gallego Plazas
Onco 2025, 5(4), 45; https://doi.org/10.3390/onco5040045 - 2 Oct 2025
Abstract
►▼
Show Figures
Colorectal liver metastases (CRLM) management remains a complex conundrum in the context of potential curable disease. The combination of systemic therapy and surgery, with overall survival outcomes up to 58% at five years, has become the gold standard. Locoregional therapies have gained evidence
[...] Read more.
Colorectal liver metastases (CRLM) management remains a complex conundrum in the context of potential curable disease. The combination of systemic therapy and surgery, with overall survival outcomes up to 58% at five years, has become the gold standard. Locoregional therapies have gained evidence in complementing surgery or even substituting it in selected cases. Adequate patient selection is paramount, but prognostic models have certain limitations that prevent their full implementation in clinical practice. A plethora of prognostic factors exists, with variable evidence supporting their definitive role. Thus, CRLM management decisions frequently vary depending on multidisciplinary team experience and hospital access to systemic and locoregional treatments. Definition of resectability has evolved in recent years due to technical developments in surgical and non-surgical approaches. Complexity is added when trying to fully understand the integration between local and systemic treatment. Whereas evidence in the context of resectable disease has been attempted in several phase III trials, definitive conclusions regarding the best approach to potentially resectable disease cannot be drawn. In addition, liver transplantation has gained evidence and is proposed in selected patients, raising a challenge regarding its integration and wider implementation. In this review, current standards in the management of CRLM regarding patient selection, resectability, surgical and non-surgical locoregional strategies, as well as the best systemic approach are covered.
Full article

Figure 1
Open AccessCommunication
Association of TP53 Arg72Pro (rs1042522) Polymorphism with Pancreatic Cancer Risk in a Patient Cohort
by
Laura Antolino, Germana de Nucci, Stefania Scarpino, Giuseppe Bianco, Gianluca Lopez, Paolo Aurello, Niccolò Petrucciani, Roberto Santoro, Giuseppe Nigri, Salvatore Agnes, Gianpiero Manes and Francesco A. D’Angelo
Onco 2025, 5(4), 44; https://doi.org/10.3390/onco5040044 - 24 Sep 2025
Abstract
Pancreatic cancer is expected to become the second leading cause of death by 2030 in Western countries. There is a need to pinpoint high-risk populations since extensive screening would be economically impractical. Methods: This study, conducted on liquid biopsies of patients affected by
[...] Read more.
Pancreatic cancer is expected to become the second leading cause of death by 2030 in Western countries. There is a need to pinpoint high-risk populations since extensive screening would be economically impractical. Methods: This study, conducted on liquid biopsies of patients affected by pancreatic ductal adenocarcinoma (PDAC), sequenced, by NGS, the main genes involved in pancreatic carcinogenesis. Results: The study was discontinued due to a low recruitment rate. NGS analysis, conducted on included patients, revealed the TP53 variant rs1042522 in 30 out of 35 patients, with a cytosine (C) replaced by a guanine (G), hence inserting an Arginine in the final protein instead of a Proline. The presence of the rs1042522 variant confers an odds ratio of 6.11 for PaC and an OR of 20 for homozygosity G/G when comparing our cohort of PaC patients to a healthy population from the 1000GenomeProject. Conclusion: These findings could identify a very-high-risk population deserving of being screened for PDAC, even though a wider validation of rs1042522 as a risk factor is needed. Impact: These preliminary data may open the way for identification of a population more prone to developing pancreatic cancer.
Full article
(This article belongs to the Special Issue Targeting of Tumor Dormancy Pathway)
Open AccessSystematic Review
Metabolic Reprogramming as a Therapeutic Target in Cancer: A Qualitative Systematic Review (QualSR) of Natural Compounds Modulating Glucose and Glutamine Pathways
by
Michael Enwere, Edward Irobi, Victoria Chime, Ada Ezeogu, Adamu Onu, Mohamed Toufic El Hussein, Gbadebo Ogungbade, Emmanuel Davies, Omowunmi Omoniwa, Charles Omale, Mercy Neufeld, Ojochide Akagwu, Terkaa Atim and Laurens Holmes, Jr.
Onco 2025, 5(3), 43; https://doi.org/10.3390/onco5030043 - 22 Sep 2025
Abstract
Background: Despite advances in gene-targeted and immunotherapies, many aggressive cancers—including glioblastoma and triple-negative breast cancer—remain refractory to treatment. Mounting evidence implicates metabolic reprogramming, especially dysregulation of glucose and glutamine metabolism, as a core hallmark of tumor progression. Natural compounds with metabolic-modulatory effects have
[...] Read more.
