Variation in Symptom Development and Infectivity of Banana Bunchy Top Disease among Four Cultivars of Musa sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. BBTV Inoculation of Banana Plants
2.3. Molecular Screening of Banana Plants and Aphids
2.4. Identification of BBTD Symptoms
2.5. Statistical Analysis
3. Results
3.1. Symptom Development and Disease Evolution in Plants with Different Genotypes
3.2. Leaf Positions at Symptom Expression
3.3. BBTV Transmission at the Cultivar Level
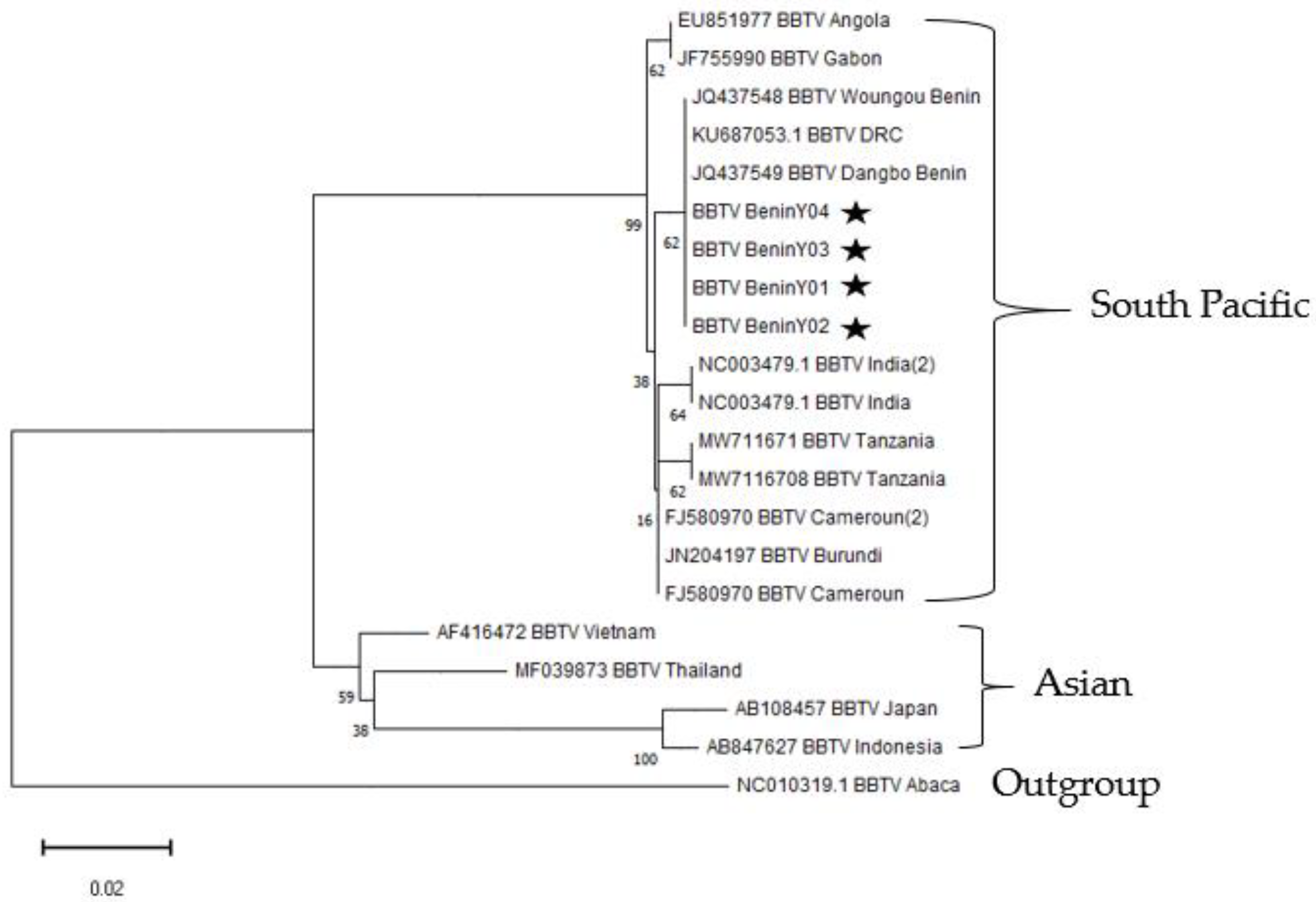
3.4. Phylogenetic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, M.-S.; Jeger, M.J. An Analytical Model of Plant Virus Disease Dynamics with Roguing and Replanting. J. Appl. Ecol. 1994, 31, 413. [Google Scholar] [CrossRef]
- Jeger, M.J.; van den Bosch, F.; McRoberts, N. Modelling Transmission Characteristics and Epidemic Development of the Tospovirus–Thrip Interaction. Arthropod-Plant Interact. 2015, 9, 107–120. [Google Scholar] [CrossRef]
- Dupuis, C. Infectious Diseases. 2021. Available online: https://www.universalis.fr/encyclopedie/maladies-infectieuses/ (accessed on 12 October 2021).
