Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. simmonsii on Tomato against Soil-Borne Fungal Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Fungal Pathogens
2.2. Isolation of Trichoderma Species
2.3. Molecular Characterisation of Trichoderma Species
2.4. Dual Culture Tests
2.5. Inoculum Preparation
2.6. Greenhouse Tests on Potted Tomato Plants
2.6.1. Fusarium
2.6.2. Rhizoctonia
2.7. Endophytic Re Isolation from Plant Tissues
2.8. Statistical Analysis
3. Results
3.1. Molecular Characterization of Trichoderma Species
3.2. Dual Culture Tests
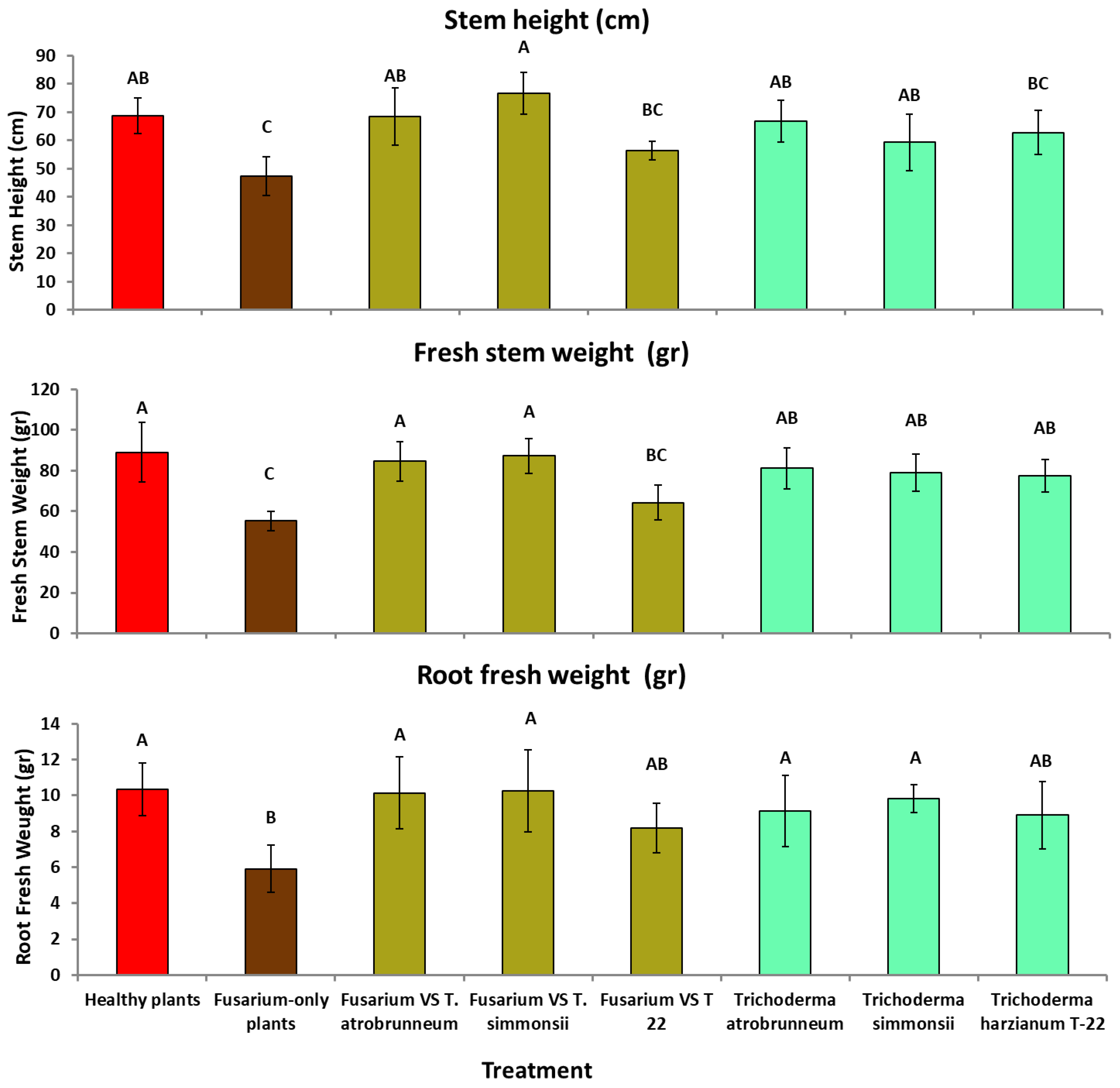
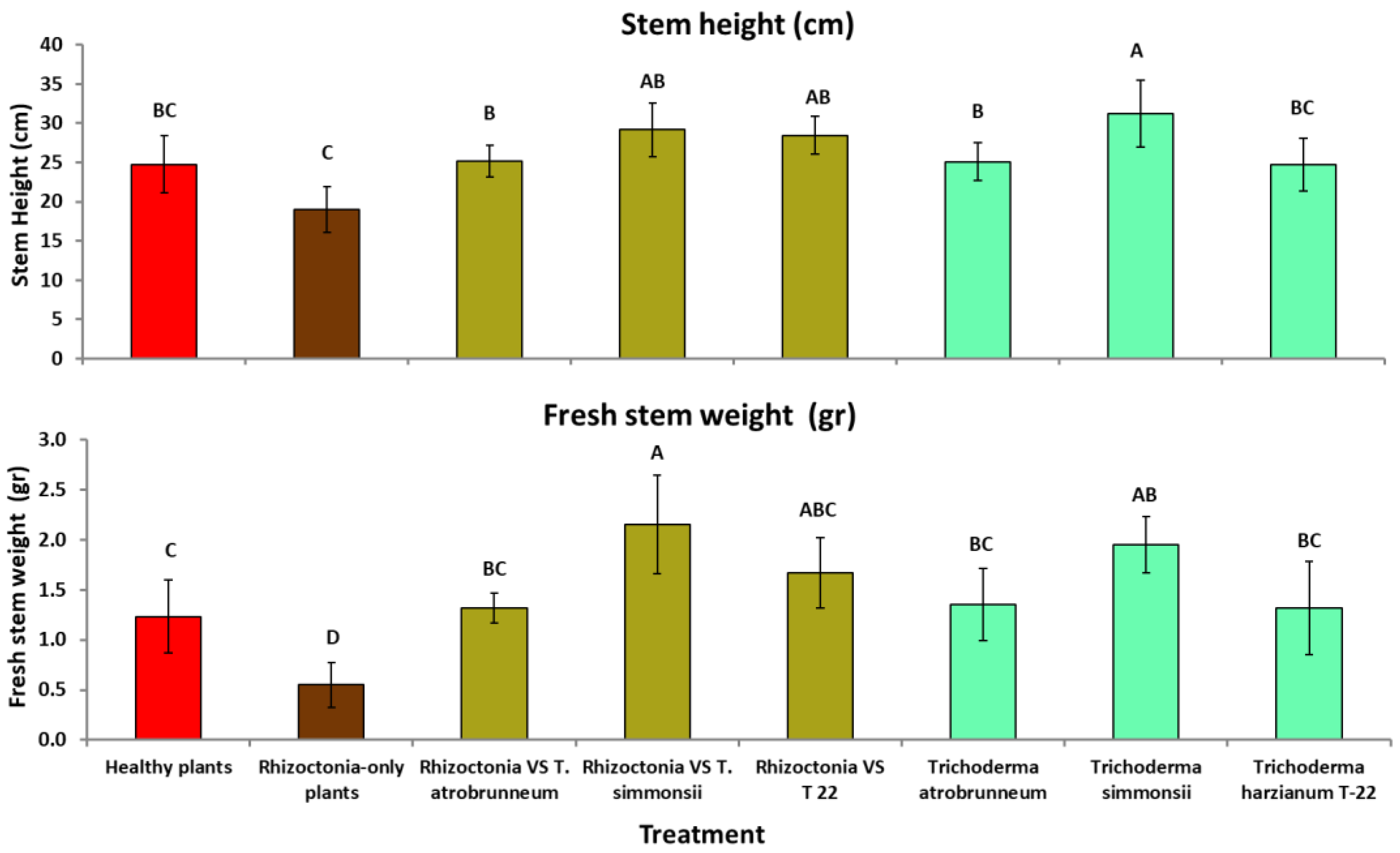
3.3. Greenhouse Tests
3.4. Re-Isolation of Trichoderma from Leaves, Stems and Roots on SDA Substrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leadbeater, A. Recent Developments and Challenges in Chemical Disease Control—A Review. Plant Protect. Sci. 2016, 51, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. Plant Health Instructor; The American Phytopathological Society: St. Paul, MN, USA, 2006; pp. 1–25. [Google Scholar] [CrossRef] [Green Version]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological Control: A Sustainable and Practical Approach for Plant Disease Management. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Asad, S.A. Mechanisms of Action and Biocontrol Potential of Trichoderma against Fungal Plant Diseases—A Review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-Based Novel Formulations: Potential Applications for Management of Next-Gen Agricultural Challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial Effects of Trichoderma Secondary Metabolites on Crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Ousley, M.A.; Lynch, J.M.; Whipps, J.M. Effect of Trichoderma on Plant Growth: A Balance between Inhibition and Growth Promotion. Microb. Ecol. 1993, 26, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ousley, M.A.; Lynch, J.M.; Whipps, J.M. Potential of Trichoderma spp. as consistent plant growth stimulators. Biol. Fertil. Soils 1994, 17, 85–90. [Google Scholar] [CrossRef]
- Saba, H. Trichoderma—A Promising Plant Growth Stimulator and Biocontrol Agent. Mycosphere 2012, 3, 524–531. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Gupta, V., Schmoll, M., Herrera-Estrella, A., Upadhyay, R., Druzhinina, I., Tuohy, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar]
- Bora, L.C.; Bora, P.; Gogoi, M. Potential of Trichoderma spp. for Pest Management and Plant Growth Promotion in NE India. In Trichoderma: Host Pathogen Interactions and Applications; Sharma, A.K., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 205–220. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 1004. [Google Scholar] [CrossRef]
