Comparison of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.) Inflorescences, Green Leaves, and Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location, Plant Material, Experimental Design, Field Preparation, and Seed Sowing
2.2. Sample Collection and Preparation
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.2. Mineral
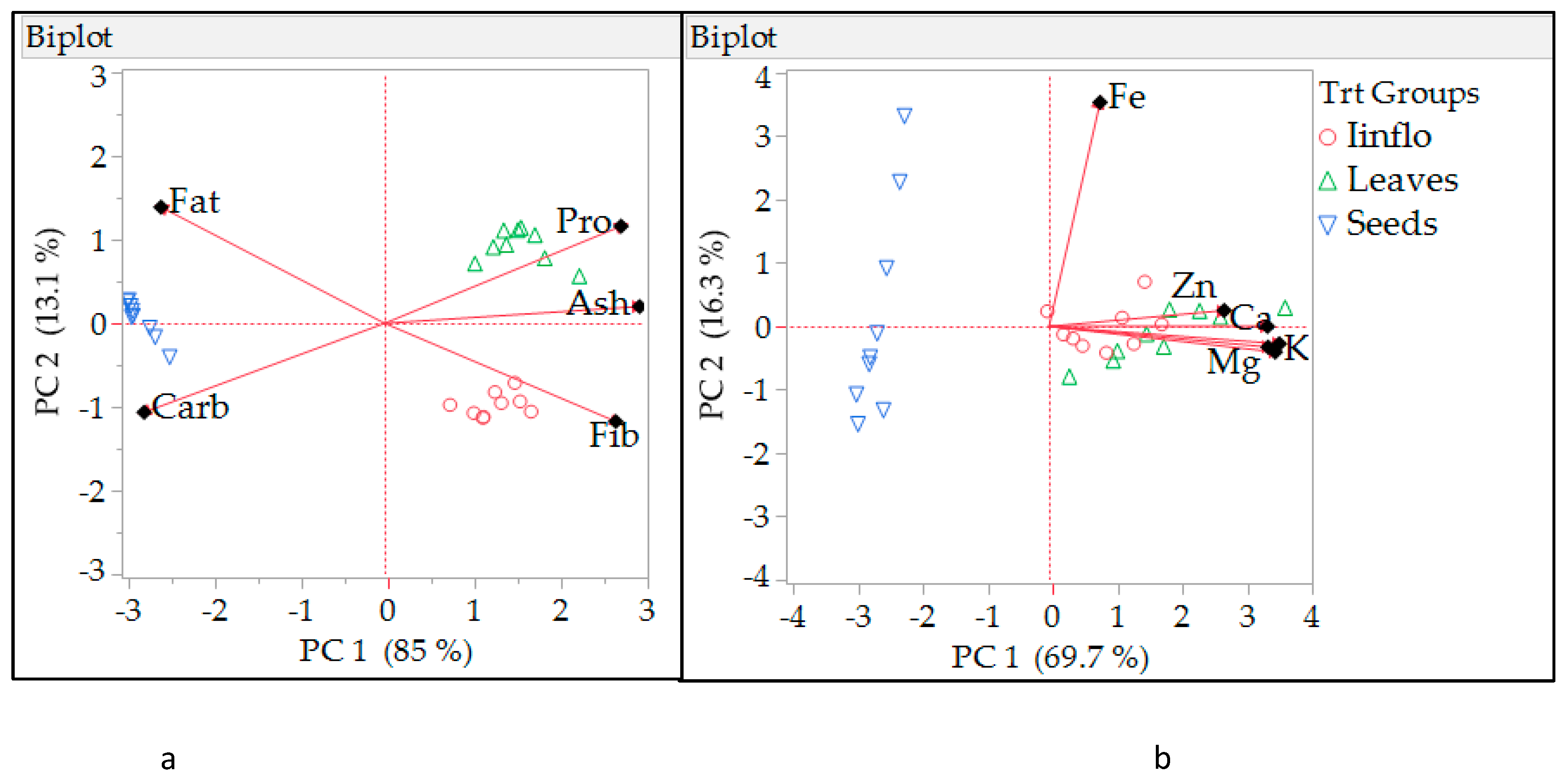