- Cuzin, L.; Delpierre, C. Epidemiology of Infectious Diseases. EMC—Mal. Infect. 2005, 2, 157–162. [Google Scholar]
- Allen, L.; Hebert, M.P. Disease Outbreaks in Plant-Vector-Virus Models with Vector Aggregation and Dispersal. AIMS 2016, 21, 2169–2191. [Google Scholar] [CrossRef]
- Jacquot, M. Genomic Diversity of Pathogenic Bacteria of the Borrelia Burgdorferi Species Complex: Evolution and Molecular Epidemiology Agricultural Science. Ph.D. Thesis, Blaise Pascal University, Clermont-Ferrand, France, 2014. [Google Scholar]
- Omondi, B.A.; Soko, M.M.; Nduwimana, I.; Delano, R.T.; Niyongere, C.; Simbare, A.; Kachigamba, D.; Staver, C. The Effectiveness of Consistent Roguing in Managing Banana Bunchy Top Disease in Smallholder Production in Africa. Plant Pathol. 2020, 69, 1754–1766. [Google Scholar] [CrossRef]
- FAOSTAT. Banana Production Data, Newsletter. 2019. Available online: www.fao.org (accessed on 13 October 2021).
- Drenth, A.; Kema, G. The Vulnerability of Bananas to Globally Emerging Disease Threats. Phytopathology 2021, 111, 2146–2161. [Google Scholar] [CrossRef]
- Ondh-Obame, J.A.; Ndong, A.N.; Ndoutoumou, P.N.; Assembe, P.C.M.; Minko, I.D.M.; Bello, K.P. Prévalence Du Banana Bunchy Top Disease (BBTD) Dans La Zone de Ntoum Au Gabon. Int. J. Bio. Chem. Sci. 2020, 14, 739–749. [Google Scholar] [CrossRef]
- Stainton, D.; Martin, D.P.; Muhire, B.M.; Lolohea, S.; Halafihi, M.; Lepoint, P.; Blomme, G.; Crew, K.S.; Sharman, M.; Kraberger, S.; et al. The Global Distribution of Banana Bunchy Top Virus Reveals Little Evidence for Frequent Recent, Human-Mediated Long Distance Dispersal Events. Virus Evol. 2015, 1, vev009. [Google Scholar] [CrossRef]
- Kitavi, M.; Downing, T.; Lorenzen, J.; Karamura, D.; Onyango, M.; Nyine, M.; Ferguson, M.; Spillane, C. The Triploid East African Highland Banana (EAHB) Genepool Is Genetically Uniform Arising from a Single Ancestral Clone That Underwent Population Expansion by Vegetative Propagation. Theor. Appl. Genet. 2016, 129, 547–561. [Google Scholar] [CrossRef]
- Simbare, A.; Sane, C.A.B.; Nduwimana, I.; Niyongere, C.; Omondi, B.A. Diminishing Farm Diversity of East African Highland Bananas in Banana Bunchy Top Disease Outbreak Areas of Burundi—The Effect of Both Disease and Control Approaches. Sustainability 2020, 12, 7467. [Google Scholar] [CrossRef]
- Jacobsen, K.; Omondi, B.A.; Almekinders, C.; Alvarez, E.; Blomme, G.; Dita, M.; Iskra-Caruana, M.-L.; Ocimati, W.; Tinzaara, W.; Kumar, P.L.; et al. Seed Degeneration of Banana Planting Materials: Strategies for Improved Farmer Access to Healthy Seed. Plant Pathol. 2019, 68, 207–228. [Google Scholar] [CrossRef]
- Kumar, P.L.; Hanna, R.; Alabi, O.J.; Soko, M.M.; Oben, T.T.; Vangu, G.H.P.; Naidu, R.A. Banana Bunchy Top Virus in Sub-Saharan Africa: Investigations on Virus Distribution and Diversity. Virus Res. 2011, 159, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Niyongere, C.; Ateka, E.; Losenge, T.; Lepoint, P. Screening Musa genotypes for Banana bunchy top disease resistance in Burundi. Acta Hortic. 2011, 897, 439–447. [Google Scholar] [CrossRef]
- Kolombia, Y.; Oviasuyi, T.; Ayisah, K.D.; Ale Gonh-Goh, A.; Atsu, T.; Oresanya, A.; Ogunsanya, P.; Alabi, T.; Kumar, P.L. First Report of Banana Bunchy Top Virus in Banana (Musa spp.) and Its Eradication in Togo. Plant Dis. 2021, 105, 3312. [Google Scholar] [CrossRef] [PubMed]
