Risk Factors and Prevention of Gastric Cancer Development—What Do We Know and What Can We Do?
Abstract
:Simple Summary
Abstract
1. Introduction
- Q1: Are there correlations between microbiota status and the development of gastric cancer?
- Q2: Can probiotic therapy have a positive impact on gastric cancer prevention?
- Q3: Can interventions be taken to reduce the risk of gastric cancer?
2. Materials and Methods
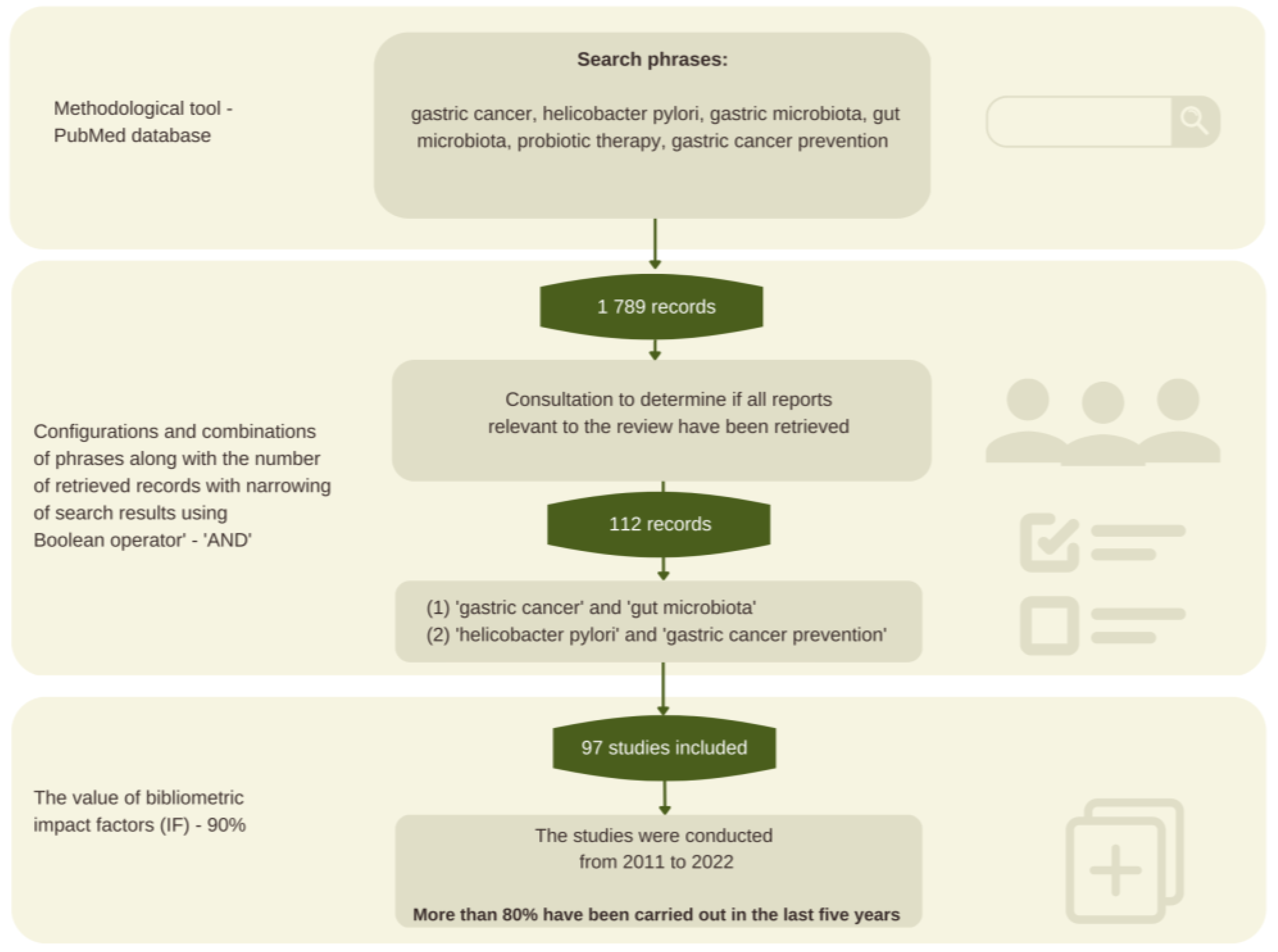
2.1. Methodology Background
2.2. Review Procedure and Search Strategy
2.3. Sources Selection
3. Gastric Cancer—Characteristics and General Classification
3.1. Helicobacter Pylori as a Carcinogen
3.2. Gastric Microbiota
3.3. Gut Microbiota and Gastric Cancer
4. Probiotics
5. Nutraceuticals with Chemopreventive Effects
5.1. Dietary Fiber
5.2. Polyunsaturated Fatty Acids
5.3. Ingredients with Immunomodulatory Effects
6. Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Piscione, M.; Mazzone, M.; Di Marcantonio, M.C.; Muraro, R.; Mincione, G. Eradication of Helicobacter pylori and Gastric Cancer: A Controversial Relationship. Front Microbiol. 2021, 12, 630852. [Google Scholar] [CrossRef]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Chiang, T.H.; Chang, W.J.; Chen, S.L.; Yen, A.M.; Fann, J.C.; Chiu, S.Y.; Chen, Y.R.; Chuang, S.L.; Shieh, C.F.; Liu, C.Y.; et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: A long-term cohort study on Matsu Islands. Gut 2021, 70, 243–250. [Google Scholar] [CrossRef]
- Waldum, H.L.; Rehfeld, J.F. Gastric cancer and gastrin: On the interaction of Helicobacter pylori gastritis and acid inhibitory induced hypergastrinemia. Scand. J. Gastroenterol. 2019, 54, 1118–1123. [Google Scholar] [CrossRef]
- Stewart, O.A.; Wu, F.; Chen, Y. The role of gastric microbiota in gastric cancer. Gut. Microbes 2020, 11, 1220–1230. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.; Carneiro, F.; Machado, J.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Kadar, Z.; Jung, I.; Orlowska, J.; Szentirmay, Z.; Sugimura, H.; Turdean, S.; Simona, G. Geographic particularities in incidence and etiopathogenesis of sporadic gastric cancer. Pol. J. Pathol. 2015, 66, 254–259. [Google Scholar] [CrossRef]
- Dai, D.; Yang, Y.; Yu, J.; Dang, T.; Qin, W.; Teng, L.; Ye, J.; Jiang, H. Interactions between gastric microbiota and metabolites in gastric cancer. Cell Death Dis. 2021, 12, 1104. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Krupa-Kotara, K.; Helisz, P.; Gwioździk, W.; Grajek, M. The importance of the microbiota in shaping women’s health—The current state of knowledge. Appl. Microbiol. 2022, 3, 11–34. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Lin, L.; Jin, D.; Yaoqiang, D.; Lyu, J. Research progress on gut microbiota in patients with gastric cancer, esophageal cancer, and small intestine cancer. Appl. Microbiol. Biotechnol 2021, 105, 4415–4425. [Google Scholar] [CrossRef]
- Gao, J.J.; Zhang, Y.; Gerhard, M.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.-L.; Bajbouj, M.; Suchanek, S.; Liu, W.-D.; et al. Association Between Gut Microbiota and Helicobacter pylori-Related Gastric Lesions in a High-Risk Population of Gastric Cancer. Front. Cell Infect. Microbiol. 2018, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Patel, T.; Bhattacharya, P.; Das, S. Gut microbiota: An Indicator to Gastrointestinal Tract Diseases. J. Gastrointest. Canc. 2016, 47, 232–238. [Google Scholar] [CrossRef]