Background: Despite advances in gene-targeted and immunotherapies, many aggressive cancers—including glioblastoma and triple-negative breast cancer—remain refractory to treatment. Mounting evidence implicates metabolic reprogramming, especially dysregulation of glucose and glutamine metabolism, as a core hallmark of tumor progression. Natural compounds with metabolic-modulatory effects have emerged as promising adjuncts in oncology. Research Question and Objectives: This review investigates the following question: How can metabolic-targeted therapies—particularly those modulating the Warburg effect and glutamine metabolism—improve cancer treatment outcomes, and what role do natural compounds play in this strategy? The objectives were to (1) evaluate the therapeutic potential of metabolic interventions targeting glucose and glutamine metabolism, (2) assess natural compounds with metabolic regulatory activity, (3) examine integration of metabolic-targeted therapies with conventional treatments, and (4) identify metabolic vulnerabilities in resistant malignancies. Methods: A qualitative systematic review (QualSR) was conducted following PRISMA guidelines. A total of 87 peer-reviewed studies published between 2000 and 2024 were included. Inclusion criteria required clearly defined mechanistic or clinical endpoints and, for clinical trials, sample sizes ≥ 30. Data extraction focused on tumor response, survival, metabolic modulation, and safety profiles. Results: Curcumin significantly reduced serum TNF-α and IL-6 (both p = 0.001) and improved antioxidant capacity (p = 0.001). EGCG downregulated ERα (p = 0.002) and upregulated tumor suppressors p53 and p21 (p = 0.001, p = 0.02). High-dose intravenous vitamin C combined with chemoradiotherapy yielded a 44.4% pathologic complete response rate in rectal cancer. Berberine suppressed Akt/mTOR signaling and glutamine transporter SLC1A5 across tumor types (q < 10−10). However, poor bioavailability (e.g., EGCG t½ = 3.4 ± 0.3 h) and systemic toxicity limit their standalone clinical application. Conclusions: Metabolic-targeted therapies—particularly natural compounds acting on glucose and glutamine pathways—offer a viable adjunct to standard cancer therapies. Clinical translation will require biomarker-driven patient stratification, improved delivery systems, and combination trials to optimize the therapeutic impact in treatment-resistant cancers.
Full article
(This article belongs to the Special Issue Targeting of Tumor Dormancy Pathway)
►▼
Show Figures

Figure 1
Open AccessReview
Precision Medicine for Older AML Patients
by
Ugo Testa, Germana Castelli and Elvira Pelosi
Onco 2025, 5(3), 42; https://doi.org/10.3390/onco5030042 - 16 Sep 2025
Cited by 1
Abstract
►▼
Show Figures
The development of molecular profiling approaches for AML patients such as whole genome sequencing, whole exome sequencing and transcriptomic sequencing have greatly contributed to better understanding of leukemia development, progression and treatment responsiveness/resistance. These studies have generated a new knowledge about driver events
[...] Read more.
The development of molecular profiling approaches for AML patients such as whole genome sequencing, whole exome sequencing and transcriptomic sequencing have greatly contributed to better understanding of leukemia development, progression and treatment responsiveness/resistance. These studies have generated a new knowledge about driver events operating in AML that can be translated into clinics, thus favoring the mutations; using this approach, more than 50% of older AML patients display molecular alterations, such as IDH1, IDH2, FLT3 (FLT3-TKD and FLT3-ITD), NPM1 and KMT2A rearrangements that can be targeted by specific drugs. Preclinical and clinical studies have supported the use of drugs targeting these molecular alterations as first-line therapy in association with induction chemotherapy in chemotherapy-fit patients or with a hypomethylating agent in association with a Bcl-2 inhibitor (Venetoclax) in chemotherapy-unfit patients. These studies have shown promising results that need to be confirmed through randomized clinical studies specifically involving the enrollment of older AML patients.
Full article

Figure 1
Open AccessReview
Exploring the Potential of Biotics in Cancer Prevention and Treatment—Mechanisms, Experimental, and Clinical Insights
by
Tia Tafla, Abinaya Balasubramanian and Janaki K. Iyer
Onco 2025, 5(3), 41; https://doi.org/10.3390/onco5030041 - 27 Aug 2025
Abstract
►▼
Show Figures
Cancer is a public health concern due to the incidence, prevalence, morbidity, and mortality associated with it. While chemotherapy, radiotherapy, surgery, and immunotherapy are common treatments, there is still ongoing research to find targeted and innovative therapies that are more efficacious. The effect
[...] Read more.
