The Dependence of Compensation Dose on Systematic and Random Interruption Treatment Time in Radiation Therapy
Abstract
:1. Introduction
| Fast (min) | Slow (h) | Totally (Not Determined) | Cell Type | Ref |
|---|---|---|---|---|
| 7–4 | 1–1.5 | CHO | [22,23] | |
| 5 | 2.5 | HF19 | [24] | |
| 19 min | ACHN Renal Cell Carcinoma | [25] | ||
| ≤60 min | Mammalian | [26] | ||
| 3–10 | 0.6–4 | - | [27] | |
| 13 | 4.5 | DT40 | [28] | |
| 7–8 | 2.5 | GM5758, GM7166, M059K, U-1810 | [29] | |
| 22 | 12 | human glioma cell line, M059-J and M059-K | [30] |
2. Materials and Methods
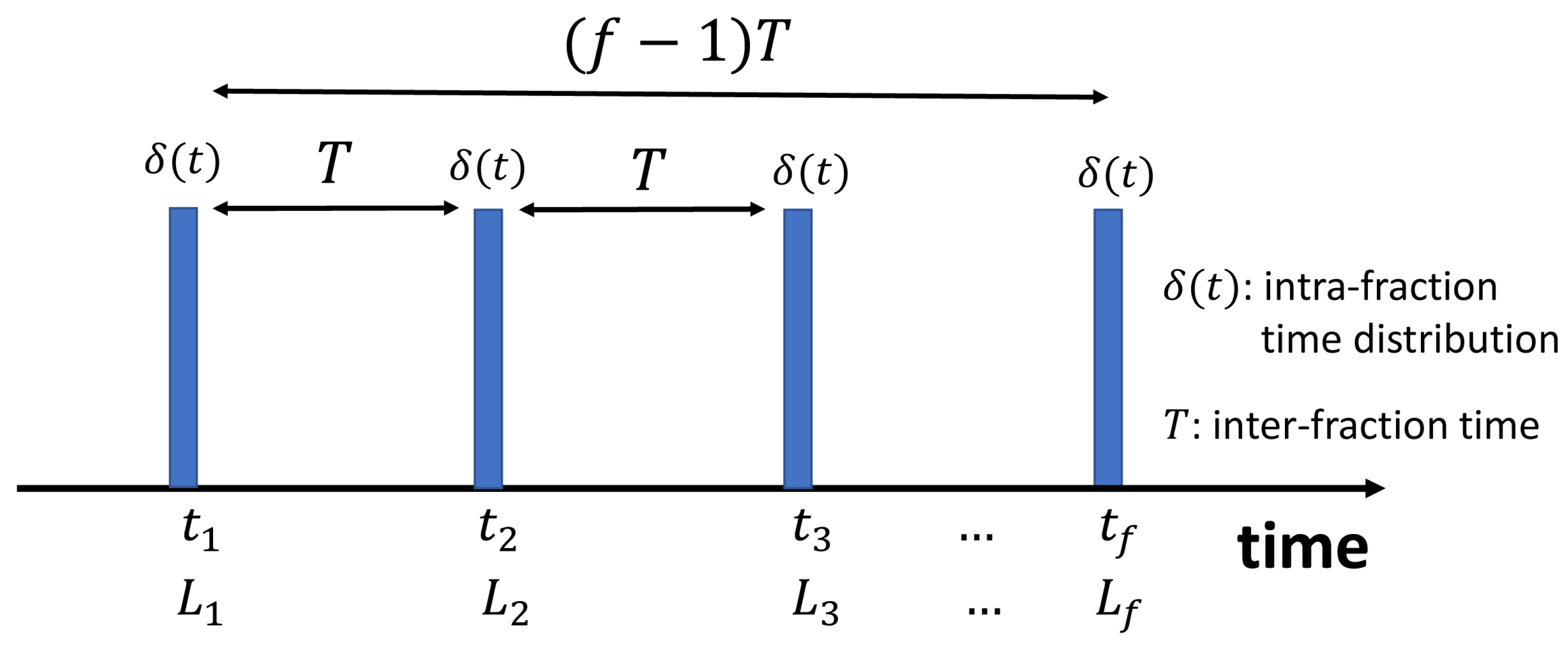
2.1. Nomenclature and Terminology
2.2. Model
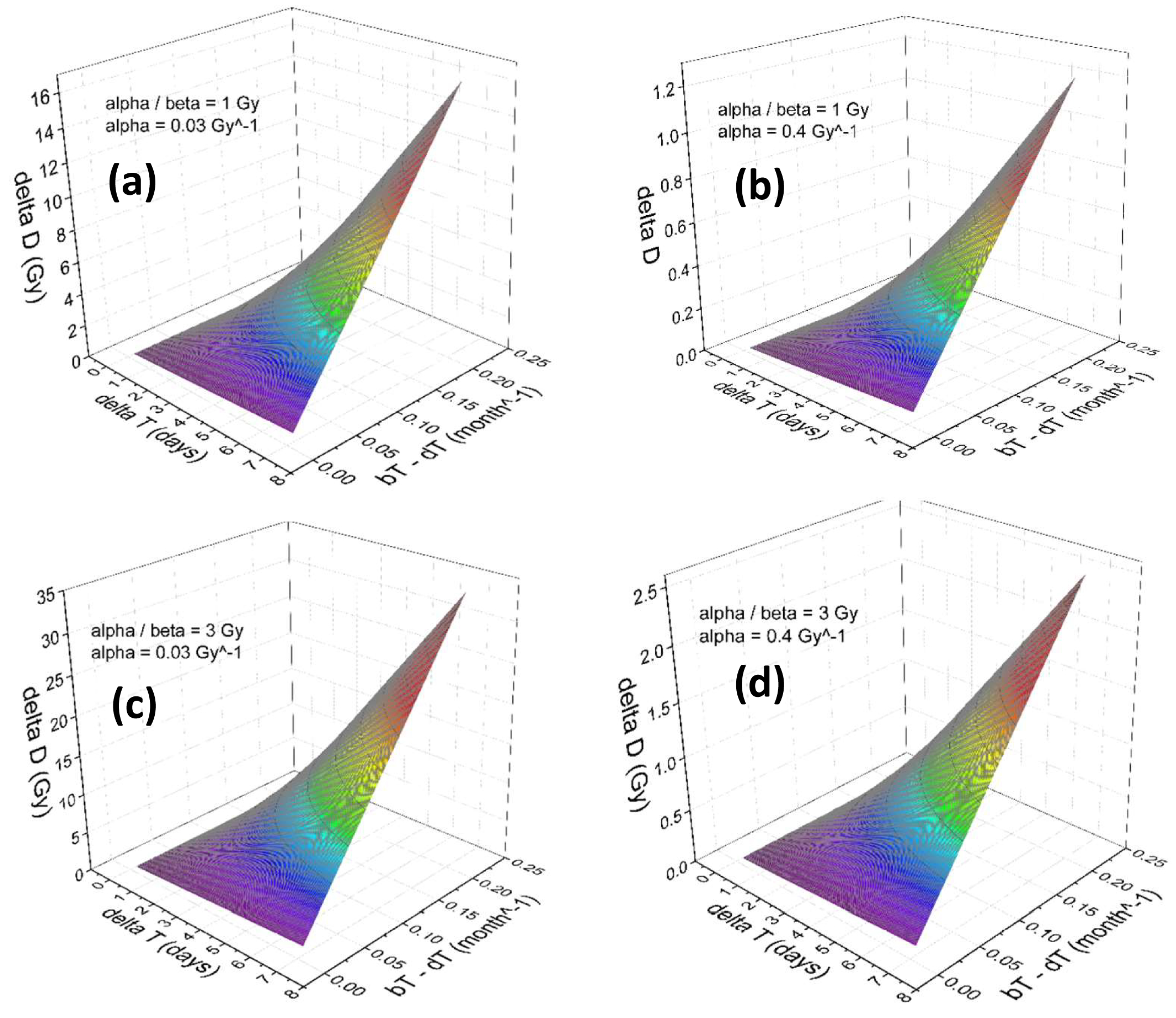
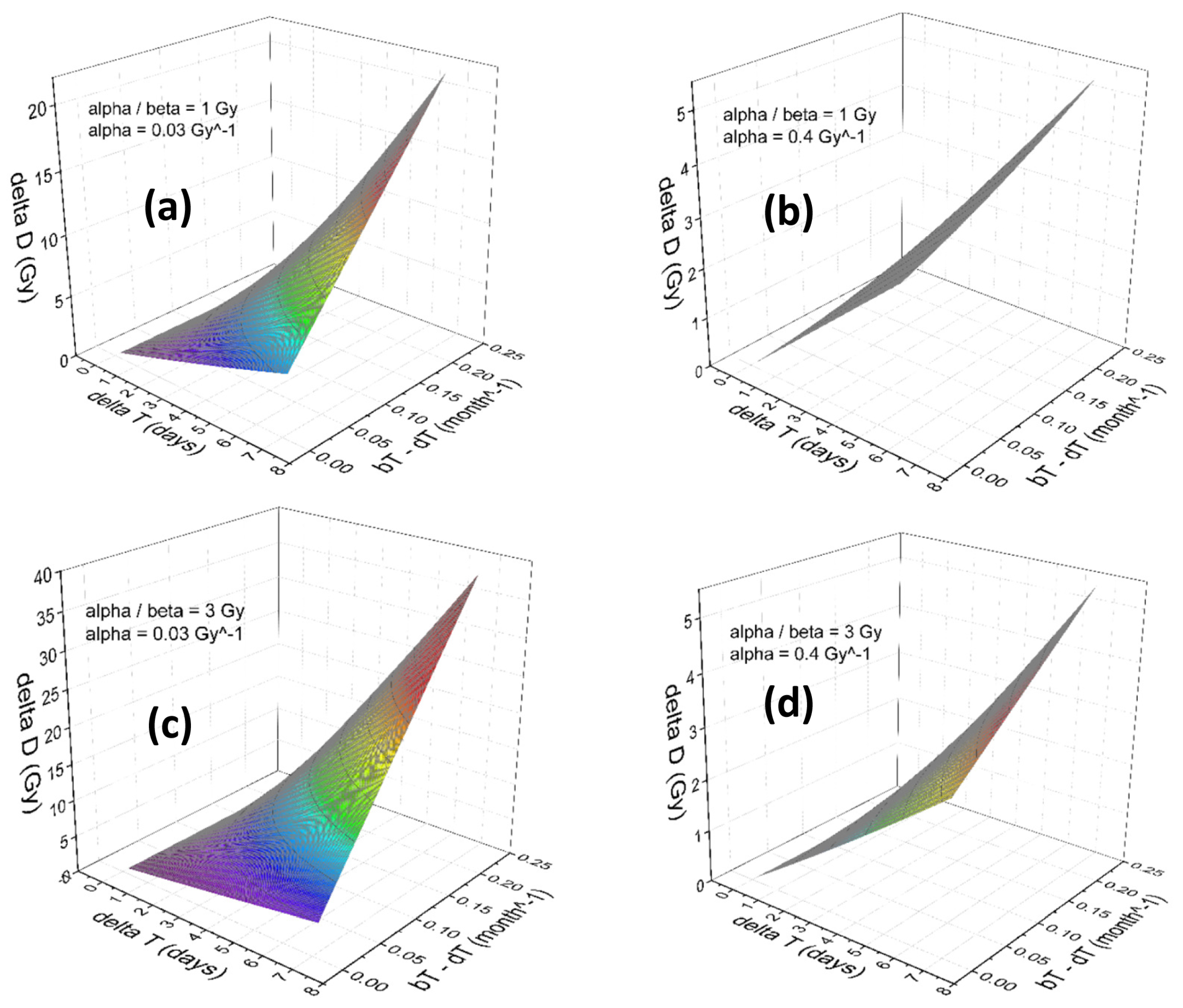
3. Results
Numerical Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Mathematical Model
Appendix A.1. SF and TCP
Appendix A.2. Calculation of
References
- Herskind, C.; Ma, L.; Liu, Q.; Zhang, B.; Schneider, F.; Veldwijk, M.R.; Wenz, F. Biology of high single doses of IORT: RBE, 5 R’s, and other biological aspects. Radiat. Oncol. 2017, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Boustani, J.; Grapin, M.; Laurent, P.A.; Apetoh, L.; Mirjolet, C. The 6th R of Radiobiology: Reactivation of Anti-Tumor Immune Response. Cancers 2019, 11, 860. [Google Scholar] [CrossRef]
- Friedl, W.; Kundrat, P. A Guide to Outcome Modeling in Radiotherapy and Oncology: Listening to the Data; Naqa, I.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- McMahon, S.J.; Prise, K.M. Mechanistic Modelling of Radiation Responses. Cancers 2019, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J.; Huang, Y.; Sachs, R.K. Evidence for formation of DNA repair centers and dose-response nonlinearity in human cells. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 893–901. [Google Scholar] [CrossRef]
- Brenner, D.J. The Linear-Quadratic Model Is an Appropriate Methodology for Determining Isoeffective Doses at Large Doses per Fraction. Semin. Radiat. Oncol. 2008, 18, 234–239. [Google Scholar] [CrossRef]