- Inbar, J.; Abramsky, M.; Cohen, D.; Chet, I. Plant Growth Enhancement and Disease Control By Trichoderma harzianum in Vegetable Seedlings Grown under Commercial Conditions. Eur. J. Plant. Pathol. 1994, 100, 337–346. [Google Scholar] [CrossRef]
- Rinu, K.; Sati, P.; Pandey, A. Trichoderma gamsii (NFCCI 2177): A Newly Isolated Endophytic, Psychrotolerant, Plant Growth Promoting, and Antagonistic Fungal Strain. J. Basic Microbiol. 2014, 54, 408–417. [Google Scholar] [CrossRef]
- Degani, O.; Rabinovitz, O.; Becher, P.; Gordani, A.; Chen, A. Trichoderma longibrachiatum and Trichoderma asperellum Confer Growth Promotion and Protection against Late Wilt Disease in the Field. J. Fungi 2021, 7, 444. [Google Scholar] [CrossRef]
- Macena, A.M.F.; Kobori, N.N.; Mascarin, G.M.; Vida, J.B.; Hartman, G.L. Antagonism of Trichoderma-Based Biofungicides against Brazilian and North American Isolates of Sclerotinia sclerotiorum and Growth Promotion of Soybean. BioControl 2020, 65, 235–246. [Google Scholar] [CrossRef]
- Shang, J.; Liu, B.; Xu, Z. Efficacy of Trichoderma asperellum TC01 against Anthracnose and Growth Promotion of Camellia sinensis Seedlings. Biol. Control 2020, 143, 104205. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, R.P.; Srivastava, A.K.; Saxena, A.K.; Arora, D.K. Growth promotion and charcoal rot management in chickpea by Trichoderma harzianum. J. Plant Prot. Res. 2008, 48, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J. Biological Control of Fusarium oxysporum f. sp. ciceri and Ascochyta rabiei Infecting Protected Geographical Indication Fuentesaúco-Chickpea by Trichoderma Species. Eur. J. Plant Pathol. 2021, 160, 825–840. [Google Scholar] [CrossRef]
- Carvalho, D.D.C.; de Mello, S.C.M.; Lobo Júnior, M.; Geraldine, A.M. Biocontrol of Seed Pathogens and Growth Promotion of Common Bean Seedlings by Trichoderma harzianum. Pesqui. Agropecu. Bras. 2011, 46, 822–828. [Google Scholar] [CrossRef] [Green Version]
- de Souza Pedro, E.A.; Harakava, R.; Lucon, C.M.M.; Guzzo, S.D. Promoção do crescimento do feijoeiro e controle da antracnose por Trichoderma spp. Pesqui. Agropecu. Bras. 2012, 47, 1589–1595. [Google Scholar] [CrossRef] [Green Version]
- Nuangmek, W.; Aiduang, W.; Kumla, J.; Lumyong, S.; Suwannarach, N. Evaluation of a Newly Identified Endophytic Fungus, Trichoderma phayaoense for Plant Growth Promotion and Biological Control of Gummy Stem Blight and Wilt of Muskmelon. Front. Microbiol. 2021, 12, 410. [Google Scholar] [CrossRef]