Cancer is a public health concern due to the incidence, prevalence, morbidity, and mortality associated with it. While chemotherapy, radiotherapy, surgery, and immunotherapy are common treatments, there is still ongoing research to find targeted and innovative therapies that are more efficacious. The effect of probiotics on cancer progression and treatment has been actively investigated using different in vitro and in vivo models. Similarly, the role of prebiotics alone or in combination with probiotics, referred to as synbiotics, has also been evaluated in the context of cancers. Recently, the therapeutical potential of postbiotics is also being determined. Many studies have demonstrated that these agents can have onco-suppressive effects and can also prevent cancer in some instances. In this review, we summarize the different studies that have utilized these therapeutics in the prevention and treatment of a variety of cancers. We also discuss the different molecular mechanisms that enable these agents to be effective against cancers. Finally, we address safety and the need for more robust clinical trials that will aid in designing strategies involving these biotics in the prevention and treatment of cancer.
Full article

Figure 1
Open AccessArticle
CRISPR-Mediated Analysis of p27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis
by
Junyan Wang, Benjamin B. Gyau, Jun Xu, Angela M. Major, John Hicks and Tsz-Kwong Man
Onco 2025, 5(3), 40; https://doi.org/10.3390/onco5030040 - 27 Aug 2025
Abstract
►▼
Show Figures
Background: Osteosarcoma (OS) is a fast-growing malignant bone tumor that occurs most often in children and teenagers. Development of pulmonary metastasis is the primary cause of treatment failure and mortality. Our previous studies demonstrated that cytoplasmic p27 interacts with PAK1, enhancing PAK1 phosphorylation
[...] Read more.
Background: Osteosarcoma (OS) is a fast-growing malignant bone tumor that occurs most often in children and teenagers. Development of pulmonary metastasis is the primary cause of treatment failure and mortality. Our previous studies demonstrated that cytoplasmic p27 interacts with PAK1, enhancing PAK1 phosphorylation and promoting OS pulmonary metastasis. However, the cellular functions of p27 and PAK1 are primarily regulated by phosphorylation, and the roles of specific phosphorylation residues in modulating OS metastatic potential remain unclear. Methods: To study tumor invasiveness and lung metastasis, we employed a CRISPR-based knock-in method to introduce specific mutations—p27-T157A, p27-T157D, PAK1-T423E, and PAK1-K299R—into the 143B OS cell line, followed by in vitro invasion and orthotopic xenograft mouse experiments. These residues were selected for their therapeutic potential, as T157 regulates p27 nuclear–cytoplasmic shuttling, while T423 and K299 modulate PAK1 kinase activity. Results: No significant differences in pulmonary metastasis were observed across p27 mutants compared to parental controls. However, the p27-T157D mutant exhibited increased cytoplasmic mislocalization, elevated PAK1-S144 phosphorylation, and enhanced in vitro invasiveness compared to the p27-T157A mutant and parental 143B cells. The PAK1-K299R mutant, designed to be kinase-dead, showed negligible S144 phosphorylation, consistent with loss of kinase activity. Unexpectedly, this mutant displayed increased T423 phosphorylation and in vitro invasiveness, and significantly enhanced pulmonary metastasis in vivo compared to the PAK1-T423E mutant and parental controls. Conclusions: These findings highlight the complexity of targeting specific p27 and PAK1 phosphorylation sites as an anti-metastatic strategy for OS. While p27-T157 phosphorylation influences cytoplasmic localization and invasiveness, it does not significantly alter metastatic outcomes. Conversely, PAK1-T423 phosphorylation is critical in driving OS metastatic potential, and the kinase-dead K299R mutant’s unexpected pro-metastatic effect suggests that kinase-independent mechanisms or compensatory pathways may contribute to metastasis. Our findings suggest the necessity for a more comprehensive understanding of the phosphorylation dynamics of p27 and PAK1 in metastatic OS. They also indicate that conventional kinase inhibition may be insufficient and underscore the potential benefits of alternative or combinatorial therapeutic strategies, such as targeting kinase-independent functions or other upstream kinases involved in these regulatory pathways.