- Carlson, D.J.; Stewart, R.D.; Li, X.A.; Jennings, K.; Wang, J.Z.; Guerrero, M. Comparison of in vitro and in vivo α/β ratios for prostate cancer. Phys. Med. Biol. 2004, 49, 4477–4491. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Baer, R.; Gautier, J. DNA double-strand break repair pathway choice and cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef]
- Borrego-Soto, G.; Ortiz-Lopez, R.; Rojas-Martínez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420. [Google Scholar] [CrossRef]
- King, R.B.; Hyl, W.B.; Cole, A.J.; Butterworth, K.T.; McMahon, S.J.; Redmond, K.M.; Trainer, C.; Prise, K.M.; McGarry, C.K.; Hounsell, A.R. An in vitro study of the radiobiological effects of flattening filter free radiotherapy treatments. Phys. Med. Biol. 2013, 58, N83. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McGarry, C.K.; Trainor, C.; O’Sullivan, J.M.; Hounsell, A.R.; Prise, K.M. A study of the biological effects of modulated 6 MV radiation fields. Phys. Med. Biol. 2010, 55, 1607–1618. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, K.T.; McGarry, C.K.; Trainor, C.; McMahon, S.J.; O’Sullivan, J.M.; Schettino, G.; Hounsell, A.R.; Prise, K.M. Dose, dose-rate and field size effects on cell survival following exposure to non-uniform radiation fields. Phys. Med. Biol. 2012, 57, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Shibamoto, Y.; Sugie, C.; Ito, M. Biological Effects of Intermittent Radiation in Cultured Tumor Cells: Influence of Fraction Number and Dose Per Fraction. J. Radiat. Res. 2005, 46, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Bewes, J.M.; Suchowerska, N.; Jackson, M.; Zhang, M.; McKenzie, D.R. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT). Phys. Med. Biol. 2008, 53, 3567–3578. [Google Scholar] [CrossRef]
- Dong, Y.; Zaorsky, N.G.; Li, T.; Churilla, T.M.; Viterbo, R.; Sobczak, M.L.; Smaldone, M.C.; Chen, D.Y.T.; Uzzo, R.G.; Hallman, M.A.; et al. Effects of Interruptions of External Beam Radiation Therapy on Outcomes in Patients with Prostate Cancer. J. Med. Imag. Radiat. Oncol. 2018, 62, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ohri, N.; Rapkin, B.D.; Guha, C.; Kalnicki, S.; Garg, M. Radiation Therapy Noncompliance and Clinical Outcomes in an Urban Academic Cancer Center. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.M.; Sutton, H. Radiation response of mammalian cells grown in culture, 1. Repair of X-ray damage in surviving Chinese hamster cells. Radiat Res. 1960, 13, 556–593. [Google Scholar] [CrossRef]
- Mu, X.; Löfroth, P.O.; Karlsson, M. The effect of fraction time in intensity modulated radiotherapy: Theoretical and experimental evaluation of an optimisation problem. Radiother Oncol. 2003, 68, 181–187. [Google Scholar] [CrossRef]
- Nakano, H.; Kawahara, D.; Ono, K.; Akagi, Y.; Hirokawa, Y. Effect of dose-delivery time for flattened and flattening filter-free photon beams based on microdosimetric kinetic model. PLoS ONE 2018, 13, 0206673. [Google Scholar] [CrossRef]
- Inaniwa, T.; Kanematsu, N.; Suzuki, M. Effects of beam interruption time on tumor control probability in single-fractionated carbon-ion radiotherapy for non-small cell lung cancer. Phys. Med. Biol. 2015, 21, 4105–4121. [Google Scholar] [CrossRef]
- Inaniwa, T.; Suzuki, M.; Furukawa, T.; Kase, Y.; Kanematsu, N.; Shirai, T.; Hawkins, R.B. Effects of dose-delivery time structure on biological effectiveness for therapeutic carbon-355 ion beams evaluated with microdosimetric kinetic model. Radiat. Res. 2013, 180, 44–59. [Google Scholar] [CrossRef]
- Metzger, L.; Iliakis, G. Kinetics of DNA Double-strand Break Repair Throughout the Cell Cycle as Assayed by Pulsed Field Gel Electrophoresis in CHO Cells. Int. J. Radiat. Biol. 1991, 59, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Matsuya, Y.; McMahon, S.J.; Tsutsumi, K.; Sasaki, K.; Okuyama, G.; Yoshii, Y.; Mori, R.; Oikawa, J.; Prise, K.M. Investigation of dose-rate effects and cell-cycle distribution under protracted exposure to ionizing radiation for various dose-rates. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gastaldo, J.; Viau, M.; Bouchot, M.; Joubert, A.; Charvet, A.M.; Foray, N. Induction and repair rate of DNA damage: A unified model for describing effects of external and internal irradiation and contamination with heavy metals. J. Theor. Biol. 2008, 251, 68–81. [Google Scholar] [CrossRef]
