Significance of PET/CT Imaging in Myeloma Assessment: Exploring Novel Applications beyond Osteolytic Lesion Detection and Treatment Response
Abstract
Simple Summary
Abstract
1. Introduction
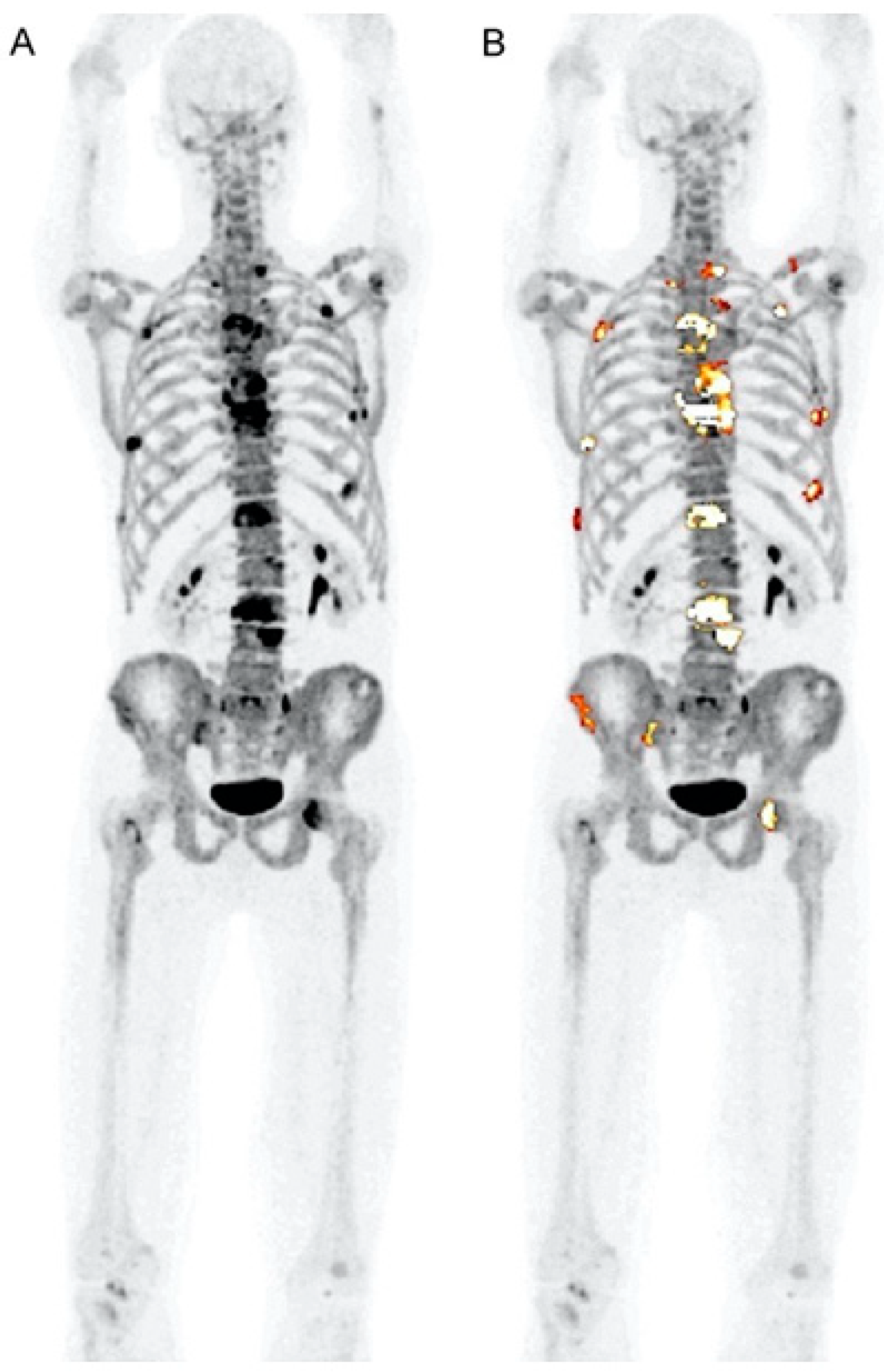
2. Assessment of Bone Disease in MM: An Examination of Osteolytic Lesions
3. Prediction of Prognosis
Correlation of [18F]FDG PET and β2-Microglobulin
4. Importance of [18F]FDG PET/CT in Assessing Treatment Efficacy
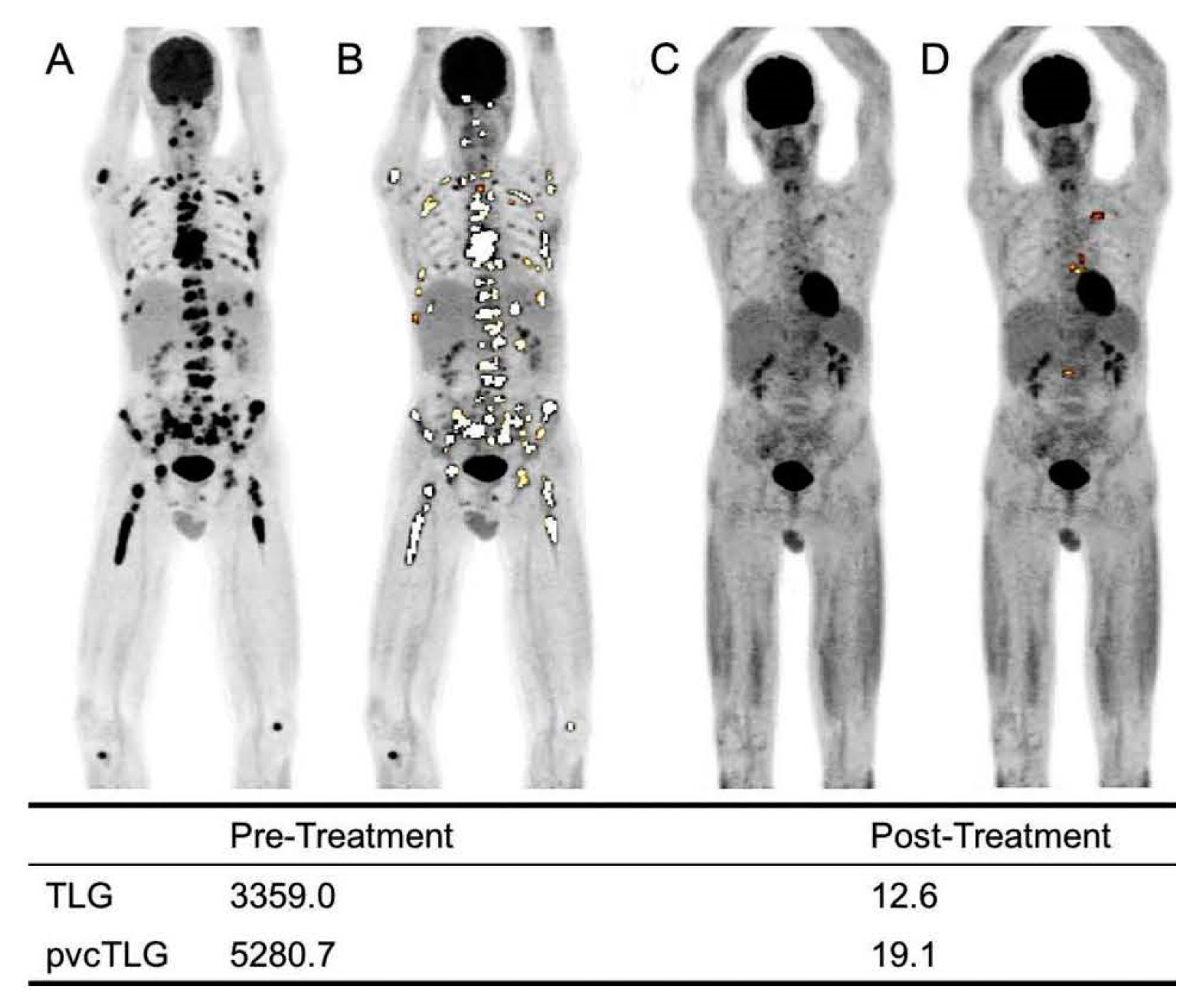
4.1. Baseline Parameters of [18F]FDG PET
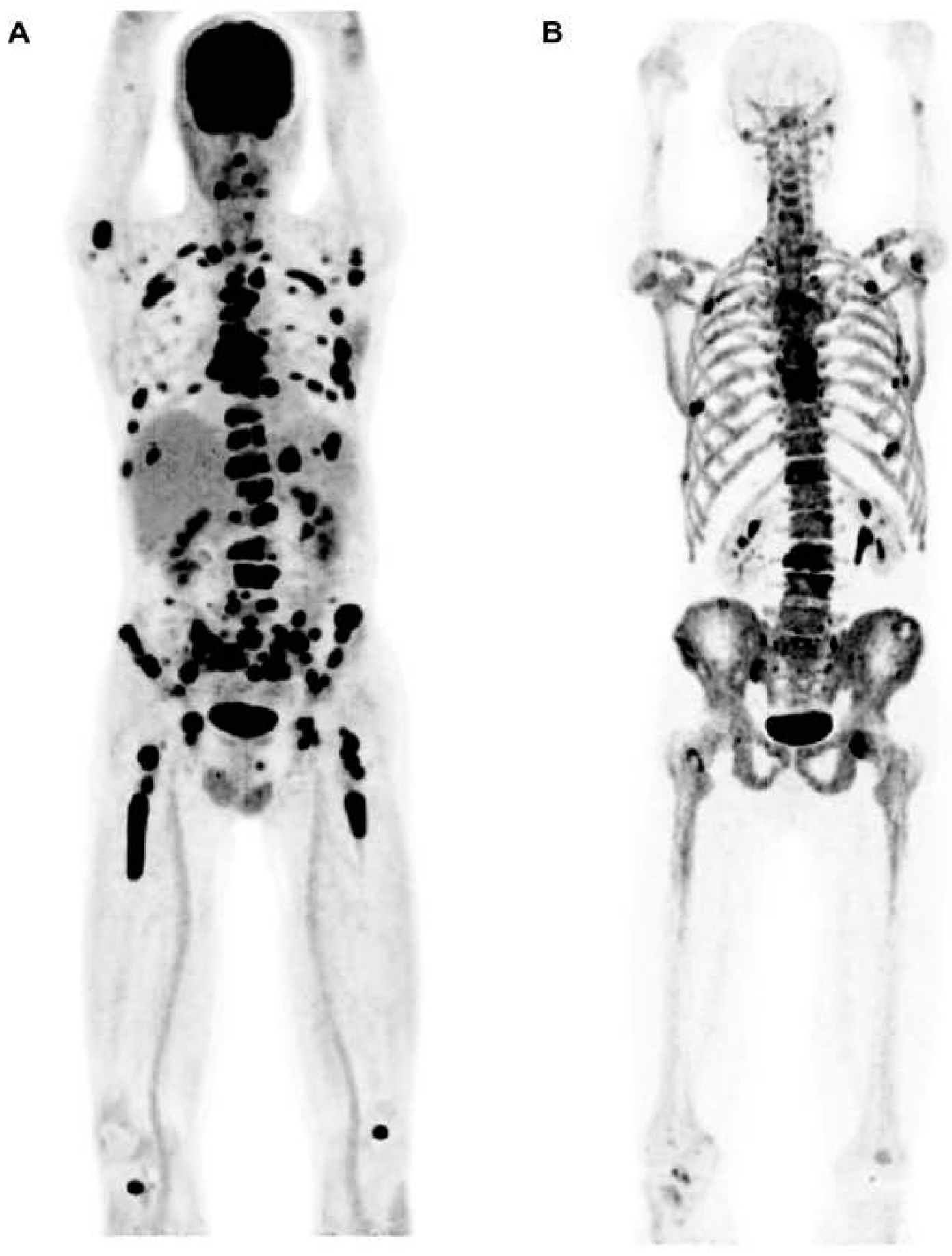
4.2. Assessment of Myeloma Patients after Treatment
5. Identification of Minimal Residual Disease (MRD) in Myeloma
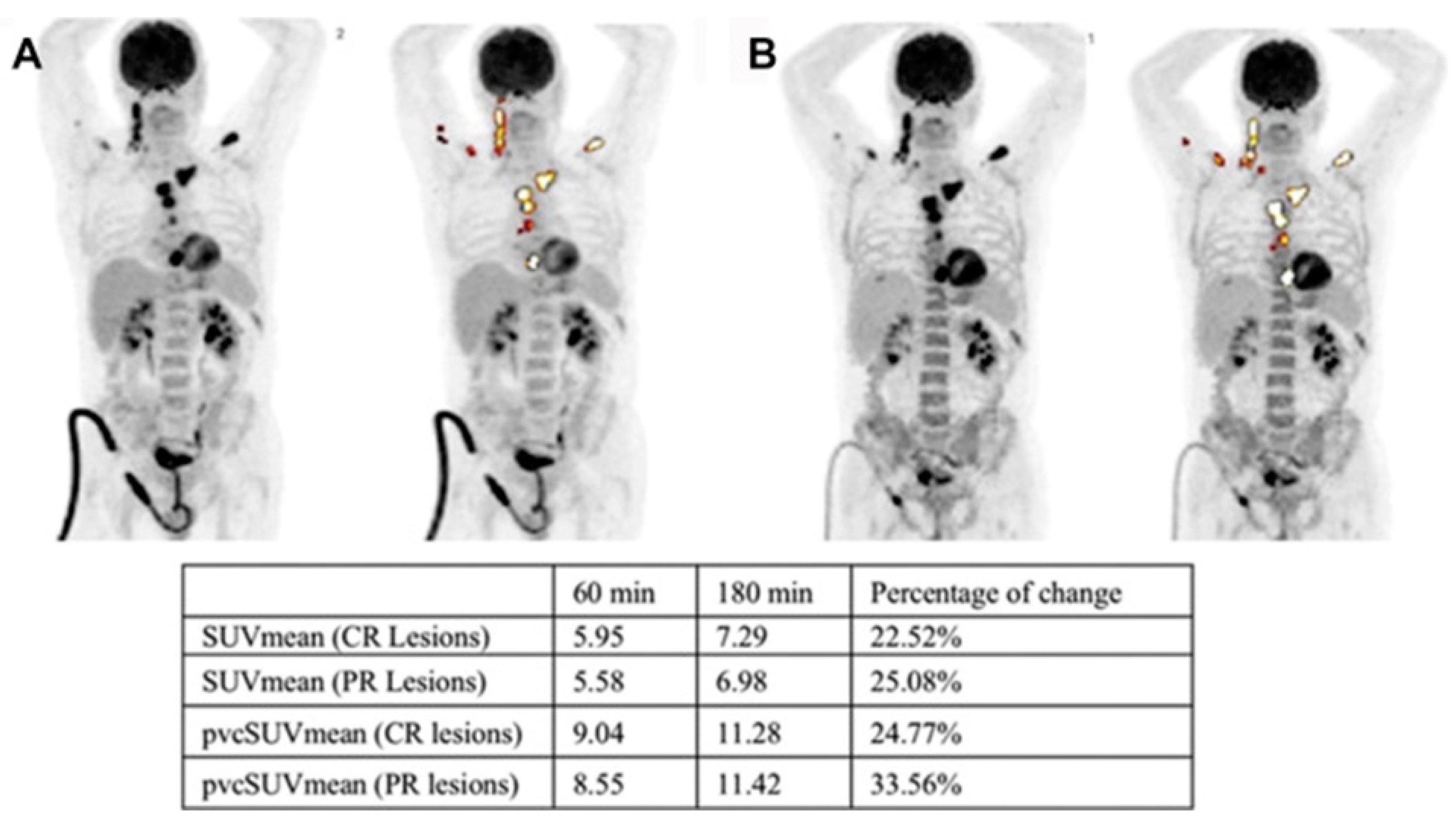
6. Dual-Time-Point Imaging (DTPI) in MM
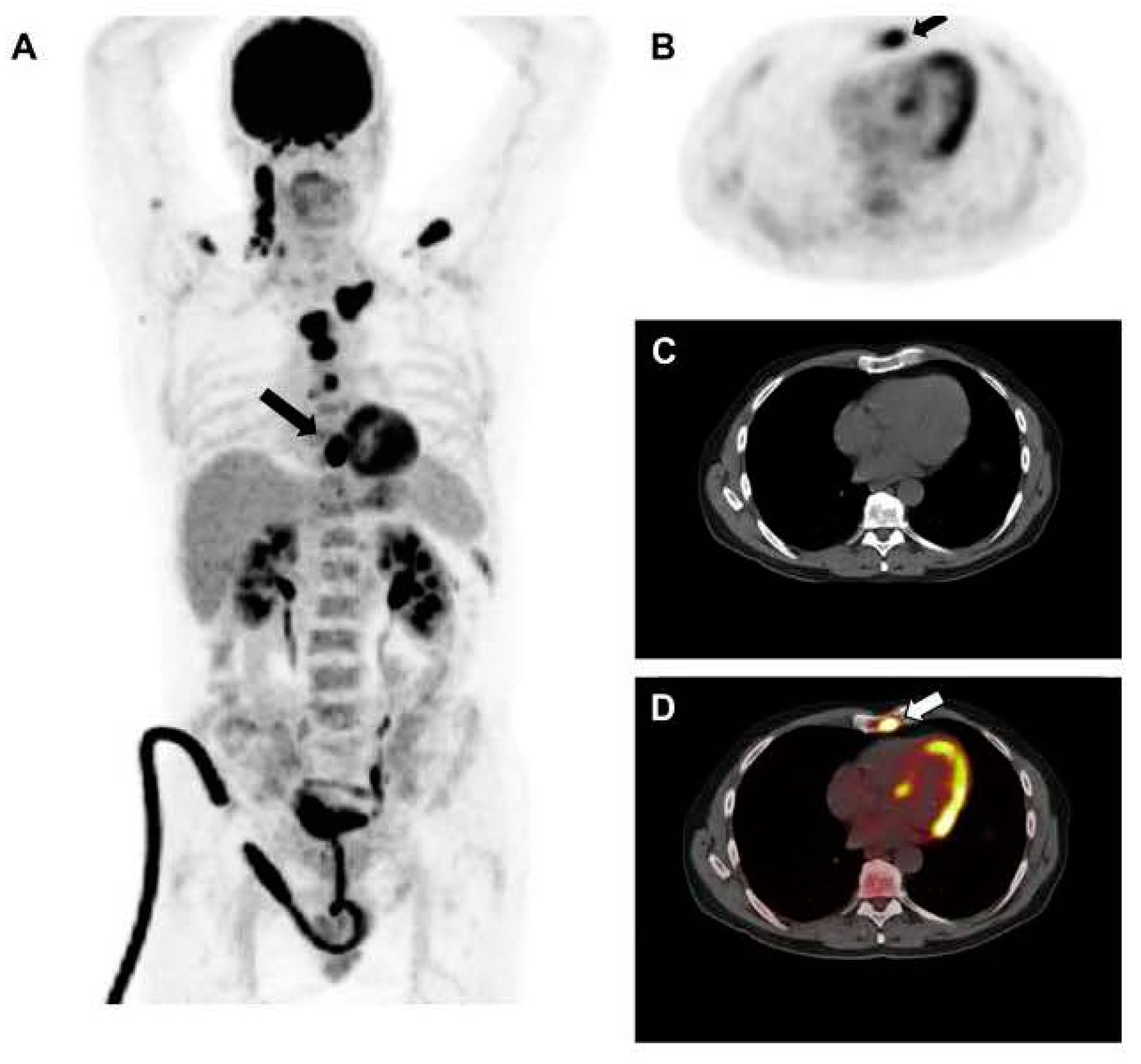
7. Assessment of Pathologic Fractures
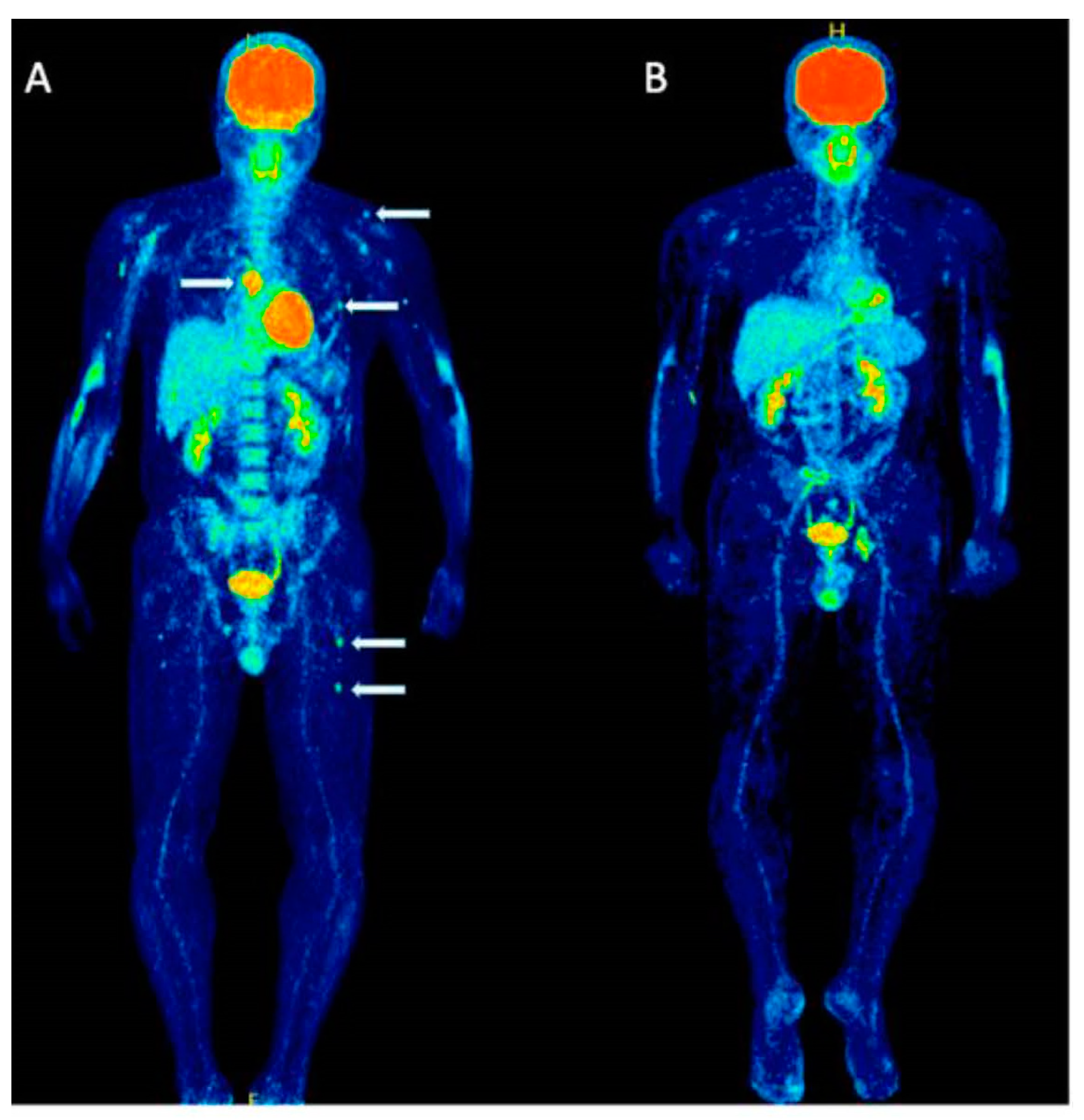
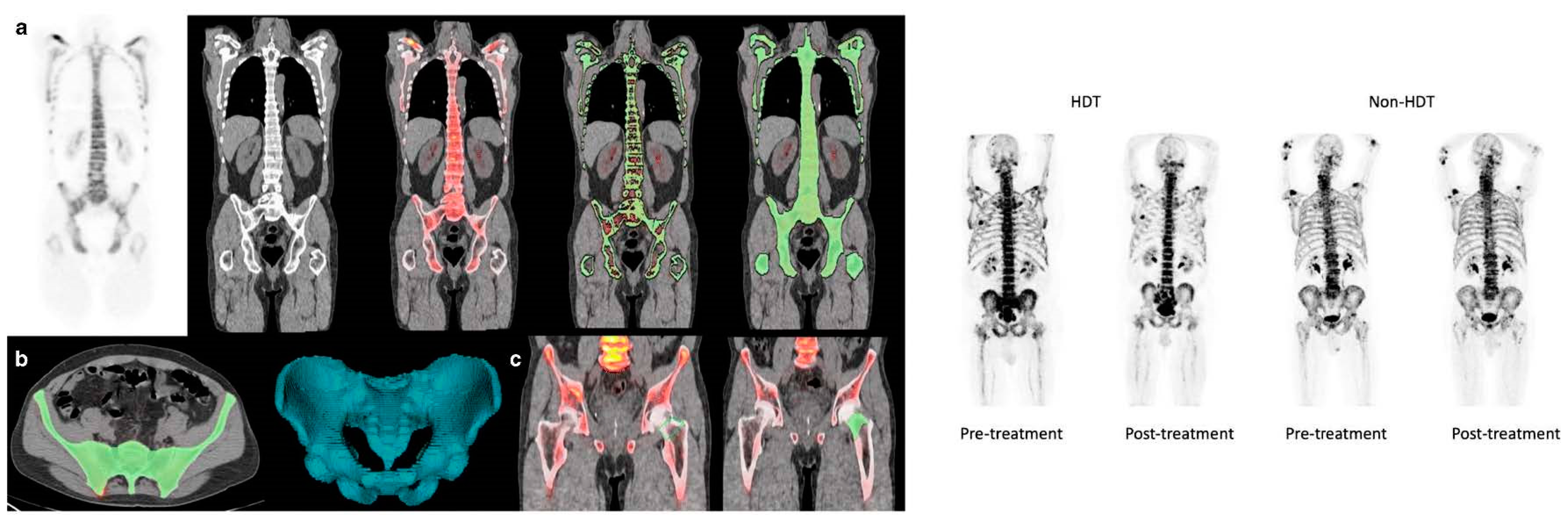
8. PET for Assessment of Bone Turnover in Myeloma Patients
9. Chemo Brain
10. [18F]FDG PET Quantification
11. Italian Myeloma Criteria for Pet Use: IMPeTUs
12. Novel PET Radiotracers
| Examples | Why? | Outcome | |
|---|---|---|---|
| Amino Acid Radiotracers | [11C]methionine 18F-fluoroethyl- tyrosine ([18F]FET) fluciclovine F18 ([18F]-FACBC) | Amino acid tracers are a promising biomarker in MM due to their likely absorption via the mechanism responsible for generating immunoglobulins in myeloma cells [53]. | In general, amino acid radiotracers have demonstrated equal or superior efficacy in evaluating patients with myeloma compared to [18F]FDG. However, the relative uptake of [18F]FET has been shown to be significantly lower in cell line data when compared to [11C]methionine and [18F]FDG. The latter two outperform [18F]FET by 7 to 20-fold and 3.5 to 5-fold, respectively [54]. |
| Lipid Radiotracers | [11C]choline, and [18F]fluorocholine ([18F]FCH) | Choline is an essential nutrient necessary for all cells due to its role in the creation of phospholipid components that form cell membranes [55]. Several radiotracers have been synthesized for choline imaging, including [11C]choline, and [18F]fluorocholine ([18F]FCH). | All of these radiotracers have been demonstrated to be superior or at least equivalent to [18F]FDG for the evaluation of MM. |
| CXCR4-targeting Radiotracers | [68Ga]Pentixafor | Many oncology studies have recognized C-X-C motif chemokine receptor 4 (CXCR4) as a potential target and an integral part of cancer progression, including aspects such as angiogenesis or other involvement leading to resistance to therapy [56,57]. An important observation from ex vivo research is the broad spectrum of solid tumors and hematological malignancies that increase the expression of CXCR4 on the tumor cell surface. This makes this G-protein coupled receptor an attractive target for both imaging and therapy [57]. | The majority of studies to date have indicated that [68Ga]Pentixafor PET/CT is equal to or superior to [18F]FDG in detecting osteolytic lesions and managing patients with MM. |