Full article

Figure 1
Open AccessCase Report
Adenoma-like Adenocarcinoma of the Colon: Case Report and Diagnostic Pitfalls of an Underrecognized Entity with Favorable Prognosis
by
Alfonso Agüera-Sánchez, Emilio Peña-Ros, Irene Martínez-Martínez and Francisco García-Molina
Onco 2025, 5(3), 39; https://doi.org/10.3390/onco5030039 - 23 Aug 2025
Abstract
►▼
Show Figures
Adenoma-like adenocarcinoma (ALAC) of the colon is a recently recognized histological subtype of colorectal adenocarcinoma, characterized by a villous architecture, low-grade cytologic atypia, and deceptive bland morphology despite its invasive potential, which can mimic non-invasive adenomas, leading to underdiagnosis in limited biopsy samples.
[...] Read more.
Adenoma-like adenocarcinoma (ALAC) of the colon is a recently recognized histological subtype of colorectal adenocarcinoma, characterized by a villous architecture, low-grade cytologic atypia, and deceptive bland morphology despite its invasive potential, which can mimic non-invasive adenomas, leading to underdiagnosis in limited biopsy samples. Herein, we report the case of an 81-year-old male presenting with right-upper-quadrant pain that was found to have a hepatic abscess and a 4 cm villous lesion in the ascending colon. Histopathological examination of the right hemicolectomy specimen revealed a villous adenocarcinoma with invasion of the muscularis propria, consistent with adenoma-like adenocarcinoma. Isolated loss of PMS2 indicated a mismatch repair deficiency. However, adjuvant therapy was not indicated. The patient remained recurrence-free for three years, until he died from unrelated causes in the context of progressive frailty and comorbidities, with no evidence of cancer progression. This case highlights the diagnostic challenges posed by ALAC and underscores the importance of recognizing its distinct morphological features. Awareness of this entity is essential to avoid misclassification and ensure adequate treatment, especially given its typically favorable prognosis with low metastatic potential.
Full article

Figure 1
Open AccessReview
Prognostic Role of B7-H3 (CD276) Expression in Initial Biopsies of Metastatic Prostate Cancer
by
Adam Yusuf and Paramahansa Pramanik
Onco 2025, 5(3), 38; https://doi.org/10.3390/onco5030038 - 14 Aug 2025
Abstract
►▼
Show Figures
Prostate cancer exhibits highly variable behavior, from slow-growing localized tumors to aggressive metastatic disease, yet early prognostic indicators remain limited. In this study, we examined B7-H3 (CD276) expression, a molecule linked to immune suppression and cancer progression in diagnostic biopsy specimens from 248
[...] Read more.
Prostate cancer exhibits highly variable behavior, from slow-growing localized tumors to aggressive metastatic disease, yet early prognostic indicators remain limited. In this study, we examined B7-H3 (CD276) expression, a molecule linked to immune suppression and cancer progression in diagnostic biopsy specimens from 248 patients with localized or metastatic prostate cancer. We found that elevated B7-H3 levels were significantly more common in metastatic cases and independently associated with reduced overall and disease-specific survival. Moreover, high B7-H3 expression correlated with increased PSA values and higher Gleason grades. These findings endorse B7-H3 as a robust prognostic marker and potential therapeutic target in advanced prostate cancer management.
Full article

Figure 1
Open AccessReview
Pancreatic Cancer: Epidemiology, Risk Factors, and Prevention
by
Sahar Mack, Thibaud Koessler, Philippe Bichard and Jean-Louis Frossard
Onco 2025, 5(3), 37; https://doi.org/10.3390/onco5030037 - 1 Aug 2025
Abstract
Pancreatic cancer (PC) is one of the most aggressive and lethal malignancies, mainly due to its late detection [...]
Full article
Open AccessReview
Imaging of Liver Metastases from GEP-NETs: A Narrative Review
by
Alessandro Posa, Enza Genco, Pierluigi Barbieri, Mario Ariano, Marcello Lippi, Alessandro Maresca and Roberto Iezzi
Onco 2025, 5(3), 36; https://doi.org/10.3390/onco5030036 - 17 Jul 2025
Abstract
►▼
Show Figures
Prompt and accurate identification of liver metastases from neuroendocrine tumors, arising from the gastrointestinal system and from the pancreas, through the means of both anatomical and functional diagnostic imaging techniques is mandatory. A patient’s prognosis and treatment planning are dependent on these diagnostic
[...] Read more.
Prompt and accurate identification of liver metastases from neuroendocrine tumors, arising from the gastrointestinal system and from the pancreas, through the means of both anatomical and functional diagnostic imaging techniques is mandatory. A patient’s prognosis and treatment planning are dependent on these diagnostic procedures. The aim of this narrative review is to depict the common appearance of liver metastases, as well as to depict atypical imaging patterns. Moreover, this review will cover the differential diagnosis between liver metastases from neuroendocrine tumors and other primary and secondary malignant liver lesions, as well as benign liver lesions.