- Khorramizadeh, M.; Saberi, A.; Tahmasebi-Birgani, M.; Shokrani, P.; Amouhedari, A. Impact of Prolonged Fraction Delivery Time Modelling Stereotactic Body Radiation Therapy with High Dose Hypofractionation on the Killing of Cultured ACHN Renal Cell Carcinoma Cell Line. J. Biomed. Phys. Eng. 2017, 7, 205–216. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Frankenberg-Schwager, M. Review of repair kinetics for DNA damage induced in eukaryotic cells in vitro by ionizing radiation. Radiother. Oncol. 1989, 14, 307–320. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Z.C.; Bui, T.A.; Sonoda, E.; Takata, M.; Takeda, S.; Iliakis, G. Efficient rejoining of radiation-induced DNA double-strand breaks in vertebrate cells deficient in genes of the RAD52 epistasis group. Oncogene 2001, 20, 2212–2224. [Google Scholar] [CrossRef]
- Karlsson, K.H.; Radulescu, I.; Rydberg, B.; Stenerlöw, B. Repair of Radiation-Induced Heat-Labile Sites is Independent of DNA-PKcs, XRCC1 and PARP. Rad. Res. 2008, 169, 506–512. [Google Scholar] [CrossRef]
- DiBiase, S.J.; Zeng, Z.C.; Chen, R.; Hyslop, T.; Curran, W.J., Jr.; Iliakis, G. DNA-dependent protein kinase stimulates an independently active, nonhomologous, end-joining apparatus. Cancer Res. 2000, 60, 1245–1253. [Google Scholar] [PubMed]
- Oliveira, S.M.; Teixeira, N.J.; Fernandes, L. What do we know about the α/β for prostate cancer? Med. Phys. 2012, 39, 1–3. [Google Scholar] [CrossRef]
- Tamponi, M.; Gabriele, D.; Maggio, A.; Stasi, M.; Meloni, G.B.; Conti, M.; Gabriele, P. Prostate cancer dose–response, fractionation sensitivity and repopulation parameters evaluation from 25 international radiotherapy outcome data sets. Br. J. Radiol. 2019, 92, 20180823. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumaier, T.; Swenson, J.; Pham, C.; Polyzos, A.; Lo, A.T.; Yang, P.; Dyball, J.; Asaithamby, A.; Chen, D.J.; Bissell, M.J.; et al. Evidence for formation of DNA repair centers and dose-response nonlinearity in human cells. Proc. Natl. Acad. Sci. USA 2012, 109, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kosti, O.; Goldman, L.; Saha, D.T.; Orden, R.A.; Pollock, A.J.; Madej, H.L.; Hsing, A.W.; Chu, L.W.; Lynch, J.H.; Goldman, R. DNA damage phenotype and prostate cancer risk. Mutat. Res. 2011, 719, 41–46. [Google Scholar] [CrossRef]