- Hicks, E.; Bienkowski, D.; Braithwaite, M.; McLean, K.; Falloon, R.; Stewart, A. Trichoderma strains suppress Rhizoctonia diseases and promote growth of potato. Phytopathol. Mediterr. 2014, 53, 502–514. [Google Scholar]
- Fontenelle, A.D.B.; Guzzo, S.D.; Lucon, C.M.M.; Harakava, R. Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Prot. 2011, 30, 1492–1500. [Google Scholar] [CrossRef]
- Zaw, M.; Matsumotom, M. Plant growth promotion of Trichoderma virens, Tv911 on some vegetables and its antagonistic effect on Fusarium wilt of tomato. Environ. Control Biol. 2020, 58, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Pavlovskaya, N.; Gneusheva, I.; Solokhina, I.; Ageeva, N. The Biological Activity of Subspecies Trichoderma harzianum against Fusarium oxysporum, the Causative Agent of Fusarium Wilt Cucumber in Vitro. BIO Web Conf. 2020, 21, 00021. [Google Scholar] [CrossRef]
- Wang, C.; Zhuang, W. Evaluating Effective Trichoderma Isolates for Biocontrol of Rhizoctonia solani Causing Root Rot of Vigna unguiculata. J. Integr. Agric. 2019, 18, 2072–2079. [Google Scholar] [CrossRef]
- Rokni, N.; Shams Alizadeh, H.; Bazgir, E.; Darvishnia, M.; Mirzaei-Najafgholi, H. The Tripartite Consortium of Serendipita indica, Trichoderma simmonsii, and Bell Pepper (Capsicum annum). Biol. Control 2021, 158, 104608. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhosseini, M.; Yaghoubian, Y.; Pirdashti, H. Plant Growth Promoting Microorganisms Can Improve Germination, Seedling Growth and Potassium Uptake of Soybean under Drought and Salt Stress. Plant Growth Regul. 2020, 90, 123–136. [Google Scholar] [CrossRef]
- Chen, L.; Bóka, B.; Kedves, O.; Nagy, V.D.; Szűcs, A.; Champramary, S.; Roszik, R.; Patocskai, Z.; Münsterkötter, M.; Huynh, T.; et al. Towards the Biological Control of Devastating Forest Pathogens from the Genus Armillaria. Forests 2019, 10, 1013. [Google Scholar] [CrossRef] [Green Version]
- Rees, H.J.; Bashir, N.; Drakulic, J.; Cromey, M.G.; Bailey, A.M.; Foster, G.D. Identification of Native Endophytic Trichoderma spp. for Investigation of In Vitro Antagonism towards Armillaria mellea Using Synthetic- and Plant-Based Substrates. J. Appl. Microbiol. 2021, 131, 392–403. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971; 237p. [Google Scholar]
- Nelson, P.E.; Tousson, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983; 206p. [Google Scholar]
- Sneh, B.; Burpee, L.; Ogoshi, A. Identification of Rhizoctonia Species; American Phytopathological Society: Saint Paul, MN, USA, 1991; 133p. [Google Scholar]
- Warcup, J.H. The Soil-Plate Method for Isolation of Fungi from Soil. Nature 1950, 166, 117–118. [Google Scholar] [CrossRef]