- Sarhadi, V.; Mathew, B.; Kokkola, A.; Karla, T.; Tikkanen, M.; Rautelin, H.; Lahti, L.; Puolakkainen, P.; Knuutila, S. Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors. Gut Pathog. 2021, 13, 11. [Google Scholar] [CrossRef]
- Yang, D.; Meng, X.-Y.; Wang, Y.; Zhang, J.; Zhao, Y.; Zheng, Z.C.; Zhang, T. Effects of probiotics on gastric cancer-related inflammation: A systematic review and meta-analysis. J. Food Biochem. 2022, 46, e14034. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Devi, T.B.; Devadas, K.; George, M.; Gandhimathi, A.; Chouhan, D.; Retnakumar, R.J.; Alexander, S.M.; Varghese, J.; Dharmaseelan, S.; Chandrika, S.K.; et al. Low Bifidobacterium Abundance in the Lower Gut Microbiota Is Associated with Helicobacter pylori-Related Gastric Ulcer and Gastric Cancer. Front. Microbiol. 2021, 12, 631140. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Tang, H.; Zhai, Q.; Liu, L.; Xia, L.; Wu, W.; Xu, Y.; Wang, J. Gut Microbiota Dysbiosis in the Development and Progression of Gastric Cancer. J. Oncol. 2022, 2022, 9971619. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Yang, Y.; Wang, H.; Wang, H.; Yu, X.; Lu, Y.; Shen, S.; Teng, L. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine 2019, 98, e16626. [Google Scholar] [CrossRef]
- Chen, C.; Shen, J.; Du, Y.; Shi, X.; Niu, Y.; Jin, G.; Liu, Y.; Shi, Y.; Lyu, J.; Lin, L. Characteristics of gut microbiota in patients with gastric cancer by surgery, chemotherapy and lymph node metastasis. Clin. Transl. Oncol. 2022, 24, 2181–2190. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Weitsman, S.; Celly, S.; Leite, G.; Mathur, R.; Sedighi, R.; Barlow, G.M.; Morales, W.; Sanchez, M.; Parodi, G.; Villanueva-Millan, M.J.; et al. Effects of Proton Pump Inhibitors on the Small Bowel and Stool Microbiomes. Dig. Dis. Sci. 2022, 67, 224–232. [Google Scholar] [CrossRef]
- Jacobs, C.; Coss Adame, E.; Attaluri, A.; Valestin, J.; Rao, S.S. Dysmotility and ppi use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment. Pharm. Ther. 2013, 37, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Krajowy Rejestr Nowotworów. Nowotwór Żołądka- Leczenie. Available online: https://onkologia.org.pl/pl/nowotwor-zoladka-leczenie#page-main-image (accessed on 9 December 2022).
- Zheng, C.; Chen, T.; Wang, Y.; Gao, Y.; Kong, Y.; Liu, Z.; Deng, X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J. Cancer 2019, 10, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Lu, Q.; Wang, H.; Zhu, X.; Guan, Z. Effects of probiotics combined with enteral nutrition on immune function and inflammatory response in postoperative patients with gastric cancer. J. Buon. 2018, 23, 678–683. [Google Scholar]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- Hijová, E.; Bertková, I.; Štofilová, J. Dietary fibre as prebiotics in nutrition. Cent. Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- McRae, M.P. The Benefits of Dietary Fiber Intake on Reducing the Risk of Cancer: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 90–96. [Google Scholar] [CrossRef]