| Immuno-PET Radiotracers | [89Zr] DFO-daratumumab [89Zr]DFO-YS5 (anti-CD46 PET radiopharmaceutical) | Immuno-PET could set a new treatment standard for MM patients by merging the specificity of a radiolabeled monoclonal antibody with the high sensitivity of PET [58]. The transmembrane glycoprotein CD38, which is the specific target of the immunotherapy drug daratumumab, is present in all myeloma cells [59,60]. | Thus far, most of the outcomes have been shown to be superior or at least equivalent to those achieved with [18F]FDG PET/CT. |
| Proliferative PET Radiotracer | 3′-Deoxy-3′-[18F] fluorothymidine ([18F]FLT) | Researchers have been focusing on studying the uptake of nucleosides as an accurate technique to assess cell growth [61]. | However, researchers from a recent study disclosed that [18F]FLT alone might not be sufficient as a PET tracer for the diagnosis of MM [62]. |
| FAPI PET: Fibroblast Activation Protein Inhibitor | [68Ga]FAPI PET | FAPI PET operates by specifically targeting fibroblast activation protein (FAP), a protein that is highly expressed in tumor stromal cells, also known as cancer-associated fibroblasts [63]. | Superior to [18F]FDG. |
13. AI for PET Quantification
14. Atherosclerosis (Limited Data Available)
15. [18F]FDG and PET Limitations
16. Summary Statements
17. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
AI Language Statement
References
- Rodriguez-Otero, P.; Paiva, B.; San-Miguel, J.F. Roadmap to cure multiple myeloma. Cancer Treat. Rev. 2021, 100, 102284. [Google Scholar] [CrossRef] [PubMed]
- Michels, T.C.; Petersen, K.E. Multiple Myeloma: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 373–383. [Google Scholar] [PubMed]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Zhuang, J.; Mundy, G.R. The pathogenesis of the bone disease of multiple myeloma. Bone 2008, 42, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Myeloma Bone Disease; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Melton, L.J., III. Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 101, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Belch, A.; Bergsagel, D.; Wilson, K.; O’Reilly, S.; Wilson, J.; Sutton, D.; Pater, J.; Johnston, D.; Zee, B. Effect of daily etidronate on the osteolysis of multiple myeloma. J. Clin. Oncol. 1991, 9, 1397–1402. [Google Scholar] [CrossRef]
- Zadeh, M.Z.; Raynor, W.Y.; Seraj, S.M.; Ayubcha, C.; Kothekar, E.; Werner, T.; Alavi, A. Evolving Roles of Fluorodeoxyglucose and Sodium Fluoride in Assessment of Multiple Myeloma Patients: Introducing a Novel Method of PET Quantification to Overcome Shortcomings of the Existing Approaches. PET Clin. 2019, 14, 341–352. [Google Scholar] [CrossRef]
- Hemrom, A.; Tupalli, A.; Alavi, A.; Kumar, R. 18F-FDG Versus Non-FDG PET Tracers in Multiple Myeloma. PET Clin. 2022, 17, 415–430. [Google Scholar] [CrossRef]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Chandler, J.C.; Costello, C.; Efebera, Y. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1685–1717. [Google Scholar] [CrossRef]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef]
- Zirakchian Zadeh, M. PET/CT in assessment of colorectal liver metastases: A comprehensive review with emphasis on 18F-FDG. Clin. Exp. Metastasis 2023, 40, 465–491. [Google Scholar] [CrossRef] [PubMed]
- Taghvaei, R.; Zadeh, M.Z.; Werner, T.J.; Alavi, A. Critical role of PET/CT-based novel quantitative techniques for assessing global disease activity in multiple myeloma and other hematological malignancies: Why it is time to abandon reliance on examining focal lesions. Eur. Radiol. 2021, 31, 149–151. [Google Scholar] [CrossRef] [PubMed]