- Gams, W.; Bissett, J. Morphology and Identification of Trichoderma. In Trichoderma and Gliocladium: Basic Biology, Taxonomy and Genetics Vol. 1; Harman, G.E., Kubicek, C.P., Eds.; Taylor and Francis: London, UK, 1998; pp. 3–34. [Google Scholar]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from Milligram Amounts of Fresh, Herbarium and Mummified Plant Tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- Destéfano, R.H.R.; Destéfano, S.A.L.; Messias, C.L. Detection of Metarhizium anisopliae var. anisopliae within Infected Sugarcane Borer Diatraea saccharalis (Lepidoptera, Pyralidae) Using Specific Primers. Genet. Mol. Biol. 2004, 27, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Druzhinina, I.S. In Honor of John Bissett: Authoritative Guidelines on Molecular Identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Morton, D.T.; Stroube, N.H. Antagonistic and stimulatory effects of microorganisms upon Sclerotium rolfsii. Phytopathology 1955, 45, 419–420. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 27.0; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- Ab Rahman, S.S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.F.; Hiller, N. Exploring the Benefits of Biocontrol for Sustainable Agriculture—A Literature Review on Biocontrol in Light of the European Green Deal; Institute for European Environmental Policy: Brussels, Belgium, 2021; pp. 1–42. [Google Scholar]
- Wilson, D. Fungal Endophytes: Out of Sight but Should Not Be out of Mind. Oikos 1993, 68, 379–384. [Google Scholar] [CrossRef]
- Heydari, A.; Pessarakli, M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Latz, M.A.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as Biological Control Agent: Scope and Prospects to Improve Efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–Plant–Pathogen Interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A Beneficial Antifungal Agent and Insights into Its Mechanism of Biocontrol Potential. Egypt J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Sivasithamparam, K. Antifungal antibiotics produced by Trichoderma spp. Soil Biol. Biochem. 1991, 23, 1011–1020. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Raza, M.; Raza, W.; Qamar, M.I. Trichoderma as Potential Biocontrol Agent, Its Exploitation in Agriculture: A Review. Plant Prot. 2018, 2, 109–135. [Google Scholar]
- Anees, M.; Tronsmo, A.; Edel-Hermann, V.; Hjeljord, L.G.; Héraud, C.; Steinberg, C. Characterization of Field Isolates of Trichoderma Antagonistic against Rhizoctonia solani. Fungal Biol. 2010, 114, 691–701. [Google Scholar] [CrossRef] [PubMed]
- de Melo, I.S.; Faull, J.L. Parasitism of Rhizoctonia solani by Strains of Trichoderma spp. Sci. agric. 2000, 57, 55–59. [Google Scholar] [CrossRef]