Full article

Figure 1
Open AccessArticle
Revolutionizing Detection of Minimal Residual Disease in Breast Cancer Using Patient-Derived Gene Signature
by
Chen Yeh, Hung-Chih Lai, Nathan Grabbe, Xavier Willett and Shu-Ti Lin
Onco 2025, 5(3), 35; https://doi.org/10.3390/onco5030035 - 12 Jul 2025
Abstract
►▼
Show Figures
Background: Many patients harbor minimal residual disease (MRD)—small clusters of residual tumor cells that survive therapy and evade conventional detection but drive recurrence. Although advances in molecular and computational methods have improved circulating tumor DNA (ctDNA)-based MRD detection, these approaches face challenges: ctDNA
[...] Read more.
Background: Many patients harbor minimal residual disease (MRD)—small clusters of residual tumor cells that survive therapy and evade conventional detection but drive recurrence. Although advances in molecular and computational methods have improved circulating tumor DNA (ctDNA)-based MRD detection, these approaches face challenges: ctDNA shedding fluctuates widely across tumor types, disease stages, and histological features. Additionally, low levels of driver mutations originating from healthy tissues can create background noise, complicating the accurate identification of bona fide tumor-specific signals. These limitations underscore the need for refined technologies to further enhance MRD detection beyond DNA sequences in solid malignancies. Methods: Profiling circulating cell-free mRNA (cfmRNA), which is hyperactive in tumor and non-tumor microenvironments, could address these limitations to inform postoperative surveillance and treatment strategies. This study reported the development of OncoMRD BREAST, a customized, gene signature-informed cfmRNA assay for residual disease monitoring in breast cancer. OncoMRD BREAST introduces several advanced technologies that distinguish it from the existing ctDNA-MRD tests. It builds on the patient-derived gene signature for capturing tumor activities while introducing significant upgrades to its liquid biopsy transcriptomic profiling, digital scoring systems, and tracking capabilities. Results: The OncoMRD BREAST test processes inputs from multiple cutting-edge biomarkers—tumor and non-tumor microenvironment—to provide enhanced awareness of tumor activities in real time. By fusing data from these diverse intra- and inter-cellular networks, OncoMRD BREAST significantly improves the sensitivity and reliability of MRD detection and prognosis analysis, even under challenging and complex conditions. In a proof-of-concept real-world pilot trial, OncoMRD BREAST’s rapid quantification of potential tumor activity helped reduce the risk of incorrect treatment strategies, while advanced predictive analytics contributed to the overall benefits and improved outcomes of patients. Conclusions: By tailoring the assay to individual tumor profiles, we aimed to enhance early identification of residual disease and optimize therapeutic decision-making. OncoMRD BREAST is the world’s first and only gene signature-powered test for monitoring residual disease in solid tumors.
Full article

Figure 1
Open AccessArticle
Multi-Task Deep Learning for Simultaneous Classification and Segmentation of Cancer Pathologies in Diverse Medical Imaging Modalities
by
Maryem Rhanoui, Khaoula Alaoui Belghiti and Mounia Mikram
Onco 2025, 5(3), 34; https://doi.org/10.3390/onco5030034 - 11 Jul 2025
Abstract
►▼
Show Figures
Background: Clinical imaging is an important part of health care providing physicians with great assistance in patients treatment. In fact, segmentation and grading of tumors can help doctors assess the severity of the cancer at an early stage and increase the chances
[...] Read more.
Background: Clinical imaging is an important part of health care providing physicians with great assistance in patients treatment. In fact, segmentation and grading of tumors can help doctors assess the severity of the cancer at an early stage and increase the chances of cure. Despite that Deep Learning for cancer diagnosis has achieved clinically acceptable accuracy, there still remains challenging tasks, especially in the context of insufficient labeled data and the subsequent need for expensive computational ressources. Objective: This paper presents a lightweight classification and segmentation deep learning model to assist in the identification of cancerous tumors with high accuracy despite the scarcity of medical data. Methods: We propose a multi-task architecture for classification and segmentation of cancerous tumors in the Brain, Skin, Prostate and lungs. The model is based on the UNet architecture with different pre-trained deep learning models (VGG 16 and MobileNetv2) as a backbone. The multi-task model is validated on relatively small datasets (slightly exceed 1200 images) that are diverse in terms of modalities (IRM, X-Ray, Dermoscopic and Digital Histopathology), number of classes, shapes, and sizes of cancer pathologies using the accuracy and dice coefficient as statistical metrics. Results: Experiments show that the multi-task approach improve the learning efficiency and the prediction accuracy for the segmentation and classification tasks, compared to training the individual models separately. The multi-task architecture reached a classification accuracy of 86%, 90%, 88%, and 87% respectively for Skin Lesion, Brain Tumor, Prostate Cancer and Pneumothorax. For the segmentation tasks we were able to achieve high precisions respectively 95%, 98% for the Skin Lesion and Brain Tumor segmentation and a 99% precise segmentation for both Prostate cancer and Pneumothorax. Proving that the multi-task solution is more efficient than single-task networks.