- Richa, S.N.; Sageena, G. Dietary factors associated with gastric cancer—A review. Transl. Med. Commun. 2022, 7, 7. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.; Ma, M.; Yang, J.; Liu, X. Dietary fiber intake reduces risk for gastric cancer: A meta-analysis. Gastroenterology 2013, 145, 113–120.e3. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kim, J. Dietary patterns and gastric cancer risk in a Korean population: A case–control study. Eur. J. Nutr. 2021, 60, 389–397. [Google Scholar] [CrossRef]
- Katagiri, R.; Goto, A.; Shimazu, T.; Yamaji, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Tsugane, S. Dietary fiber intake and risk of gastric cancer: The Japan Public Health Center-based prospective study. Int. J. Cancer 2021, 148, 2664–2673. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.S.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, F.; Schaltenberg, N.; Villablanca, E.J.; Huber, S.; Gagliani, N. Dietary Habits and Intestinal Immunity: From Food Intake to CD4+ T H Cells. Front. Immunol. 2019, 9, 3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi, F.; Sahmani, M.; Moghbelinejad, S.; Azad, M.; Rahmani, B.; Pishkhan, S.; Gholamzadeh Khoei, S.; Mohammadi Goldar, Z.; Gheibi, N. Changes of WIF-1 and WT-1 genes expression following the anti-cancer effects of omega-3 and omega-6 on gastric cancer cells. Gene Rep. 2020, 21, 100826. [Google Scholar] [CrossRef]
- Lee, H.J.; Young-Min, H.; Jeong, M.A.; Kang, E.A.; Park, Y.J.; Cha, J.Y.; Hahm, K.B. Role of omega-3 polyunsaturated fatty acids in preventing gastrointestinal cancers: Current status and future perspectives. Expert Rev. Anticancer. Ther. 2018, 18, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.R.; Kim, E.; Fan, Y.Y.; Chapkin, R.S. Omega-3 fatty acids, membrane remodeling and cancer prevention. Mol. Asp. Med. 2018, 64, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Feijó, P.M.; Rodrigues, V.D.; Viana, M.S.; Dos Santos, M.P.; Abdelhay, E.; Viola, J.P.; de Pinho, N.B.; Martucci, R.B. Effects of ω-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: A randomized controlled trial. Nutrition 2019, 61, 125–131. [Google Scholar] [CrossRef]
- Nemati, A.; Nachvak, S.M.; Djafarian, K.; Faizi-Khankandi, I. Effect of omega-3 fatty acid supplementation on nutritional status in patients with gastric cancer during chemotherapy. J. Nutr. Sci. Diet. 2015, 1, 2–8. [Google Scholar]
- Liu, Z.; Ge, X.; Chen, L.; Sun, F.; Ai, S.; Kang, X.; Lv, B.; Lu, X. The Addition of ω-3 Fish Oil Fat Emulsion to Parenteral Nutrition Reduces Short-Term Complications after Laparoscopic Surgery for Gastric Cancer. Nutr. Cancer 2021, 73, 2469–2476. [Google Scholar] [CrossRef]
- Mocellin, M.C.; de Quadros Camargo, C.; de Souza Fabre, M.E.; de Moraes Trindade, E.B.S. Fish oil effects on quality of life, body weight and free fat mass change in gastrointestinal cancer patients undergoing chemotherapy: A triple blind, randomized clinical trial. J. Funct. Foods 2017, 31, 113–122. [Google Scholar] [CrossRef]