- van Lammeren-Venema, D.; Regelink, J.C.; Riphagen, I.I.; Zweegman, S.; Hoekstra, O.S.; Zijlstra, J.M. 18F-fluoro-deoxyglucose positron emission tomography in assessment of myeloma-related bone disease: A systematic review. Cancer 2012, 118, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Regelink, J.C.; Minnema, M.C.; Terpos, E.; Kamphuis, M.H.; Raijmakers, P.G.; Pieters-van den Bos, I.C.; Heggelman, B.G.; Nievelstein, R.J.; Otten, R.H.; van Lammeren-Venema, D.; et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: A systematic review. Br. J. Haematol. 2013, 162, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Steinbach, L.; Caputo, G.; Segall, G.; Hawkins, R. Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am. J. Roentgenol. 2005, 184, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Nanni, C.; Patriarca, F.; Englaro, E.; Castellucci, P.; Geatti, O.; Tosi, P.; Tacchetti, P.; Cangini, D.; Perrone, G.; et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica 2007, 92, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Westerland, O.; Amlani, A.; Kelly-Morland, C.; Fraczek, M.; Bailey, K.; Gleeson, M.; El-Najjar, I.; Streetly, M.; Bassett, P.; Cook, G.J.R.; et al. Comparison of the diagnostic performance and impact on management of 18F-FDG PET/CT and whole-body MRI in multiple myeloma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2558–2565. [Google Scholar] [CrossRef]
- Mesguich, C.; Hulin, C.; Latrabe, V.; Lascaux, A.; Bordenave, L.; Hindié, E.; Marit, G. Prospective comparison of 18FDG PET/CT and whole-body diffusion-weighted MRI in the assessment of multiple myeloma. Ann. Hematol. 2020, 99, 2869–2880. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Merz, M.; Raab, M.S.; Bertsch, U.; Weru, V.; Kopp-Schneider, A.; Jauch, A.; Goldschmidt, H.; Dimitrakopoulou-Strauss, A. The prognostic significance of [(18)F]FDG PET/CT in multiple myeloma according to novel interpretation criteria (IMPeTUs). EJNMMI Res. 2021, 11, 100. [Google Scholar] [CrossRef]
- Ho, C.-L.; Chen, S.; Cheng, K.C.; Leung, Y.L.; Wong, K.-N.; Cheung, W.; Chim, J. Assessment of tumor burden in multiple myeloma by correlation between dual-tracer PET/CT and β2-microglobulin. J. Nucl. Med. 2013, 54 (supplement 2), 435. [Google Scholar]
- Zirakchian Zadeh, M. Clinical Application of 18F-FDG-PET Quantification in Hematological Malignancies: Emphasizing Multiple Myeloma, Lymphoma and Chronic Lymphocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2023, 23, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Karlin, L.; Garderet, L.; Facon, T.; Macro, M.; Benboubker, L.; Caillot, D.; Escoffre, M.; Stoppa, A.-M. Prospective evaluation of MRI and PET-CT at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial. Blood 2015, 126, 395. [Google Scholar] [CrossRef]
- Bartel, T.B.; Haessler, J.; Brown, T.L.; Shaughnessy, J.D., Jr.; van Rhee, F.; Anaissie, E.; Alpe, T.; Angtuaco, E.; Walker, R.; Epstein, J. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood J. Am. Soc. Hematol. 2009, 114, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Mitchell, A.; Waheed, S.; Crowley, J.; Hoering, A.; Petty, N.; Brown, T.; Bartel, T.; Anaissie, E.; van Rhee, F. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood J. Am. Soc. Hematol. 2013, 121, 1819–1823. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A.; Hoffmann, M.; Bergner, R.; Uppenkamp, M.; Haberkorn, U.; Strauss, L.G. Prediction of progression-free survival in patients with multiple myeloma following anthracycline-based chemotherapy based on dynamic FDG-PET. Clin. Nucl. Med. 2009, 34, 576–584. [Google Scholar] [CrossRef]