- Niyongere, C.; Ateka, E.; Nkezabahizi, D.; Losenge, T.; Blomme, G.; Lepoint, P. Occurrence and Distribution of Banana Bunchy Top Disease in the Great Lakes Region of Africa. Tree For. Sci. Biotechnol. 2012, 6, 102–107. [Google Scholar]
- Abiola, A.; Zandjanakou-Tachin, M.; Aoudji, K.N.A.; Avocevou-Ayisso, C.; Kumar, P.L. Adoption of Roguing to Contain Banana Bunchy Top Disease in South-East Bénin: Role of Farmers’ Knowledge and Perception. Int. J. Fruit Sci. 2020, 20, 720–736. [Google Scholar] [CrossRef]
- Allen, R.N. Further Studies on Epidemiological Factors Influencing Control of Banana Bunchy Top Disease and Evaluation of Control Measures by Computer Simulation. Aust. J. Agric. Res. 1987, 38, 373–382. [Google Scholar]
- Ngatat, S.; Hanna, R.; Lienou, J.; Ghogomu, R.T.; Nguidang, S.P.K.; Enoh, A.C.; Ndemba, B.; Korie, S.; Fotso Kuate, A.; Nanga Nanga, S.; et al. Musa Germplasm A and B Genomic Composition Differentially Affects Their Susceptibility to Banana Bunchy Top Virus and its Aphid Vector, Pentalonia nigronervosa. Plants 2022, 11, 1206. [Google Scholar] [CrossRef]
- Ngatat, S.; Hanna, R.; Kumar, P.L.; Gray, S.M.; Cilia, M.; Ghogomu, R.T.; Fontem, D.A. Relative Susceptibility of Musa Genotypes to Banana Bunchy Top Disease in Cameroon and Implication for Disease Management. Crop Prot. 2017, 101, 116–122. [Google Scholar] [CrossRef]
- Onyango, M.; Karamura, D.; Keeley, S.; Manshardt, R.; Haymer, D. Morphological characterisation of east african aab and aa dessert bananas (Musa spp.). Acta Hortic. 2011, 897, 95–105. [Google Scholar] [CrossRef]
- Chabi, M.C.; Dassou, A.G.; Dossou-Aminon, I.; Ogouchoro, D.; Omondi, B.A.; Dansi, A. Banana and Plantain Production Systems in Benin: Ethnobotanical Investigation, Varietal Diversity, Pests, and Implications for Better Production. J. Ethnobiol. Ethnomed. 2018, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Robbertse, N.; Omondi, B.A.; Millar, I.M.; Krüger, K.; Jooste, A.E.C. Non-Destructive DNA Extraction from Aphids: The Application in Virus—Vector Studies of Banana Bunchy Top Virus (BBTV). Eur. J. Plant Pathol. 2019, 153, 571–582. [Google Scholar] [CrossRef]
- Foottit, R.G.; Maw, H.E.L.; Pike, K.S.; Miller, R.H. The Identity of Pentalonia nigronervosa Coquerel and P. Caladii van Der Goot (Hemiptera: Aphididae) Based on Molecular and Morphometric Analysis. Zootaxa 2010, 2358, 25. [Google Scholar] [CrossRef]
- Omondi, B.A.; Obeng-Ofori, D.; Kyerematen, R.A.; Danquah, E.Y. Host Preference and Suitability of Selected Crops for Two Biotypes of Bemisia Tabaci in Ghana. J. Ghana. Sci. Assoc. 2004, 1, 105–116. [Google Scholar] [CrossRef]
- Mahadev, S.J.; Amin, I.A.; Khatri, A.I.; Khan, S.; Raza, Y.; Usuf, Z.; Bridon, R.B. PCR Detection of Banana Bunchy Top Virus (BBTV) at Tissue Culture Level for the Production of Virus-Free Planting Materials. Int. Res. J. Biol. Sci. 2013, 2, 22–26. [Google Scholar]
- Mansoor, S.J.; Amin, I.A.; Khatri, A.I.; Khan, S.; Raza, Y.; Yusuf, Z.; Bridon, B.W. A PCR-Based Method, with Internal Control, for the Detection of Banana Bunchy Top Virus in Banana. Mol. Biotechnol. 2005, 30, 167–170. [Google Scholar] [CrossRef]
- Kumar, P.L. Virus Detection in Banana. 2016. Available online: http://newint.iita.org/wp-content/uploads/2016/06/Virus-detection-in-banana-a-laboratory-manual.pdf (accessed on 25 October 2021).