- Eltweri, A.M.; Thomas, A.L.; Metcalfe, M.; Calder, P.C.; Dennison, A.R.; Bowrey, D.J. Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin. Nutr. 2017, 36, 65–78. [Google Scholar] [CrossRef]
- Camargo, C.Q.; Mocellin, M.C.; Brunetta, H.S.; Chagas, T.R.; Fabre, M.E.S.; Trindade, E.B.S.M.; Silva, E.L.D.; Nunes, E.A. Fish oil decreases the severity of treatment-related adverse events in gastrointestinal cancer patients undergoing chemotherapy: A randomized, placebo-controlled, triple-blind clinical trial. Clin. Nutr. ESPEN 2019, 31, 61–70. [Google Scholar] [CrossRef]
- Finglas, P.; Roe, M.; Pinchen, H. McCance and Widdowson’s The Composition of Foods Integrated Dataset. Public Health England: London, UK, 2021. Available online: http://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid?utm_source (accessed on 21 December 2022).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny: Warsaw, Poland, 2020; Volume 83. [Google Scholar]
- Irún, P.; Lanas, A.; Piazuelo, E. Omega-3 Polyunsaturated Fatty Acids and Their Bioactive Metabolites in Gastrointestinal Malignancies Related to Unresolved Inflammation. A Review. Front. Pharmacol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Ikezaki, H.; Furusyo, N.; Jacques, P.F.; Shimizu, M.; Murata, M.; Schaefer, E.J.; Urita, Y.; Hayashi, J. Higher dietary cholesterol and ω-3 fatty acid intakes are associated with a lower success rate of Helicobacter pylori eradication therapy in Japan. Am. J. Clin. Nutr. 2017, 106, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Correia, M.; Michel, V.; Matos, A.A.; Carvalho, P.; Oliveira, M.J.; Ferreira, R.M.; Dillies, M.A.; Huerre, M.; Seruca, R.; Figueiredo, C.; et al. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization. PLoS ONE 2012, 7, e35072. [Google Scholar] [CrossRef]
- Han, Y.M.; Kim, K.J.; Jeong, M.; Park, J.; Go, E.; Kang, J.X.; Pyo Hong, S.; Baik Hahm, K. Suppressed Helicobacter pylori-associated gastric tumorigenesis in Fat-1 transgenic mice producing endogenous ω-3 polyunsaturated fatty acids. Oncotarget 2016, 11, 66606–66622. [Google Scholar] [CrossRef] [Green Version]
- Zare Javid, A.; Maghsoumi-Norouzabad, L.; Bazyar, H.; Aghamohammadi, V.; Alavinejad, P. Effects of Concurrent Omega-3 and Cranberry Juice Consumption Along with Standard Antibiotic Therapy on the Eradication of Helicobacter pylori, Gastrointestinal Symptoms, Some Serum Inflammatory and Oxidative Stress Markers in Adults with Helicobacter pylori Infection: A Study Protocol for a Randomized Controlled Trial. Infect. Drug Resist. 2020, 15, 3179–3185. [Google Scholar] [CrossRef]
- Park, J.M.; Jeong, M.; Kim, E.H.; Han, Y.M.; Kwon, S.H.; Hahm, K.B. Omega-3 Polyunsaturated Fatty Acids Intake to Regulate Helicobacter pylori-Associated Gastric Diseases as Nonantimicrobial Dietary Approach. Biomed. Res. Int. 2015, 2015, 712363. [Google Scholar] [CrossRef] [Green Version]