3.3. Principal Component Analysis (PCA)
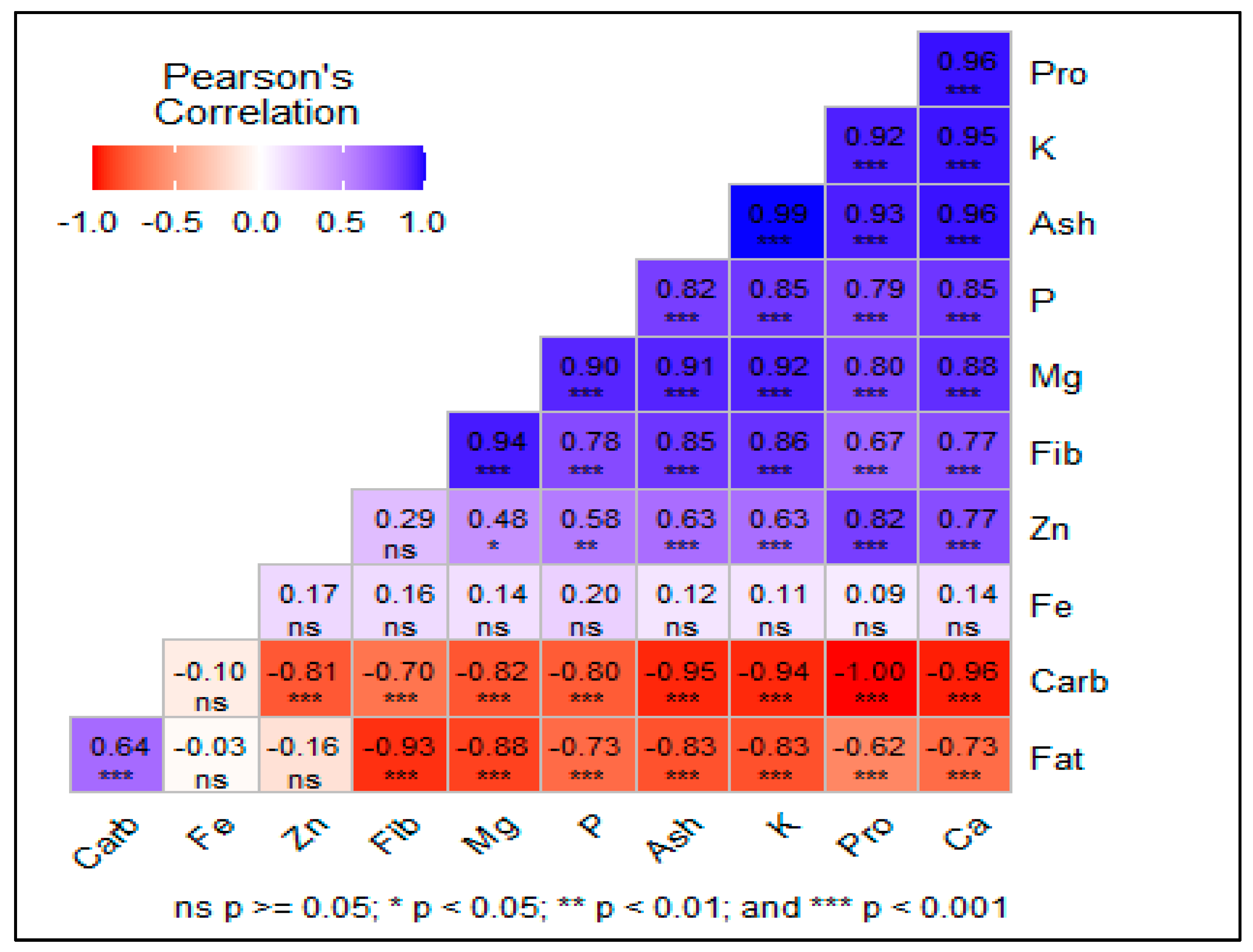
3.4. Correlation of Traits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alandia, G.; Rodriguez, J.; Jacobsen, S.-E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- FAOSTAT. FAOSTAT Database. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 March 2023).