Full article

Figure 1
Open AccessReview
The Role of Tissue Factor-Positive Microparticles in Gynecological Cancer-Associated Disseminated Intravascular Coagulation: Molecular Mechanisms and Clinical Implications
by
Muqaddas Qureshi, Muhammad Tanveer Alam and Ahsanullah Unar
Onco 2025, 5(3), 33; https://doi.org/10.3390/onco5030033 - 10 Jul 2025
Cited by 1
Abstract
►▼
Show Figures
Gynecological malignancies (ovarian, endometrial, and cervical cancers), including disseminated intravascular coagulation (DIC), often provoke systemic coagulopathy. In recent years, tumor-derived, tissue factor–positive microparticles (TF+ MPs) have emerged as potent drivers of cancer-associated thrombosis and possibly DIC. These small (0.1–1 µm) membrane vesicles
[...] Read more.
Gynecological malignancies (ovarian, endometrial, and cervical cancers), including disseminated intravascular coagulation (DIC), often provoke systemic coagulopathy. In recent years, tumor-derived, tissue factor–positive microparticles (TF+ MPs) have emerged as potent drivers of cancer-associated thrombosis and possibly DIC. These small (0.1–1 µm) membrane vesicles bud from cancer cell surfaces and carry procoagulant factors (phosphatidylserine and TF) on their surface. We review how TF+ MPs are generated by tumor cells and amplify the extrinsic coagulation cascade, potentially triggering DIC in patients with advanced gynecologic cancers. Clinical studies have linked el evated TF+ MP levels and activity to venous thromboembolism (VTE) in cancer, and small case series suggest dramatically high MP–TF activity in cancer-related DIC. We summarize evidence that TF+ MPs from ovarian tumors carry exceptionally high TF procoagulant activity (median ~80 pg/mL), and nearly all patients with cancer-associated VTE or DIC have MP–TF levels above normal. This review discusses diagnostic implications (e.g., measuring MP–TF activity as a biomarker) and treatment strategies (through the reduction in tumors, anticoagulation, and experimental TF inhibitors) in this setting. We also identify gaps in knowledge (standardized MP assays, prospective studies) and propose future directions (targeting MP formation or TF signaling). Two summary tables highlight recent studies of TF+ MPs in gynecologic cancer and their clinical outcomes. Illustrative figures depict the TF+ MP-triggered coagulation cascade and a conceptual framework for clinical management. Understanding TF+ MPs in gynecological cancer could improve the prediction and management of DIC and related thromboses.
Full article
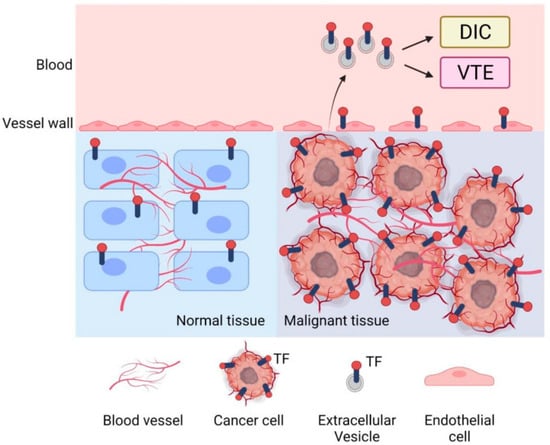
Figure 1
Open AccessCommunication
Are Mitochondria the True Origin of Cancer? A Hypothesis-Driven Perspective
by
Sergio Da Silva
Onco 2025, 5(3), 32; https://doi.org/10.3390/onco5030032 - 1 Jul 2025
Abstract
Conventional wisdom holds that nuclear oncogenes and tumor suppressors initiate malignant transformation. However, mounting research suggests that mitochondrial dysfunction—rooted in the unique evolutionary history and genetic autonomy of mitochondria—may serve as a more fundamental driver of oncogenesis. This paper proposes a “mitochondria-first” hypothesis
[...] Read more.