- Krupa-Kotara, K.; Grajek, M.; Wypych-Ślusarska, A.; Martynus-Depta, S.; Oleksiuk, K.; Głogowska-Ligus, J.; Szczepańska, E.; Słowiński, J. Properties of Polyunsaturated Fatty Acids in Primary and Secondary Prevention of Cardiovascular Diseases in the View of Patients (Silesia, Poland). Nurs. Rep. 2022, 12, 980–992. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Jagielski, P.; Wnęk, D.; Łuszczki, E.; Bolesławska, I.; Micek, A.; Kozioł-Kozakowska, A.; Piórecka, B.; Koczur, K.; Jankowska, K.; Gaździńska, A.; et al. Proposition of a New POLA Index to Assess the Immunomodulatory Properties of the Diet and Its Relationship with the Gut Microbiota, Using the Example of the Incidence of COVID-19 in a Group of People without Comorbidities. Nutrients 2022, 14, 4227. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef]
- Cherayil, B.J.; Ellenbogen, S.; Shanmugam, N.N. Iron and intestinal immunity. Curr. Opin. Gastroenterol. 2011, 27, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhou, Y.; He, H.; Chen, W.; Lenahan, C.; Li, X.; Deng, Y.; Shao, A.; Huang, J. Crosstalk between Macrophages, T Cells, and Iron Metabolism in Tumor Microenvironment. Oxid. Med. Cell Longev. 2021, 2, 8865791. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Story, M.J. Essential sufficiency of zinc, ω-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer. Biochimie 2021, 187, 94–109. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium-Calcineurin-NFAT Signaling Pathway in Health and Autoimmune Diseases. Front Immunol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Kaschek, L.; Zöphel, S.; Knörck, A.; Hoth, M. A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity. Semin. Cell. Dev. Biol. 2021, 115, 10–18. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin, C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Prieto, L.I.; Baker, D.J. Cellular Senescence and the Immune System in Cancer. Gerontology 2019, 65, 505–512. [Google Scholar] [CrossRef]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic Acid and an Acidic Tumor Microenvironment suppress Anticancer Immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Yetkin, D.; Balli, E.; Ayaz, F. Antiproliferative activity of Tamoxifen, Vitamin D3 and their concomitant treatment. EXCLI J. 2021, 20, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Helisz, P.; Dziubanek, G.; Krupa-Kotara, K.; Gwioździk, W.; Grajek, M.; Głogowska-Ligus, J. Colorectal Cancer and the Role of the Gut Microbiota—Do Medical Students Know More Than Other Young People?—Cross-Sectional Study. Nutrients 2022, 14, 4185. [Google Scholar] [CrossRef] [PubMed]
- Bara, T., Jr.; Gurzu, S.; Sugimura, H.; Bara, T.; Beleaua, M.A.; Jung, I. A systematic review of the possible carcinogenic role of the aristolochic acid. Rom. J. Morphol. Embryol. 2017, 58, 41–44. [Google Scholar] [PubMed]
- Yassibas, E.; Arslan, P.; Yalcin, S. Evaluation of dietary and life-style habits of patients with gastric cancer: A case-control study in Turkey. Asian Pac. J. Cancer Prev. 2012, 13, 2291–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakseresht, M.; Forman, D.; Malekzadeh, R.; Yazdanbod, A.; West, R.M.; Greenwood, D.C.; Crabtree, J.E.; Cade, J.E. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 2011, 22, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Vahid, F.; Davoodi, S.H. Nutritional Factors Involved in the Etiology of Gastric Cancer: A Systematic Review. Nutr. Cancer 2021, 73, 376–390. [Google Scholar] [CrossRef]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Microbiome-based personalized nutrition as a result of the 4.0 technological revolution: A mini literature review. Process. Biochem. 2022, 121, 257–262. [Google Scholar] [CrossRef]