- Beksac, M.; Gunduz, M.; Ozen, M.; Ozturk, S.M.B.; Kucuk, O.; Ozkan, E. Impact of PET-CT response on survival parameters following autologous stem cell transplantation among patients with multiple myeloma: Comparison of two cut-off values. Blood 2014, 124, 3983. [Google Scholar] [CrossRef]
- Korde, N.; Roschewski, M.; Zingone, A.; Kwok, M.; Manasanch, E.E.; Bhutani, M.; Tageja, N.; Kazandjian, D.; Mailankody, S.; Wu, P. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015, 1, 746–754. [Google Scholar] [CrossRef]
- Cascini, G.L.; Falcone, C.; Console, D.; Restuccia, A.; Rossi, M.; Parlati, A.; Tamburrini, O. Whole-body MRI and PET/CT in multiple myeloma patients during staging and after treatment: Personal experience in a longitudinal study. La Radiol. Medica 2013, 118, 930–948. [Google Scholar] [CrossRef]
- Nanni, C.; Zamagni, E.; Celli, M.; Caroli, P.; Ambrosini, V.; Tacchetti, P.; Brioli, A.; Zannetti, B.; Pezzi, A.; Pantani, L. The value of 18F-FDG PET/CT after autologous stem cell transplantation (ASCT) in patients affected by multiple myeloma (MM): Experience with 77 patients. Clin. Nucl. Med. 2013, 38, e74–e79. [Google Scholar] [CrossRef]
- Spinnato, P.; Bazzocchi, A.; Brioli, A.; Nanni, C.; Zamagni, E.; Albisinni, U.; Cavo, M.; Fanti, S.; Battista, G.; Salizzoni, E. Contrast enhanced MRI and 18F-FDG PET-CT in the assessment of multiple myeloma: A comparison of results in different phases of the disease. Eur. J. Radiol. 2012, 81, 4013–4018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Nanni, C.; Mancuso, K.; Tacchetti, P.; Pezzi, A.; Pantani, L.; Zannetti, B.; Rambaldi, I.; Brioli, A.; Rocchi, S. PET/CT improves the definition of complete response and allows to detect otherwise unidentifiable skeletal progression in multiple myeloma. Clin. Cancer Res. 2015, 21, 4384–4390. [Google Scholar] [CrossRef]
- Zirakchian Zadeh, M.; Raynor, W.Y.; Østergaard, B.; Hess, S.; Yellanki, D.P.; Ayubcha, C.; Mehdizadeh Seraj, S.; Acosta-Montenegro, O.; Borja, A.J.; Gerke, O.; et al. Correlation of whole-bone marrow dual-time-point 18F-FDG, as measured by a CT-based method of PET/CT quantification, with response to treatment in newly diagnosed multiple myeloma patients. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 257–264. [Google Scholar] [PubMed]
- Zhuang, H.; Pourdehnad, M.; Lambright, E.S.; Yamamoto, A.J.; Lanuti, M.; Li, P.; Mozley, P.D.; Rossman, M.D.; Albelda, S.M.; Alavi, A. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J. Nucl. Med. 2001, 42, 1412–1417. [Google Scholar] [PubMed]
- Taghvaei, R.; Oestergaard, B.; Zadeh, M.Z.; Raynor, W.; Paydary, K.; Acosta-Montenegro, O.; Nielsen, A.; Werner, T.; Abildgaard, N.; Hoilund-Carlsen, P.F.; et al. Correlation of Dual Time Point FDG-PET with Response to Chemotherapy in Multiple Myeloma. J. Nucl. Med. 2017, 58 (Suppl. 1), 188. [Google Scholar]