- Bazile, D.; Baudron, F. The dynamics of the global expansion of quinoa growing in view of its high biodiversity. In State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO: Rome, Italy; CIRAD: Rome, Italy, 2015; pp. 42–55. Available online: https://fao.org/3/a-i4042e.pdf (accessed on 25 October 2023).
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide evaluations of quinoa: Preliminary results from post international year of quinoa FAO projects in nine countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Khatkar, B.S. Pseudocereals nutritional composition functional properties and food applications. Food Bioact. Funct. Appl. Hum. Health 2019, 28, 410. [Google Scholar]
- Abd El-Samad, E.; Hussin, S.; El-Naggar, A.; El-Bordeny, N.; Eisa, S. The potential use of quinoa as a new non-traditional leafy vegetable crop. Biosci. Res. 2018, 15, 3387–3403. [Google Scholar]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Chacaliaza-Rodríguez, L.; Espinoza-Begazo, G.; Ramos-Escudero, F.; Servan, K. Proximate chemical composition and content of biologically active components in leaves of two quinoa cultivars (Salcedo and Altiplano) produced in Peru. Res. J. Med. Plant. 2016, 10, 450–456. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lan, X.-Z.; Wu, Y.-Y.; Ou, Y.-W.; Chen, T.C.; Wu, W.-T. The antioxidant activity and nitric oxide production of extracts obtained from the leaves of Chenopodium quinoa Willd. Biomedicine 2017, 7, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Choque-Quispe, D.; LigardaSamanez, C.A.; RamosPacheco, B.S.; Legu’ıaDamiano, S.; CallaFlórez, M.; ZamalloaPuma, L.M.; ColqueCondeña, L. Phenolic compounds, antioxidant capacity, and protein content of three varieties of germinated quinoa (Chenopodium quinoa Willd). Ing. Investig. 2021, 41, e89831. [Google Scholar] [CrossRef]
- Debski, B.; Gralak, M.A.; Bertrandt, J.; Klos, A. Comparison of antioxidant potential and mineral composition of quinoa and lamb’s quarters weed (Chenopodium album). Probl. High Epidemiol. 2018, 99, 88–93. [Google Scholar]
- Olivera, L.; Best, I.; Paredes, P.; Perez, N.; Chong, L.; Marzano, A. Nutritional value, methods for extraction and bioactive compounds of quinoa. In Pseudocereals; IntechOpen: London, UK, 2022; p. 107. [Google Scholar]
- FAO. Resolution. Quinoa: An Ancient Crop to Contribute with World Food Security, FAO Regional Office for Latin America and the Caribbean Ed. Santiago de Chile. 2011. Available online: http://www.fao.org/3/aq287e/aq287e.pdf (accessed on 28 October 2023).
- Rojas, W.; Pinto, M.; Alanoca, C.; Gómez, P.L.; Leon-Lobos, P.; Alercia, A.; Diulgheroff, S.; Padulosi, S.; Bazile, D. Quinoa genetic resources and ex-situ conservation. In State of the Art Report on Quinoa around the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO: Rome, Italy, 2015; pp. 56–82. ISBN 978-92-5-108558-5. Available online: http://fao.org/quinoa-2013/publications/detail/en/item/278923/icode/?no_mobile=1 (accessed on 11 October 2023).
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “golden grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Schlick, G.; Bebenheim, D.L. Quinoa—An Emerging New Crop with Potential CELSS. NASA Technical Report. NASA-TP-3422. 1993. Available online: http://ntrs.nasa.gov/citations/19940015664 (accessed on 10 September 2023).