- Deloose, E.; Janssen, P.; Depoortere, I.; Tack, J. The migrating motor complex: Control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 271–285. [Google Scholar] [CrossRef]
- Sun, C.-Q.; Chang, Y.-B.; Cui, L.-L.; Chen, J.-J.; Sun, N.; Zhang, W.-J.; Dai, L.-P. A population-based case-control study on risk factors for gastric cardia cancer in rural areas of Linzhou. Asian Pac. J. Cancer Prev. 2013, 14, 2897–2901. [Google Scholar] [CrossRef] [Green Version]
- Behnampour, N.; Hajizadeh, E.; Zayeri, F.; Semnani, S. Modeling of influential predictors of gastric cancer incidence rates in Golestan province, North Iran. Asian Pac. J. Cancer Prev. 2014, 15, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Krupa-Kotara, K.; Dakowska, D. Impact of obesity on the risk of cancer. Cent. Eur. J. Public Health 2021, 29, 38–44. [Google Scholar] [CrossRef]
- Grajek, M.; Krupa-Kotara, K.; Rozmiarek, M.; Sobczyk, K.; Działach, E.; Górski, M.; Kobza, J. The Level of COVID-19 Anxiety among Oncology Patients in Poland. Int. J. Environ. Res. Public Health 2022, 19, 11418. [Google Scholar] [CrossRef]
- Górski, M.; Garbicz, J.; Buczkowska, M.; Marsik, G.; Grajek, M.; Całyniuk, B.; Polaniak, R. Depressive disorders among long-term care residents in the face of isolation due to COVID-19 pandemic. Psychiatr. Polska 2022, 56, 101–114. [Google Scholar] [CrossRef]
| Food Product | FA Content (g/100 g of Product) | EPA 1 (mg/100 g FA 3) | EPA (mg/100 g of Product) | DHA 2 (mg/100 g FA 3) | DHA (mg/100 g of Product) |
|---|---|---|---|---|---|
| Mussels, cooked | 2.20 | 22,350 | 491.7 | 8350 | 183.70 |
| Prawns, cooked | 0.90 | 18,250 | 164.25 | 14,110 | 126.99 |
| Cod, baked | 0.50 | 8290 | 41.45 | 25,700 | 128.50 |
| Mackerel, grilled | 22.40 | 5820 | 1303.68 | 9160 | 2051.84 |
| Haddock, steamed | 0.60 | 11,260 | 67.56 | 29,740 | 178.44 |
| Chicken, breast, grilled without skin | 2.2 | 470 | 10.34 | 1010 | 22.22 |
| Tuna, canned in sunflower oil, drained | 6.4 | 400 | 25.6 | 2250 | 144 |
| Salmon, pink, canned in brine, drained | 4.8 | 8000 | 384 | 14,650 | 703.2 |
| RecommendedDHA + EPAintakeforadults: 250 mg perday | |||||
| Nutrient | Immune Function |
|---|---|
| Iron | It likely influences B-cell function and Th1/Th2 lymphocyte balance [63]. Affects the intestinal microbiota, by influencing the growth and survival of microorganisms inhabiting the human body [64]. Increased iron most likely promotes intestinal inflammation, while low Fe levels positively correlate with intestinal infections [64,65]. Potential anticancer effects through immune modulation and a hypothetical component of supporting cancer treatment in terms of tumor suppression (importance of ferroptosis) [66]. |
| Zinc | Zinc deficiencies contribute to increased production of pro-inflammatory cytokines such as interleukin-1β, interleukin-6, and TNF-α [67,68]. Deficiency results in decreased numbers of granulocytes, lymphocytes, and NK cells [67,68,69]. An essential micronutrient in both acquired and innate immunity processes [68,69]. Anti-inflammatory activity via regulatory T cell (Treg) function and by inhibiting NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) [69]. Exhibits indirect anti-tumor activity mediated by Th17 cells, NK cells, and T cells (Treg) [67]. |