- Zadeh, M.Z.; Seraj, S.M.; Østergaard, B.; Mimms, S.; Raynor, W.Y.; Aly, M.; Borja, A.J.; Arani, L.S.; Gerke, O.; Werner, T.J.; et al. Prognostic significance of 18F-sodium fluoride in newly diagnosed multiple myeloma patients. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 151–160. [Google Scholar]
- Ak, İ.; Onner, H.; Akay, O.M. Is there any complimentary role of F-18 NaF PET/CT in detecting of osseous involvement of multiple myeloma? A comparative study for F-18 FDG PET/CT and F-18 FDG NaF PET/CT. Ann. Hematol. 2015, 94, 1567–1575. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Goldschmidt, H.; Hose, D.; Pan, L.; Cheng, C.; Kopka, K.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. PET/CT studies of multiple myeloma using 18F-FDG and 18F-NaF: Comparison of distribution patterns and tracers’ pharmacokinetics. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1343–1353. [Google Scholar] [CrossRef]
- Raynor, W.; Houshmand, S.; Gholami, S.; Emamzadehfard, S.; Rajapakse, C.S.; Blomberg, B.A.; Werner, T.J.; Høilund-Carlsen, P.F.; Baker, J.F.; Alavi, A. Evolving role of molecular imaging with 18F-sodium fluoride PET as a biomarker for calcium metabolism. Curr. Osteoporos. Rep. 2016, 14, 115–125. [Google Scholar] [CrossRef]
- Zirakchian Zadeh, M.; Østergaard, B.; Raynor, W.Y.; Revheim, M.E.; Seraj, S.M.; Acosta-Montenegro, O.; Ayubcha, C.; Yellanki, D.P.; Al-Zaghal, A.; Nielsen, A.L.; et al. Comparison of 18F-sodium fluoride uptake in the whole bone, pelvis, and femoral neck of multiple myeloma patients before and after high-dose therapy and conventional-dose chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Lenzi, R.; Theriault, R.L.; Davis, R.N.; Meyers, C.A. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 2004, 100, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Janelsins, M.C.; Mohile, S.G.; Holmes, H.M.; Hsu, T.; Inouye, S.K.; Karuturi, M.S.; Kimmick, G.G.; Lichtman, S.M.; Magnuson, A.; et al. Chemotherapy-related cognitive impairment in older patients with cancer. J. Geriatr. Oncol. 2016, 7, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan Shamchi, S.; Zirakchian Zadeh, M.; Østergaard, B.; Kim, J.; Raynor, W.Y.; Khosravi, M.; Taghvaei, R.; Nielsen, A.L.; Gerke, O.; Werner, T.J.; et al. Brain glucose metabolism in patients with newly diagnosed multiple myeloma significantly decreases after high-dose chemotherapy followed by autologous stem cell transplantation. Nucl. Med. Commun. 2020, 41, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Ziai, P.; Hayeri, M.R.; Salei, A.; Salavati, A.; Houshmand, S.; Alavi, A.; Teytelboym, O.M. Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. Radiographics 2016, 36, 481–496. [Google Scholar] [CrossRef]
- Kwee, T.C.; Cheng, G.; Lam, M.G.E.H.; Basu, S.; Alavi, A. SUVmax of 2.5 should not be embraced as a magic threshold for separating benign from malignant lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Zirakchian Zadeh, M.; Ayubcha, C.; Raynor, W.Y.; Werner, T.J.; Alavi, A. A review of different methods used for quantification and assessment of FDG-PET/CT in multiple myeloma. Nucl. Med. Commun. 2022, 43, 378–391. [Google Scholar] [CrossRef]
- McDonald, J.E.; Kessler, M.M.; Gardner, M.W.; Buros, A.F.; Ntambi, J.A.; Waheed, S.; van Rhee, F.; Zangari, M.; Heuck, C.J.; Petty, N.; et al. Assessment of Total Lesion Glycolysis by (18)F FDG PET/CT Significantly Improves Prognostic Value of GEP and ISS in Myeloma. Clin. Cancer Res. 2017, 23, 1981–1987. [Google Scholar] [CrossRef]