- Asaithamby, A.; Uematsu, N.; Chatterjee, A.; Story, M.D.; Burma, S.; Chen, D.J. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat. Res. 2008, 169, 437–446. [Google Scholar] [CrossRef]
- Turner, H.C.; Shuryak, I.; Taveras, M.; Bertucci, A.; Perrier, J.R.; Chen, C.; Elliston, C.D.; Johnson, G.W.; Smilenov, L.B.; Amundson, S.A.; et al. Effect of dose rate on residual γ-H2AX levels and frequency of micronuclei in X-irradiated mouse lymphocytes. Radiat. Res. 2015, 183, 315–324. [Google Scholar] [CrossRef]
- Frankenburg-Schwager, M.; Harbich, R.; Beckonert, S.; Frankenberg, D. Half-life values for DNA double-strand break rejoining in yeast can vary by more than an order of magnitude depending on the irradiation conditions. Int. J. Radiat. Biol. 1994, 66, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiat. Res. 1998, 150, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef]
- Waters, C.A.; Str, N.T.; Wyatt, D.W.; Pryor, J.M.; Ramsden, D.A. Nonhomologous end joining: A good solution for bad ends. DNA Repair 2014, 17, 39–51. [Google Scholar] [CrossRef]
- Krejci, L.; Altmannova, V.; Spirek, M.; Zhao, X. Homologous recombination and its regulation. Nucleic Acids Res. 2012, 40, 5795–5818. [Google Scholar] [CrossRef]
- Deriano, L.; Roth, D.B. Modernizing the nonhomologous end-joining repertoire: Alternative and classical NHEJ share the stage. Annu. Rev. Genet. 2013, 47, 433–455. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Prise, K.M. A Mechanistic DNA Repair and Survival Model (Medras): Applications to Intrinsic Radiosensitivity, Relative Biological Effectiveness and Dose-Rate. Front. Oncol. 2021, 29, 689112. [Google Scholar] [CrossRef] [PubMed]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Ma, A.G.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995, 336, 69–77. [Google Scholar] [CrossRef]