Conventional wisdom holds that nuclear oncogenes and tumor suppressors initiate malignant transformation. However, mounting research suggests that mitochondrial dysfunction—rooted in the unique evolutionary history and genetic autonomy of mitochondria—may serve as a more fundamental driver of oncogenesis. This paper proposes a “mitochondria-first” hypothesis of cancer, emphasizing the pivotal role of mitochondrial DNA (mtDNA) mutations, metabolic reprogramming, and immune evasion. By examining the evolutionary conflict between host and mitochondria, evaluating high mtDNA mutation rates, and highlighting the disruptive potential of mitochondrial transfer to immune cells, we outline robust mechanisms through which mitochondria could ignite cancer development. We also discuss emerging diagnostic and therapeutic approaches that target mitochondrial integrity, offering a potential paradigm shift in oncology.
Full article
(This article belongs to the Special Issue The Evolving Landscape of Contemporary Cancer Therapies)
Open AccessSystematic Review
Prostate Cancer and Dietary Sugar Intake: A Systematic Review
by
Karim Khaled, Hala Jardaly and Orouba Almilaji
Onco 2025, 5(3), 31; https://doi.org/10.3390/onco5030031 - 30 Jun 2025
Abstract
►▼
Show Figures
Background: Prostate cancer is a leading malignancy among men globally, with its incidence expected to rise due to aging populations and shifting lifestyles. While established risk factors include age, ethnicity, and genetics, the role of modifiable dietary factors, particularly sugar intake, remains
[...] Read more.
Background: Prostate cancer is a leading malignancy among men globally, with its incidence expected to rise due to aging populations and shifting lifestyles. While established risk factors include age, ethnicity, and genetics, the role of modifiable dietary factors, particularly sugar intake, remains less clear. Emerging evidence suggests that high sugar consumption may promote carcinogenesis through insulin resistance, chronic inflammation, and hormonal dysregulation. This systematic review aimed to evaluate the current evidence on the association between dietary sugar intake and prostate cancer risk. Methods: A systematic search was conducted across six databases for observational studies published between January 2005 and April 2025. Eligible studies assessed the associations between quantitative sugar intake and prostate cancer outcomes. Screening, data extraction, and a risk of bias assessment (using ROBINS-E) were performed independently by multiple reviewers. Results: Six studies met the inclusion criteria, comprising four prospective cohorts, one case–control study, and one cross-sectional study, with a combined sample of 11,583 men from the USA, Canada, Sweden, and France. Three studies reported a significant positive association between a high intake of dietary sugars and prostate cancer risk, two found no association, and one showed mixed findings depending on the type of sugar. Heterogeneity in the exposure assessments and confounder control limited the comparability. Conclusions: This review suggests a possible association between high dietary sugar intake and increased prostate cancer risk, especially from added sugars and sugar-sweetened beverages. However, inconsistent findings and methodological limitations highlight the need for robust, prospective studies with standardized assessments to understand this relationship better.
Full article

Figure 1
Open AccessArticle
Racial Diversity in the Decline in Hepatocellular Carcinoma and Increasing Age at Diagnosis in a Primarily African American Medical Center Population
by
Gabriel Boudagh, Ahmad Alnasart, Kenan Abou Chaer, Paul Naylor and Milton Mutchnick
Onco 2025, 5(3), 30; https://doi.org/10.3390/onco5030030 - 30 Jun 2025
Abstract
►▼
Show Figures
Background: Hepatocellular carcinoma (HCC) remains a significant global health burden, particularly among vulnerable populations. This retrospective study investigates trends in HCC incidence and age at diagnosis within an urban medical center population, focusing on the impact of hepatitis C virus (HCV) treatment and
[...] Read more.
Background: Hepatocellular carcinoma (HCC) remains a significant global health burden, particularly among vulnerable populations. This retrospective study investigates trends in HCC incidence and age at diagnosis within an urban medical center population, focusing on the impact of hepatitis C virus (HCV) treatment and racial disparities. Methods: The study includes 484 patients diagnosed with HCC between 2000 and 2023. Results: A significant decline in HCC incidence was observed with a peak in incidences between 2015 and 2017 (p < 0.02). The increase and subsequent decline were driven by a decline in HCV-related cases, particularly among the African American (AA) population. This trend was not seen for patients with other risk factors for HCC. An increase in age at diagnosis in HCV patients but not other risk patients was observed in AA (62 vs. 69 years p = 0.001) but not non-AA patients (66 vs. 67 p = 0.16). This increase in age for AA HCV patients could be due to an aging population, changing risk factor profiles, and/or limitations in surveillance and early detection of HCC. Conclusions: This study highlights the critical role of HCV treatment in reducing HCC incidence, particularly within the AA population. These findings emphasize the need for sustained efforts in surveillance, early detection, and targeted prevention strategies to address the evolving epidemiology of HCC and improve outcomes across all populations.