- Nanni, C.; Versari, A.; Chauvie, S.; Bertone, E.; Bianchi, A.; Rensi, M.; Bellò, M.; Gallamini, A.; Patriarca, F.; Gay, F.; et al. Interpretation criteria for FDG PET/CT in multiple myeloma (IMPeTUs): Final results. IMPeTUs (Italian myeloma criteria for PET USe). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 712–719. [Google Scholar] [CrossRef]
- Nanni, C. PET-FDG: Impetus. Cancers 2020, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R. Amino Acid and Proliferation PET/CT for the Diagnosis of Multiple Myeloma. Front. Nucl. Med. 2022, 1, 796357. [Google Scholar] [CrossRef]
- Lückerath, K.; Lapa, C.; Spahmann, A.; Jörg, G.; Samnick, S.; Rosenwald, A.; Einsele, H.; Knop, S.; Buck, A.K. Targeting paraprotein biosynthesis for non-invasive characterization of myeloma biology. PLoS ONE 2013, 8, e84840. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline transport for phospholipid synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 2014, 124, 31–82. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Serfling, S.E.; Lindner, T.; Hänscheid, H.; Schirbel, A.; Hahner, S.; Fassnacht, M.; Einsele, H.; Werner, R.A. CXCR4-targeted theranostics in oncology. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Chalopin, B.; Gouard, S.; Carlier, T.; Saëc, P.R.; Marionneau-Lambot, S.; Moreau, P.; Touzeau, C.; Kraeber-Bodere, F.; Bodet-Milin, C.; et al. ImmunoPET in Multiple Myeloma-What? So What? Now What? Cancers 2020, 12, 1467. [Google Scholar] [CrossRef]
- Pandit-Taskar, N. Functional Imaging Methods for Assessment of Minimal Residual Disease in Multiple Myeloma: Current Status and Novel ImmunoPET Based Methods. Semin. Hematol. 2018, 55, 22–32. [Google Scholar] [CrossRef]
- Caserta, E.; Chea, J.; Minnix, M.; Poku, E.K.; Viola, D.; Vonderfecht, S.; Yazaki, P.; Crow, D.; Khalife, J.; Sanchez, J.F.; et al. Copper 64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 2018, 131, 741–745. [Google Scholar] [CrossRef]
- Peck, M.; Pollack, H.A.; Friesen, A.; Muzi, M.; Shoner, S.C.; Shankland, E.G.; Fink, J.R.; Armstrong, J.O.; Link, J.M.; Krohn, K.A. Applications of PET imaging with the proliferation marker [18F]-FLT. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 95–104. [Google Scholar]
- Sachpekidis, C.; Goldschmidt, H.; Kopka, K.; Kopp-Schneider, A.; Dimitrakopoulou-Strauss, A. Assessment of glucose metabolism and cellular proliferation in multiple myeloma: A first report on combined 18F-FDG and 18F-FLT PET/CT imaging. EJNMMI Res. 2018, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Dendl, K.; Cardinale, J.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology 2023, 306, e220749. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Schreder, M.; Schirbel, A.; Samnick, S.; Kortüm, K.M.; Herrmann, K.; Kropf, S.; Einsele, H.; Buck, A.K.; Wester, H.-J.; et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - Comparison to [18F]FDG and laboratory values. Theranostics 2017, 7, 205–212. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Enqvist, O.; Ulén, J.; Kopp-Schneider, A.; Pan, L.; Jauch, A.; Hajiyianni, M.; John, L.; Weinhold, N.; Sauer, S.; et al. Application of an artificial intelligence-based tool in [18F]FDG PET/CT for the assessment of bone marrow involvement in multiple myeloma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.M.; Bauckneht, M.; Masoero, G.; Mach, F.; Quercioli, A.; Seitun, S.; Balbi, M.; Brunelli, C.; Parodi, A.; Nencioni, A.; et al. The vulnerable coronary plaque: Update on imaging technologies. Thromb. Haemost. 2013, 110, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.A.; Benz, D.C.; Gräni, C.; Buechel, R.R. Imaging the event-prone coronary artery plaque. J. Nucl. Cardiol. 2019, 26, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Arani, L.S.; Zirakchian Zadeh, M.; Saboury, B.; Revheim, M.-E.; Øestergaard, B.; Borja, A.J.; Samadi Samarin, D.; Mehdizadeh Seraj, S.; Kalbush, E.; Ayubcha, C.; et al. Assessment of atherosclerosis in multiple myeloma and smoldering myeloma patients using 18F- sodium fluoride PET/CT. J. Nucl. Cardiol. 2021, 28, 3044–3054. [Google Scholar] [CrossRef]
- Messiou, C.; Porta, N.; Sharma, B.; Levine, D.; Koh, D.-M.; Boyd, K.; Pawlyn, C.; Riddell, A.; Downey, K.; Croft, J.; et al. Prospective Evaluation of Whole-Body MRI versus FDG PET/CT for Lesion Detection in Participants with Myeloma. Radiol. Imaging Cancer 2021, 3, e210048. [Google Scholar] [CrossRef]
- Nanni, C.; Kobe, C.; Baeßler, B.; Baues, C.; Boellaard, R.; Borchmann, P.; Buck, A.; Buvat, I.; Chapuy, B.; Cheson, B.D.; et al. European Association of Nuclear Medicine (EANM) Focus 4 consensus recommendations: Molecular imaging and therapy in haematological tumours. Lancet Haematol. 2023, 10, e367–e381. [Google Scholar] [CrossRef]

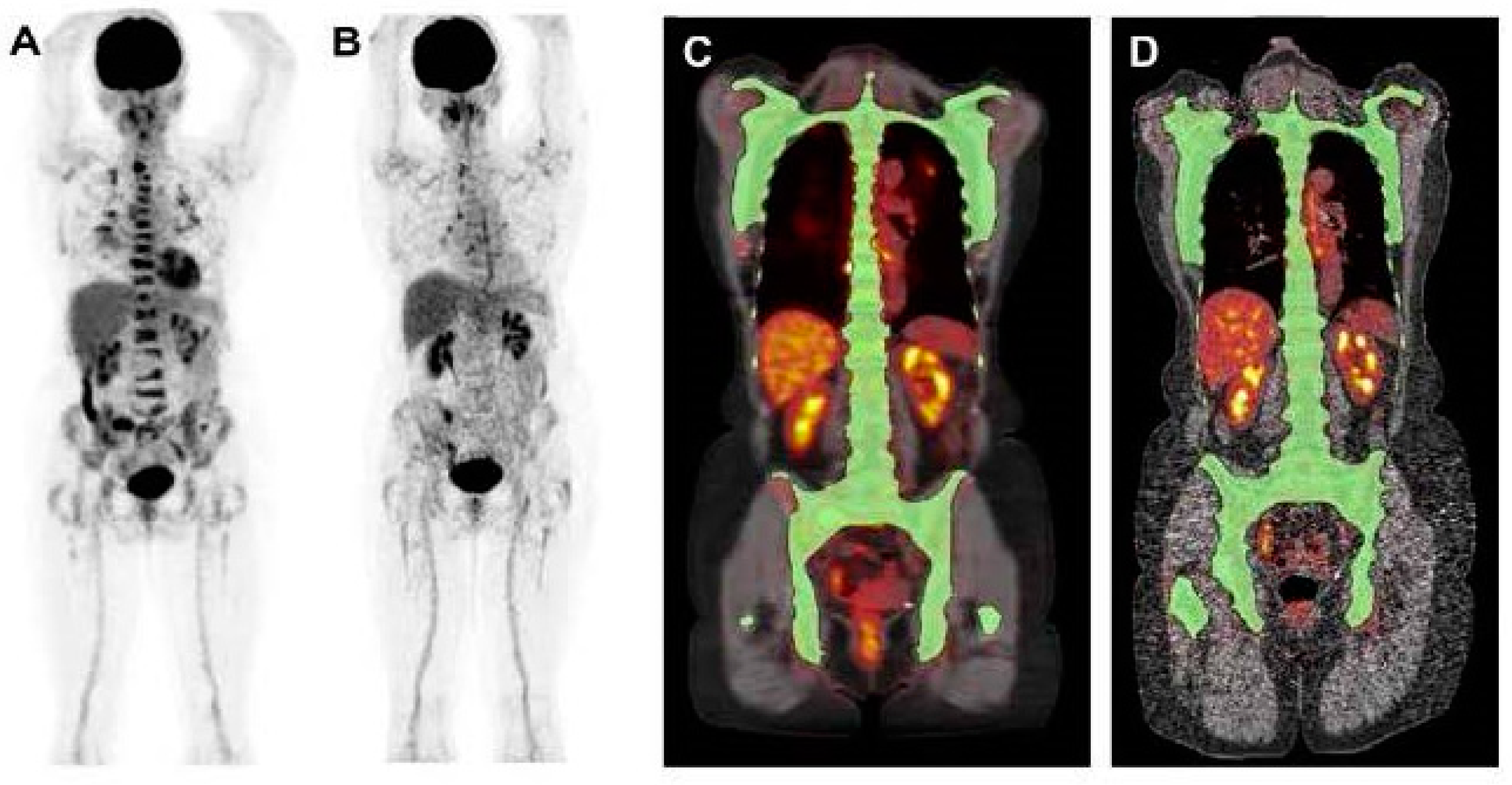
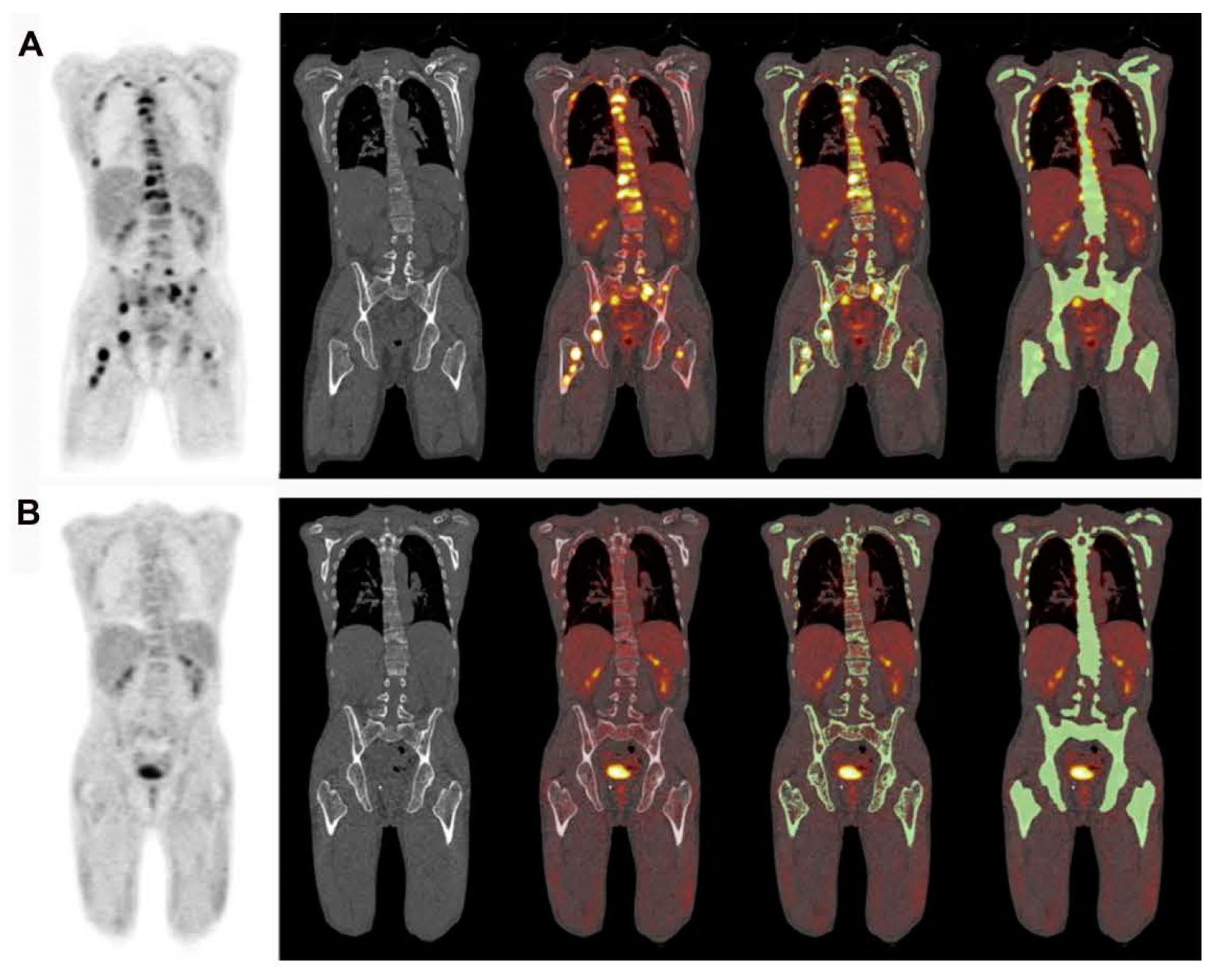

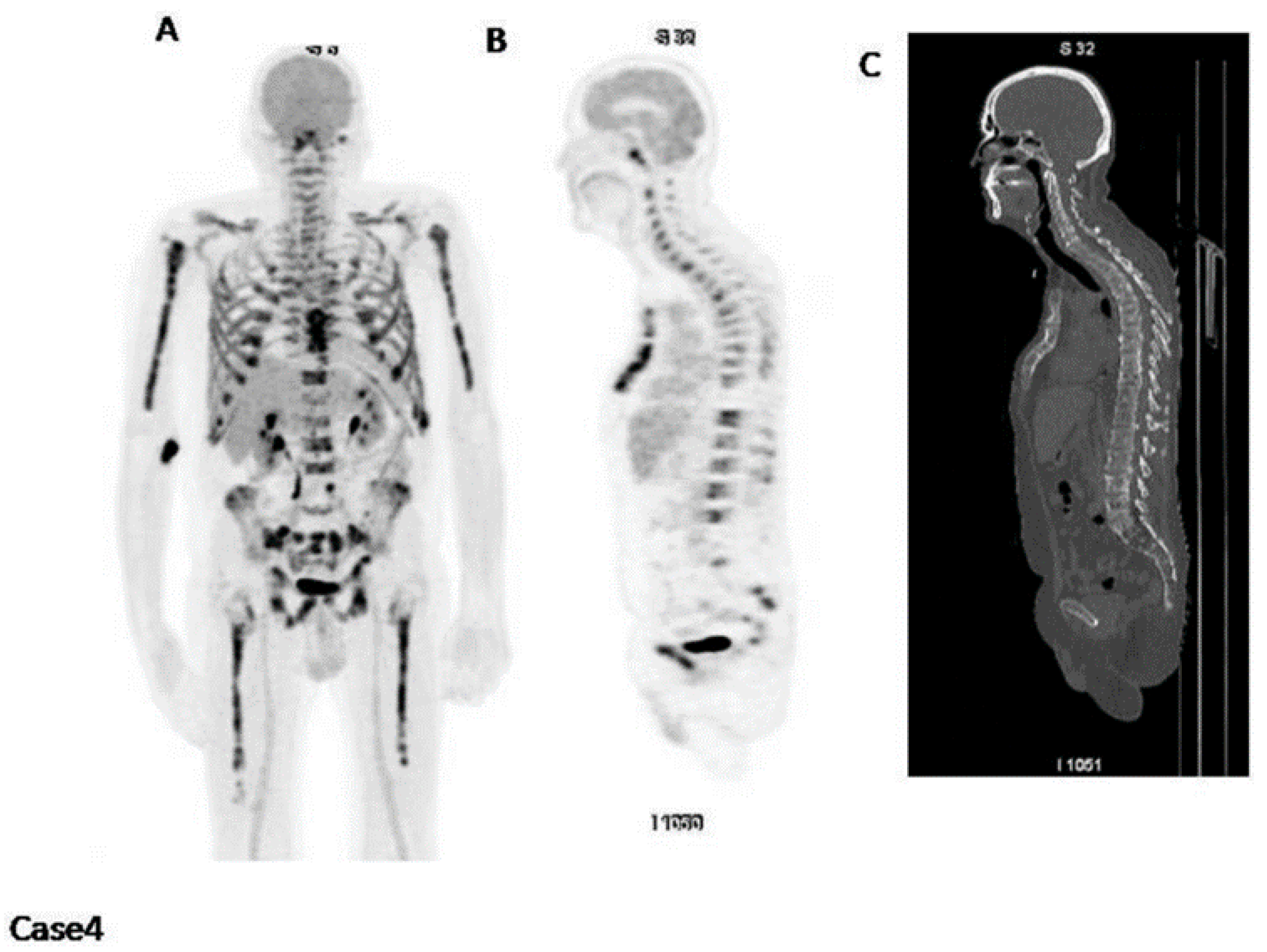


| Parameter | ICC | 95% CI |
|---|---|---|
| Whole bone (pre-treatment) | 0.983 | 0.965–0.992 |
| Whole bone (post-treatment) | 0.989 | 0.978–0.995 |
| Whole pelvis (pre-treatment) | 0.998 | 0.996–0.999 |
| Whole pelvis (post-treatment) | 0.996 | 0.991–0.998 |
| Assessment of Bone Disease in Multiple Myeloma | Clinical and Research * | [18F]FDG PET/CT demonstrates high sensitivity in detecting osteolytic lesions. The sensitivity of PET can be enhanced with delayed imaging. However, the waiting time interval between the two scans (early and delayed) presents a significant challenge. |
| Prediction of Prognosis | Mostly Research | [18F]FDG PET stands out as one of the top imaging modalities for forecasting outcomes in various cancers because it delivers quantitative parameters. |
| Effectiveness of Therapy and Assessment of Residual Disease | Clinical and Research | In comparison to anatomical imaging methods such as CT and MRI, [18F]FDG PET stands out for these purposes because it offers molecular insights detectable prior to visible structural alterations. As a result, many guidelines recommend PET as the preferred imaging method for these specific areas. |
| Assessment of Pathologic Fractures | Research | An Na[18F]F radiotracer, indicative of osteoblastic activity, is the appropriate choice for this task, not [18F]FDG. |
| PET for Assessment of Bone Turnover in Myeloma Patients | Research | In a recent study by Zirakchian Zadeh and colleagues, Na[18F]F PET was demonstrated to effectively evaluate bone activity in myeloma patients post-treatment. |