- Halifu, S.; Deng, X.; Song, X.; Song, R.; Liang, X. Inhibitory Mechanism of Trichoderma virens ZT05 on Rhizoctonia solani. Plants 2020, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Jiang, D.; Fu, Y. Trichoderma spp. as antagonist of Rhizoctonia solani. J. Plant Pathol. Microbiol. 2017, 8, 1–9. [Google Scholar]
- Trillas, M.I.; Casanova, E.; Cotxarrera, L.; Ordovás, J.; Borrero, C.; Avilés, M. Composts from Agricultural Waste and the Trichoderma asperellum Strain T-34 Suppress Rhizoctonia solani in Cucumber Seedlings. Biol. Control 2006, 39, 32–38. [Google Scholar] [CrossRef]
- Mayo, S.; Gutierrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.S.; Zvenyika, Z. Integrated control of Rhizoctonia solani and Fusarium solani in tobacco transplants with Trichoderma hurziunum and triadimenol. Plant Pathol. 1988, 37, 271–277. [Google Scholar] [CrossRef]
- Herrera, W.; Valbuena, O.; Pavone-Maniscalco, D. Formulation of Trichoderma asperellum TV190 for biological control of Rhizoctonia solani on corn seedlings. Egypt J. Biol. Pest Control 2020, 30, 44. [Google Scholar] [CrossRef]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Mycoparasitic Activity of Indigenous Trichoderma virens Strains Against Mungbean Soil Borne Pathogen Rhizoctonia solani: Hyperparasite and Hydrolytic Enzyme Production. AGRIVITA J. Agric. Sci. 2020, 42, 229–242. [Google Scholar] [CrossRef]
- Barari, H. Biocontrol of Tomato Fusarium Wilt by Trichoderma Species under in Vitro and in Vivo Conditions. Cercet. Agron. Mold. 2016, 49, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Prasad, L.; Chaudhary, S.; Sagar, S.; Tomar, A. Mycoparasitic Capabilities of Diverse Native Strain of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici. J. Appl. Nat. Sci. 2016, 8, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Moosa, A.; Sahi, S.T.; Haq, I.-U.; Farzand, A.; Khan, S.A.; Javaid, K. Antagonistic Potential of Trichoderma Isolates and Manures Against Fusarium Wilt of Tomato. Int. J. Veg. Sci. 2017, 23, 207–218. [Google Scholar] [CrossRef]
- Babychan, M.; Simon, S. Efficacy of Trichoderma spp. against Fusarium oxysporum f. Sp. lycopersici. (FOL) Infecting Pre-and Post-Seedling of Tomato. J. Pharmacogn. Phytochem. 2017, 6, 616–619. [Google Scholar]
- Patel, S.; Saraf, M. Biocontrol efficacy of Trichoderma asperellum MSST against tomato wilting by Fusarium oxysporum f. sp. lycopersici. Arch. Phytopathol. Plant Prot. 2017, 50, 228–238. [Google Scholar] [CrossRef]
- Sallam, N.M.A.; Eraky, A.M.I.; Sallam, A. Effect of Trichoderma spp. on Fusarium Wilt Disease of Tomato. Mol. Biol. Rep. 2019, 46, 4463–4470. [Google Scholar] [CrossRef]