| Calcium | It contributes to cellular functions such as proliferation, apoptosis, and gene transcription [70]. Calcium ions contribute to the proper functioning of cytotoxic T lymphocytes and NK cells, which may be important in terms of anticancer therapies [71]. |
| Dietary fiber | The function of pectins includes strengthening the mucus layer, increasing epithelial integrity, and activating or inhibiting dendritic cell and macrophage responses. Pectins can strengthen the intestinal immune barrier by promoting the adhesion of commensal bacteria and inhibiting the adhesion of pathogens to epithelial cells [72]. A high intake of dietary fiber leads to an increase in the number of intestinal bacterial species responsible for the production of SCFAs, essential for the proper functioning of the immune system and inflammatory disease prevention [73]. |
| Omega-3 fatty acids | It contributes to the activation of immune cells, including by regulating cell membranes [74]. It influences changes in gene expression in macrophages [74]. It contributes to the reduction in inflammation through properties that reduce the secretion of IL-1β, TNF-α, and IL-6 [74]. It most likely contributes to T-cell modulation [74]. |
| Vitamin D | It affects the immune system, both specific and non-specific immunity [75]. Exhibits anti-tumor activity, through the regulation of tumorigenesis [76]. Contributes to the regulation of the inflammatory microenvironment, via mechanisms such as inhibition of NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) pathways, and regulation of immune cell cytokine levels [77]. |
| Vitamin C | It supports epithelial barrier functions against pathogens [78]. Plays a key role in immune cell proliferation and differentiation, among other things, and is a cofactor in gene transcription and immune cell signaling [79]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helisz, P.; Gwioździk, W.; Krupa-Kotara, K.; Grajek, M.; Głogowska-Ligus, J.; Słowiński, J. Risk Factors and Prevention of Gastric Cancer Development—What Do We Know and What Can We Do? Onco 2023, 3, 26-42. https://doi.org/10.3390/onco3010003
Helisz P, Gwioździk W, Krupa-Kotara K, Grajek M, Głogowska-Ligus J, Słowiński J. Risk Factors and Prevention of Gastric Cancer Development—What Do We Know and What Can We Do? Onco. 2023; 3(1):26-42. https://doi.org/10.3390/onco3010003
Chicago/Turabian StyleHelisz, Paulina, Weronika Gwioździk, Karolina Krupa-Kotara, Mateusz Grajek, Joanna Głogowska-Ligus, and Jerzy Słowiński. 2023. "Risk Factors and Prevention of Gastric Cancer Development—What Do We Know and What Can We Do?" Onco 3, no. 1: 26-42. https://doi.org/10.3390/onco3010003
APA StyleHelisz, P., Gwioździk, W., Krupa-Kotara, K., Grajek, M., Głogowska-Ligus, J., & Słowiński, J. (2023). Risk Factors and Prevention of Gastric Cancer Development—What Do We Know and What Can We Do? Onco, 3(1), 26-42. https://doi.org/10.3390/onco3010003