- Abbotts, R.; Wilson, D.M., 3rd. Coordination of DNA single strand break repair. Free Radic. Biol. Med. 2017, 107, 228–244. [Google Scholar] [CrossRef]
- Caldecott, K.W. XRCC1 protein; Form and function. DNA Repair 2019, 81, 102664. [Google Scholar] [CrossRef]
- Pascal, J.M. The comings and goings of PARP-1 in response to DNA damage. DNA Repair 2018, 71, 177–182. [Google Scholar] [CrossRef]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 3, 239–254.e6. [Google Scholar] [CrossRef]
- Iliakis, G.; Wang, H.; Perrault, A.R.; Boecker, W.; Rosidi, B.; Windhofer, F.; Wu, W.; Guan, J.; Terzoudi, G.; Pantelias, G. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet. Genome Res. 2004, 104, 14–20. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Li, Y.; Pandey, N.K.; Chen, T.; Wang, L.; Amador, E.H.; Chen, W.; Liu, F.; Xiao, E.; et al. Study of copper-cysteamine based X-ray induced photodynamic therapy and its effects on cancer cell proliferation and migration in a clinical mimic setting. Bioact. Mater. 2022, 7, 504–514. [Google Scholar] [CrossRef]
- Abolfath, R.; Helo, Y.; Bronk, L.; Carabe, A.; Grosshans, D.; Mohan, R. Renormalization of radiobiological response functions by energy loss fluctuations and complexities in chromosome aberration induction: Deactivation theory for proton therapy from cells to tumor control. Eur. Phys. J. D 2019, 73, 64. [Google Scholar] [CrossRef]
- Sachs, R.K.; Hahnfeldt, P.; Brenner, D.J. The link between low-LET dose-response relations and the underlying kinetics of damage production/repair/misrepair. Int. J. Radiat. Biol. 1997, 72, 351. [Google Scholar] [PubMed]
- Hawkins, R.B. A microdosimetric-kinetic theory of the dependence of the RBE for cell death on LET. Med. Phys. 1998, 25, 1157. [Google Scholar] [CrossRef] [PubMed]
- Niemierko, A.; Goitein, M. Calculation of normal tissue complication probability and dose-volume histogram reduction schemes for tissues with a critical element architecture. Radiother. Oncol. 1991, 20, 166–176. [Google Scholar] [CrossRef]