- Jagraj, S.; Vipul, K.; Seweta, S.; Adesh, K.; Vinit, P.S. In vitro evaluation of Trichoderma species against Fusarium oxysporum f. sp. lycopersici causing tomato wilt. Plant Pathol. J. 2018, 17, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Ghazalibiglar, H.; Kandula, D.R.W.; Hampton, J.G. Biological Control of Fusarium Wilt of Tomato by Trichoderma Isolates. N. Z. Plant Prot. 2016, 69, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Jamil, A. Antifungal and plant growth promoting activity of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici colonizing tomato. J. Plant Prot. Res. 2021, 61, 243–253. [Google Scholar]
- Hewedy, O.A.; Mansour, A.; Ali, M.G.; Lateif, K.S.A.; Ismaiel, M.H.; El-Meihy, R.M. Comprehensive Characterization and Screening of Different Trichoderma Isolates as Plant Growth Promoters: Insight to Metal Solubilization, Enzymatic Activity, and Antagonistic Effect. Rev. Res. Sq. 2022; Preprint. [Google Scholar] [CrossRef]
- Monfil, V.O.; Casas-Flores, S. Chapter 32—Molecular Mechanisms of Biocontrol in Trichoderma spp. and Their Applications in Agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 429–453. [Google Scholar] [CrossRef]
- Anil, K.; Lakshmi, T. Phosphate Solubilization Potential and Phosphatase Activity Of Rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010, 41, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Shanmuga Arasu, V.; Kathiresan, K. Effect of Trichoderma on Soil Phosphate Solubilization and Growth Improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- França, D.V.C.; Kupper, K.C.; Magri, M.M.R.; Gomes, T.M.; Rossi, F. Trichoderma spp. isolates with potential of phosphate solubilization and growth promotion in cherry tomato. Pesqui. Agropecuária Trop. 2017, 47, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Aishwarya, S.; Viswanath, H.S.; Singh, A.; Singh, R. Biosolubilization of different nutrients by Trichoderma spp. and their mechanisms involved: A review. Int. J. Adv. Agric. Sci. Tech. 2020, 7, 34–39. [Google Scholar]
- Aban, J.L.; Barcelo, R.C.; Oda, E.E.; Reyes, G.A.; Balangcod, T.D.; Gutierrez, R.M.; Hipol, R.M. Dominant Root Associated Fungi (RAF) from Drynaria quercifolia L. Either Induce or Retard Growth of PSB Rc10 Rice (Oryza sativa L.) in Gibberellic Acid-Inhibited Medium. App. Environ. Res. 2017, 39, 89–98. [Google Scholar] [CrossRef]
- Berg, G. Plant-Microbe Interactions Promoting Plant Growth and Health: Perspectives for Controlled Use of Microorganisms in Agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Rhizobacteria (PGPR): Their Potential as Antagonists and Biocontrol Agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Samuels, G.; Hebbar, P. Trichoderma Identification and Agricultural Applications; American Phytopathological Society Press: St. Paul, NY, USA, 2015; 204p. [Google Scholar]