Full article
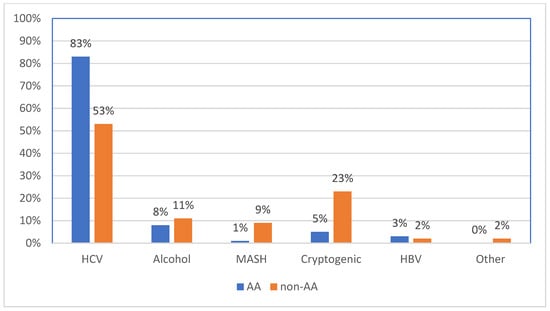
Figure 1
Open AccessArticle
Comparative Analysis of Predictive Models for Individual Cancer Risk: Approaches and Applications
by
Philippe Westerlinck
Onco 2025, 5(2), 29; https://doi.org/10.3390/onco5020029 - 17 Jun 2025
Abstract
►▼
Show Figures
Introduction: This article provides a comprehensive analysis of predictive models for individual cancer risk, examining their development, application, and evaluation. The study covers various cancer types, highlighting the diversity and sophistication of models over time. Methods: Utilizing data from PubMed, Web of Science,
[...] Read more.
Introduction: This article provides a comprehensive analysis of predictive models for individual cancer risk, examining their development, application, and evaluation. The study covers various cancer types, highlighting the diversity and sophistication of models over time. Methods: Utilizing data from PubMed, Web of Science, and Scopus, the research includes models developed for 22 cancer types, with significant emphasis on breast and colorectal cancers due to their prevalence and early detection benefits. Results: The analysis reveals an uneven distribution of models, often concentrated in the United States and the United Kingdom, with a notable gap in models for rarer cancers. Key methodologies such as logistic regression and Cox proportional hazards models dominate the field, reflecting a preference for established statistical techniques. The study underscores the importance of incorporating multiple risk factors, including genetic, environmental, lifestyle, and clinical data, to enhance predictive accuracy. Despite advancements, the article identifies a critical need for external validation and standardization in reporting practices to improve model reliability and generalizability. Conclusions The findings emphasize the potential of these models in personalized cancer prevention and early detection, while also calling for continued research and methodological harmonization to address existing gaps and challenges.
Full article

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cancers, Current Oncology, JCM, Medicina, Onco
Cancer Biology and Radiation Therapy: 2nd Edition
Topic Editors: Chang Ming Charlie Ma, Ka Yu Tse, Ming-Yii Huang, Mukund SeshadriDeadline: 25 July 2026
Topic in
Biomolecules, Molecules, Nanomaterials, Onco, Pharmaceutics, Cancers, Applied Nano, Antioxidants
Advanced Nanocarriers for Targeted Drug and Gene Delivery
Topic Editors: Rita Cortesi, Maddalena Sguizzato, Francesca FerraraDeadline: 31 December 2026
Topic in
Biomedicines, Cancers, Current Oncology, JCM, Onco
Cancer Genomics: Emerging Trends and Technological Advances
Topic Editors: Martha Patricia Gallegos-Arreola, Luis Eduardo Figuera Villanueva, Hector Barajas-MartinezDeadline: 31 May 2027

Conferences
Special Issues
Special Issue in
Onco
Targeting of Tumor Dormancy Pathway
Guest Editors: Athanassios Kotsinas, Constantin N. BaxevanisDeadline: 31 December 2025
Special Issue in
Onco
Immune–Cancer Cell Interactions: Impact on Clinical Outcomes and Opportunities for Therapy
Guest Editors: Sophia Karagiannis, Ourania E. TsitsilonisDeadline: 7 February 2026
Special Issue in
Onco
Biomarkers for the Detection of Cancer and Monitoring Response to Treatment
Guest Editor: Barbara GuinnDeadline: 25 February 2026
Special Issue in
Onco
Liquid Biopsy and Peripheral Immune Status in Cancer Therapy Response
Guest Editors: Galatea Kallergi, Anastasia Xagara, Constantin N. BaxevanisDeadline: 31 May 2026