- Baurens, F.-C.; Noyer, J.-L.; Lanaud, C.; Lagoda, P.J.L. Assessment of a Species-Specific Element (Brep 1) in Banana. Theor. Appl. Genet. 1997, 95, 922–931. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Acides Nucléiques Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Sharman, M.; Thomas, J.E.; Skabo, S.; Holton, T.A. Abacá Bunchy Top Virus, a New Member of the Genus Babuvirus (Family Nanoviridae). Arch. Virol. 2008, 153, 135–147. [Google Scholar] [CrossRef]
- Nelson, S.C. Banana Bunchy Top; Detailed Signs and Symptoms, College of Tropical Agriculture and Human Resources (UHCTAHR), University of Hawaii. Banana Bunchy Top Virus. Disease Spread and Development. 2004. Available online: http://www.ctahr.hawaii.edu/bbtd/downloads/BBTV-details.pdf (accessed on 25 October 2021).





| Symptoms | Cultivars | Remarks | |||
|---|---|---|---|---|---|
| FHIA 25 | Ebenga | Aloga | Sotoumon | ||
| Dark streaks along the petiole and young leaf | 3 weeks | 4 weeks | 4 weeks | - | Presence of streaks only on the petiole of FHIA 25 plants |
| Dark streaks along the pseudostem | 7 weeks | 9 weeks | 12 weeks | - | Presence of streaks on the petiole and on the plant blades |
| Marginal chlorosis of the leaf blade margin with normal size | 8 weeks | 12 weeks | 14 weeks | - | Absence of marginal chlorosis of the leaf blade margin with normal size in Sotoumon |
| Reduction in the size of newly emerged blades | 10 weeks | 15 weeks | 15 weeks | 15 weeks | Presence of striae plus reduction in leaf size in all cultivars |
| Leaves form a rosette-like cluster-Bunchy top | 12 weeks | 17 weeks | 17 weeks | 19 weeks | Presence of bunchy top in all cultivars |
| Leaf Number at Onset of Typical Symptom 1 | |||
|---|---|---|---|
| Cultivar | Morse Code | Marginal Chlorosis | Stunting Onset |
| FHIA 25 | 3.59 ± 0.48 c | 4.59 ± 0.48 c | 5.59 ± 0.48 a |
| Ebenga | 5.23 ± 0.37 b | 6.18 ± 0.30 b | 6.77 ± 0.49 b |
| Aloga | 5.09 ± 0.17 b | 6.09 ± 0.17 b | 7.09 ± 0.17 c |
| Sotoumon | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 10.47 ± 0.56 d |
| Treatment (Cultivars) | Percentage Transmission Per Fortnight (Weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | |
| FHIA 25 | 0 | 30 | 30 | 37 | 44 | 56 | 69 | 81 | 87 | 92 |
| Ebenga | 0 | 27 | 27 | 36 | 45 | 64 | 73 | 73 | 82 | 86 |
| Aloga | 0 | 20 | 20 | 30 | 40 | 60 | 70 | 70 | 80 | 83 |
| Sotoumon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabi, M.; Dassou, A.G.; Adoukonou-Sagbadja, H.; Thomas, J.; Omondi, A.B. Variation in Symptom Development and Infectivity of Banana Bunchy Top Disease among Four Cultivars of Musa sp. Crops 2023, 3, 158-169. https://doi.org/10.3390/crops3020016
Chabi M, Dassou AG, Adoukonou-Sagbadja H, Thomas J, Omondi AB. Variation in Symptom Development and Infectivity of Banana Bunchy Top Disease among Four Cultivars of Musa sp. Crops. 2023; 3(2):158-169. https://doi.org/10.3390/crops3020016
Chicago/Turabian StyleChabi, Modeste, Anicet Gbèblonoudo Dassou, Hubert Adoukonou-Sagbadja, John Thomas, and Aman Bonaventure Omondi. 2023. "Variation in Symptom Development and Infectivity of Banana Bunchy Top Disease among Four Cultivars of Musa sp." Crops 3, no. 2: 158-169. https://doi.org/10.3390/crops3020016
APA StyleChabi, M., Dassou, A. G., Adoukonou-Sagbadja, H., Thomas, J., & Omondi, A. B. (2023). Variation in Symptom Development and Infectivity of Banana Bunchy Top Disease among Four Cultivars of Musa sp. Crops, 3(2), 158-169. https://doi.org/10.3390/crops3020016