- Manoharachary, C.; Singh, H.B.; Varma, A. Trichoderma: Agricultural Applications and Beyond; Springer: Cham, Switzerland, 2020; 367p. [Google Scholar] [CrossRef]
- Sid Ahmed, A.; Ezziyyani, M.; Pérez Sánchez, C.; Candela, M.E. Effect of Chitin on Biological Control Activity of Bacillus spp. and Trichoderma harzianum against Root Rot Disease in Pepper (Capsicum annuum) Plants. Eur. J. Plant Pathol. 2003, 109, 633–637. [Google Scholar] [CrossRef]
- Rabeendran, N.; Jones, E.E.; Moot, D.J.; Stewart, A. Biocontrol of Sclerotinia Lettuce Drop by Coniothyrium minitans and Trichoderma hamatum. Biol. Control 2006, 39, 352–362. [Google Scholar] [CrossRef]
- Villalta, O.N.; Wite, D.; Hunt, J.; Stewart, A.; Porter, I.J. Biological control of Sclerotinia minor on lettuce using Trichoderma and Coniothyrium species. Acta Hortic. 2012, 944, 51–58. [Google Scholar] [CrossRef]
- Blaya, J.; López-Mondéjar, R.; Lloret, E.; Pascual, J.A.; Ros, M. Changes Induced by Trichoderma harzianum in Suppressive Compost Controlling Fusarium Wilt. Pestic. Biochem. Physiol. 2013, 107, 112–119. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Del Mar Alguacil, M.; Pascual, J.A.; Van Wees, S.C.M. Phytohormone Profiles Induced by Trichoderma Isolates Correspond with Their Biocontrol and Plant Growth-Promoting Activity on Melon Plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]

| Species | Collection Site | Blast ID Number | DNA Sequence | Identity (%) | GenBank Closest Hit |
|---|---|---|---|---|---|
| Trichoderma simmonsii | Devon, United Kingdom from soil of abandoned mine | GMMBSMTJ01R | GGAGGGCATTACCGAGTTTACAACTCCCAAACCCAATGTGAACGTTACCAAACTGTTGCCTCGGCGGGATCTCTGCCCCGGGTGCGTCGCAGCCCCGGACCAAGGCGCCCGCCGGAGGACCAACCTAAAACTCTTATTGTATACCCCCTCGCGGGTTTTTTTATAATCTGAGCCTTTCTCGGCGCCTCTCGTAGGCGTTTCGAAAATGAATCAAAACTTTCAACAACGGATCTCTTGGTTCTGGCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACATTGCGCCCGCCAGTATTCTGGCGGGCATGCCTGTCCGAGCGTCATTTCAACCCTCGAACCCCTCCGGGGGGTCGGCGTTGGGGATCGGCCCTCCCTTAGCGGGTGGCCGTCTCCGAAATACAGTGGCGGTCTCGCCGCAGCCTCTCCTGCGCAGTAGTTTGCACACTCGCATCGGGAGCGCGGCGCGTCCACAGCCGTTAAACACCCAACTTCTGAAATGTTGACCTCGGATCAGGTAGGAATACCCGCTGAACTTAAGCATATCA | 99.16 | NR_137297.1 |