- Bhargava, A.; Shukla, S.; Deepak, O. Chenopodium quinoa: An Indian perspective. Ind. Crops Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Craine, E.B.; Murphy, K.M. Seed composition and amino acid profiles for quinoa grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B. Quinoa (Chenopodium quinoa Willd.) as source of bioactive compounds: A review. Bioact. Comp. Health Dis. 2019, 2, 27–47. [Google Scholar] [CrossRef]
- Jancurová, M.; Minarovičová, L.; Dandár, A. Quinoa-A Review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Functional components and anti-nutritional factors in gluten-free grains: A focus on quinoa seeds. Foods 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Du, J.; Charrondière, R.J. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Pathan, S.; Ndunguru, G.; Islam, M.R.; Jhumur, S.; Ayele, A. Production of quinoa leafy greens in high tunnel for season extension in Missouri. Horticulturae 2023, 9, 209. [Google Scholar] [CrossRef]
- Pathan, S.; Eivazi, F.; Valliyodan, B.; Paul, K.; Ndunguru, G.; Clark, K. Nutritional composition of the green leaves of quinoa (Chenopodium quinoa Willd.). J. Food Res. 2019, 8, 55–65. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R. Nutritional composition and bioactive components in quinoa greens—A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. Anticancer, antimicrobial and antioxidant compounds of quinoa inflorescence. Adv. Life Sci. 2020, 8, 68–72. [Google Scholar]
- Lau, B.F.; Kong, K.W.; Leong, K.H.; Sun, J.; He, X.; Wang, Z.; Mustafa, M.R.; Ling, T.C.; Ismail, A. Banana inflorescence: Its bio-prospects as an ingredient for functional foods. Trends Food Sci. Technol. 2020, 97, 14–28. [Google Scholar] [CrossRef]
- Femi, B.; Malhija, N.; Mary, T.S.; Kumaran, T. Coconut (Cocos nucifera) inflorescences sap nutritional characteristics and health effects. J. Basic Appl. Res. Biomed. 2020, 6, 50–52. [Google Scholar]
- Okechukwu, K.E.; Lawretta, P.I.; Eberechukwu, A.C.; Stanley, O.K.; Egbuta, K.C.; Joel, N.C. Nutritive and anti-nutritive evaluation of cocoyam (Colocasia Esculenta L. Schott) inflorescence. Am. J. Food Sci. Technol. 2020, 8, 42–48. [Google Scholar]
- Barros, L.; Carvalho, A.M.; Ferreira, I. The nutritional composition of fennel (Foeniculum vulgare): Shoots, leaves, stems and inflorescences. LWT-Food Sci. Technol. 2010, 43, 814–818. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Sobolev, A.P.; Giusti, A.M.; Vinci, G.; Cammarone, S.; Tortora, C.; Lamelza, L.; Prencipe, S.A.; et al. Industrial hemp (Cannabis sativa L.) inflorescences as novel food: The effect of different agronomical practices on chemical profile. Foods 2022, 11, 3658. [Google Scholar] [CrossRef]
- Meireles, D.; Gomes, J.; Lopes, L. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Tradit. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Upadhyay, P.; Yadav, M.K.; Mishra, S.; Sharma, P.; Purohit, S. Moringa oleifera: A review of the medical evidence for its nutritional and pharmacological properties. Int. J. Res. Pharm. Sci. 2015, 5, 12–16. [Google Scholar]
- Batista, C.; Barros, L.; Carvalho, A.M.; Ferreira, I. Nutritional, and nutraceutical potential of rape (Brassica napus L., var. napus) and ‘tronchuda’ cabbage (Brassica oleraceae L., var. costata) inflorescences. Food Chem. Toxicol. 2011, 49, 1208–1214. [Google Scholar] [CrossRef]
- Wijekoon, M.M.; Karim, A.A.; Bhat, R. Evaluation of nutritional quality of torch ginger (Etlingera elatior Jack.) inflorescence. Int Food Res. J. 2011, 18, 1415–1420. [Google Scholar]
- Pathan, S.; Ndunguru, G.; Clark, K.; Ayele, A. Yield and nutritional responses of quinoa (Chenopodium quinoa Willd.) genotypes to irrigated, rainfed, and droughts tress environments. Front. Sustain. Food Syst. 2023, 7, 1242187. [Google Scholar] [CrossRef]
- Indrayan, A.K.; Sharma, S.; Durgapal, D.; Kumar, N.; Kumar, M. Determination of nutritive value and analysis of mineral elements for somemedicinally valued plants from Uttaranchal. Curr. Sci. 2005, 89, 1252–1255. [Google Scholar]
- SAS Software, Version 9.3; SAS: Cary, NC, USA, 2011.
- JMP, Version Pro 13; The SAS Institute Inc.: Cary, NC, USA, 2013.