- Young, A.; Berry, R.; Holloway, A.F.; Blackburn, N.B.; Dickinson, J.L.; Skala, M.; Phillips, J.L.; Brettingham-Moore, K.H. RNA-seq profiling of a radiation resistant and radiation sensitive prostate cancer cell line highlights opposing regulation of DNA repair and targets for radiosensitization. BMC Cancer 2014, 14, 808. [Google Scholar] [CrossRef]
- Thames, H.D.; Zhang, M.; Tucker, S.L.; Liu, H.H.; Dong, L.; Mohan, R. Cluster models of dose–volume effects. Int. Radiat. Oncol. Biol. Phys. 2004, 59, 1491–1504. [Google Scholar] [CrossRef]
- Zaider, M.; Amols, H.I. Practical considerations in using calculated healthy-tissue complication probabilities for treatment-plan optimization. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 439–447. [Google Scholar] [CrossRef]
- Kellerer, A.M. Fundamentals of Microdosimetry The Dosimetry of Ionizing Radiation; Kase, K.R., Ed.; Academic: London, UK, 1985; Volume 1, pp. 77–161. [Google Scholar]
- Rossi, H.H.; Zaider, M. Microdosimetry and Its Applications; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Kellerer, A.M.; Rossi, H.H. A Generalized Formulation of Dual Radiation Action. Radiat. Res. 2012, 178, AV204–AV213. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abolfath, R.; Khalili, M.; Senejani, A.G.; Kodery, B.; Ivker, R. The Dependence of Compensation Dose on Systematic and Random Interruption Treatment Time in Radiation Therapy. Onco 2022, 2, 264-281. https://doi.org/10.3390/onco2030015
Abolfath R, Khalili M, Senejani AG, Kodery B, Ivker R. The Dependence of Compensation Dose on Systematic and Random Interruption Treatment Time in Radiation Therapy. Onco. 2022; 2(3):264-281. https://doi.org/10.3390/onco2030015
Chicago/Turabian StyleAbolfath, Ramin, Mitra Khalili, Alireza G. Senejani, Balachandran Kodery, and Robert Ivker. 2022. "The Dependence of Compensation Dose on Systematic and Random Interruption Treatment Time in Radiation Therapy" Onco 2, no. 3: 264-281. https://doi.org/10.3390/onco2030015
APA StyleAbolfath, R., Khalili, M., Senejani, A. G., Kodery, B., & Ivker, R. (2022). The Dependence of Compensation Dose on Systematic and Random Interruption Treatment Time in Radiation Therapy. Onco, 2(3), 264-281. https://doi.org/10.3390/onco2030015