| Trichoderma atrobrunneum | Serres, Greece from corn combs | GMMU60DF013 | GGAGGGCATTACCGAGTTTACAACTCCCAAACCCAATGTGAACGTTACCAAACTGTTGCCTCGGCGGGATCTCTGCCCCGGGTGCGTCGCAGCCCCGGACCAAGGCGCCCGCCGGAGGACCAACCAAAACTCTTATTGTATACCCCCTCGCGGGTTTTTTTTATAATCTGAGCCTTCTCGGCGCCTCTCGTAGGCGTTTCGAAAATGAATCAAAACTTTCAACAACGGATCTCTTGGTTCTGGCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACATTGCGCCCGCCAGTATTCTGGCGGGCATGCCTGTCCGAGCGTCATTTCAACCCTCGAACCCCTCCGGGGGGTCGGCGTTGGGGATCGGCCCTGCCTTGGCGGTGGCCGTCTCCGAAATACAGTGGCGGTCTCGCCGCAGCCTCTCCTGCGCAGTAGTTTGCACACTCGCATCGGGAGCGCGGCGCGTCCACAGCCGTTAAACACCCAACTTCTGAGTTTGCACACTCGCATCGGGAGCGCGGCGCGTCCACAGCCGTTAAACACCCAACTTCTGA | 99.49 | NR_137298.1 |
| Fungus | Time | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| F. oxysporum only a | 0.57 ± 0.10 aE * | 1.08 ± 0.12 aD | 1.77 ± 0.15 aC | 2.38 ± 0.15 aB | 2.92 ± 0.10 aA |
| F. oxysporum and T. harzianum b | 0.55 ± 0.10 aD | 1.07 ± 0.12 aC | 1.70 ± 0.17 aB | 1.87 ± 0.08 bAB | 1.90 ± 0.09 bA |
| F. oxysporum and T. atrobrunneum | 0.55 ± 0.05 aC | 1.05 ± 0.05 aB | 1.60 ± 0.09 aA | 1.65 ± 0.05 cA | 1.68 ± 0.06 cA |
| F. oxysporum and T. simmonsii | 0.55 ± 0.05 aD | 1.05 ± 0.08 aC | 1.75 ± 0.10 aB | 1.93 ± 0.05 bA | 1.95 ± 0.05 bA |
| Fungus | Time | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| R. solani only a | 1.10 ± 0.09 aE * | 3.03 ± 0.08 aD | 5.17 ± 0.14 aC | 6.57 ± 0.08 aB | 7.82 ± 0.08 aA |
| R. solani and T. harzianum b | 0.97 ± 0.05 bC | 2.03 ± 0.16 bB | 3.17 ± 0.08 bA | 3.20 ± 0.09 bA | 3.22 ± 0.10 bA |
| R. solani and T.atrobrunneum | 0.88 ± 0.08 bcC | 2.05 ± 0.05 bB | 3.08 ± 0.08 bA | 3.12 ± 0.12 bA | 3.15 ± 0.10 bA |
| R. solani and T. simmonsii | 0.85 ± 0.05 cC | 1.95 ± 0.10 bB | 3.15 ± 0.12 bA | 3.28 ± 0.15 bA | 3.30 ± 0.13 bA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natsiopoulos, D.; Tziolias, A.; Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A. Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. simmonsii on Tomato against Soil-Borne Fungal Pathogens. Crops 2022, 2, 202-217. https://doi.org/10.3390/crops2030015
Natsiopoulos D, Tziolias A, Lagogiannis I, Mantzoukas S, Eliopoulos PA. Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. simmonsii on Tomato against Soil-Borne Fungal Pathogens. Crops. 2022; 2(3):202-217. https://doi.org/10.3390/crops2030015
Chicago/Turabian StyleNatsiopoulos, Dimitrios, Apostolos Tziolias, Ioannis Lagogiannis, Spyridon Mantzoukas, and Panagiotis A. Eliopoulos. 2022. "Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. simmonsii on Tomato against Soil-Borne Fungal Pathogens" Crops 2, no. 3: 202-217. https://doi.org/10.3390/crops2030015
APA StyleNatsiopoulos, D., Tziolias, A., Lagogiannis, I., Mantzoukas, S., & Eliopoulos, P. A. (2022). Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. simmonsii on Tomato against Soil-Borne Fungal Pathogens. Crops, 2(3), 202-217. https://doi.org/10.3390/crops2030015