- White, P.J. Calcium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 165–198. [Google Scholar]
- Grzebisz, W. Crop response to magnesium fertilization as affected by nitrogen supply. Plant Soil 2013, 368, 23–39. [Google Scholar] [CrossRef]
| This Study | Published | ||||
|---|---|---|---|---|---|
| Traits | Inflor. | Leaves | Grains | 1 Leaves | 2 Grains Range |
| Protein | 25.09 b | 33.40 a | 14.74 c | 37.05 | 9.10–15.70 |
| Moisture | 7.38 b | 9.07 a | 6.90 b | 4.00 | 8.20–13.10 |
| Fat | 2.80 c | 4.16 b | 6.06 a | 4.50 | 4.00–7.60 |
| Fiber | 11.43 a | 8.40 b | 2.71 c | 6.93 | 1.00–9.20 |
| Ash | 15.37 b | 16.91 a | 4.31 c | 20.04 | 2.00–7.70 |
| Carbohydrate | 49.36 b | 36.46 c | 67.98 a | 34.03 | 48.50–69.80 |
| Energy | 323 | 317 | 385 | 325 | 331–381 |
| (kcal/100 g) | |||||
| This Study | Published | ||||
|---|---|---|---|---|---|
| Amino Acids | Inflo. | Leaves | Grains | Leaves 1 | Grains 2 Mean, Range |
| His | 2.09 b | 2.14 b | 2.92 a | 1.89 | 2.70, 1.40–5.40 |
| Ile | 3.71 b | 4.57 a | 3.83 b | 4.35 | 3.10, 0.80–7.40 |
| Leu | 5.53 c | 7.46 a | 5.95 b | 7.15 | 6.00, 2.30–9.40 |
| Lys | 5.24 c | 6.07 a | 5.69 b | 5.1 | 4.80, 2.40–7.80 |
| Met | 1.31 c | 1.78 b | 1.93 a | 1.62 | 1.90, 0.30–9.10 |
| Phe | 4.14 b | 4.84 a | 3.84 c | 4.83 | 6.30, 2.70–10.30 |
| Thr | 3.26 c | 4.01 a | 3.38 b | 3.91 | 3.70, 2.10–8.90 |
| Trp | 1.47 b | 1.81 a | 1.24 c | 3.32 | 0.90, 0.60–1.90 |
| Val | 4.20 c | 5.14 a | 4.45 b | 4.97 | 3.70, 0.80–6.10 |
| Total EAAs | 30.95 | 37.82 | 33.23 | 37.14 | |
| This Study | Published | ||||
|---|---|---|---|---|---|
| Minerals | Inflor. | Leaves | Grains | 1 Leaves | 2 Grains Range |
| Ca | 835.78 b | 1109.56 a | 76.67 c | 1535.00 | 28–149 |
| K | 6125.56 b | 6755.56 a | 1516.67 c | 8769.00 | 207–502 |
| Mg | 622.78 a | 575.11 b | 268.33 c | 902.00 | 656–1475 |
| P | 615.33 a | 625.56 a | 511.33 b | 405.62 | 350–482 |
| Fe | 10.30 a | 9.88 a | 9.56 a | 11.55 | 2.60–15.00 |
| Zn | 6.94 b | 9.22 a | 6.58 b | 6.79 | 0.79–4.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathan, S.; Ndunguru, G.; Ayele, A.G. Comparison of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.) Inflorescences, Green Leaves, and Grains. Crops 2024, 4, 72-81. https://doi.org/10.3390/crops4010006
Pathan S, Ndunguru G, Ayele AG. Comparison of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.) Inflorescences, Green Leaves, and Grains. Crops. 2024; 4(1):72-81. https://doi.org/10.3390/crops4010006
Chicago/Turabian StylePathan, Safiullah, Grato Ndunguru, and Addissu G. Ayele. 2024. "Comparison of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.) Inflorescences, Green Leaves, and Grains" Crops 4, no. 1: 72-81. https://doi.org/10.3390/crops4010006
APA StylePathan, S., Ndunguru, G., & Ayele, A. G. (2024). Comparison of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.) Inflorescences, Green Leaves, and Grains. Crops, 4(1), 72-81. https://doi.org/10.3390/crops4010006