| Chemo Brain | Research | [18F]FDG PET is capable of measuring alterations in brain glucose metabolism using quantitative analysis. Thus, it is suitable for examining the effects of “chemo brain.” |
| FDG PET Quantification | Mostly Research | Quantification using [18F]FDG PET in MM is generally challenging. While SUVmax has proven to be more effective than other PET metrics for this application, it only reflects metabolic activity in a limited region. Although MTV and TLG are employed as volumetric PET metrics, their effectiveness can be reduced due to the presence of numerous lesions in some cases. Some institutions are now leaning toward CT-based segmentations; these techniques are consistently reproducible, yet their clinical patient assessment merits further study. The IMPetus approach offers another perspective, primarily visual-based. While this method is thorough, its effectiveness can be influenced by the expertise of the individual interpreting the results. |
| Novel PET Radiotracers | Mostly Research | [18F]FDG, being a non-specific radiotracer, is susceptible to both false positive and negative outcomes. This underscores the need to research more targeted radiotracers for evaluating myeloma disease. Many of these newer PET radiotracers have demonstrated results that are either on par with or superior to [18F]FDG PET in identifying osteolytic lesions. |
| AI for PET Quantification | Research | The AI approach to evaluate PET results in myeloma using this new concept has shown encouraging outcomes thus far. The investigators argue that this method can address the challenges associated with inconsistent evaluations of PET results in patients with myeloma. |
| Atherosclerosis | Research (limited data available) | Typically, Na[18F]F, rather than [18F]FDG, is suggested for examining atherosclerosis. Recent research indicates that Na[18F]F PET might be an effective imaging technique for evaluating atherosclerosis in individuals with myeloma. Further studies are recommended by the authors to assess the correlation between myeloma and atherosclerosis. |
| PET Limitation | N/A | The lower spatial resolution of PET compared to anatomical imaging might impede the evaluation of tiny lesions. Additional challenges include patient preparation and radiation exposure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirakchian Zadeh, M. Significance of PET/CT Imaging in Myeloma Assessment: Exploring Novel Applications beyond Osteolytic Lesion Detection and Treatment Response. Onco 2024, 4, 15-36. https://doi.org/10.3390/onco4010002
Zirakchian Zadeh M. Significance of PET/CT Imaging in Myeloma Assessment: Exploring Novel Applications beyond Osteolytic Lesion Detection and Treatment Response. Onco. 2024; 4(1):15-36. https://doi.org/10.3390/onco4010002
Chicago/Turabian StyleZirakchian Zadeh, Mahdi. 2024. "Significance of PET/CT Imaging in Myeloma Assessment: Exploring Novel Applications beyond Osteolytic Lesion Detection and Treatment Response" Onco 4, no. 1: 15-36. https://doi.org/10.3390/onco4010002
APA StyleZirakchian Zadeh, M. (2024). Significance of PET/CT Imaging in Myeloma Assessment: Exploring Novel Applications beyond Osteolytic Lesion Detection and Treatment Response. Onco, 4(1), 15-36. https://doi.org/10.3390/onco4010002





