Genotypic Variability in Root Morphological Traits in Canola (Brassica napus L.) at the Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Canola Genotypes
2.2. Root Phenotyping System
2.3. Experiment Layouts and Performance
2.4. Data Collection
2.5. Image and Data Analysis
3. Results
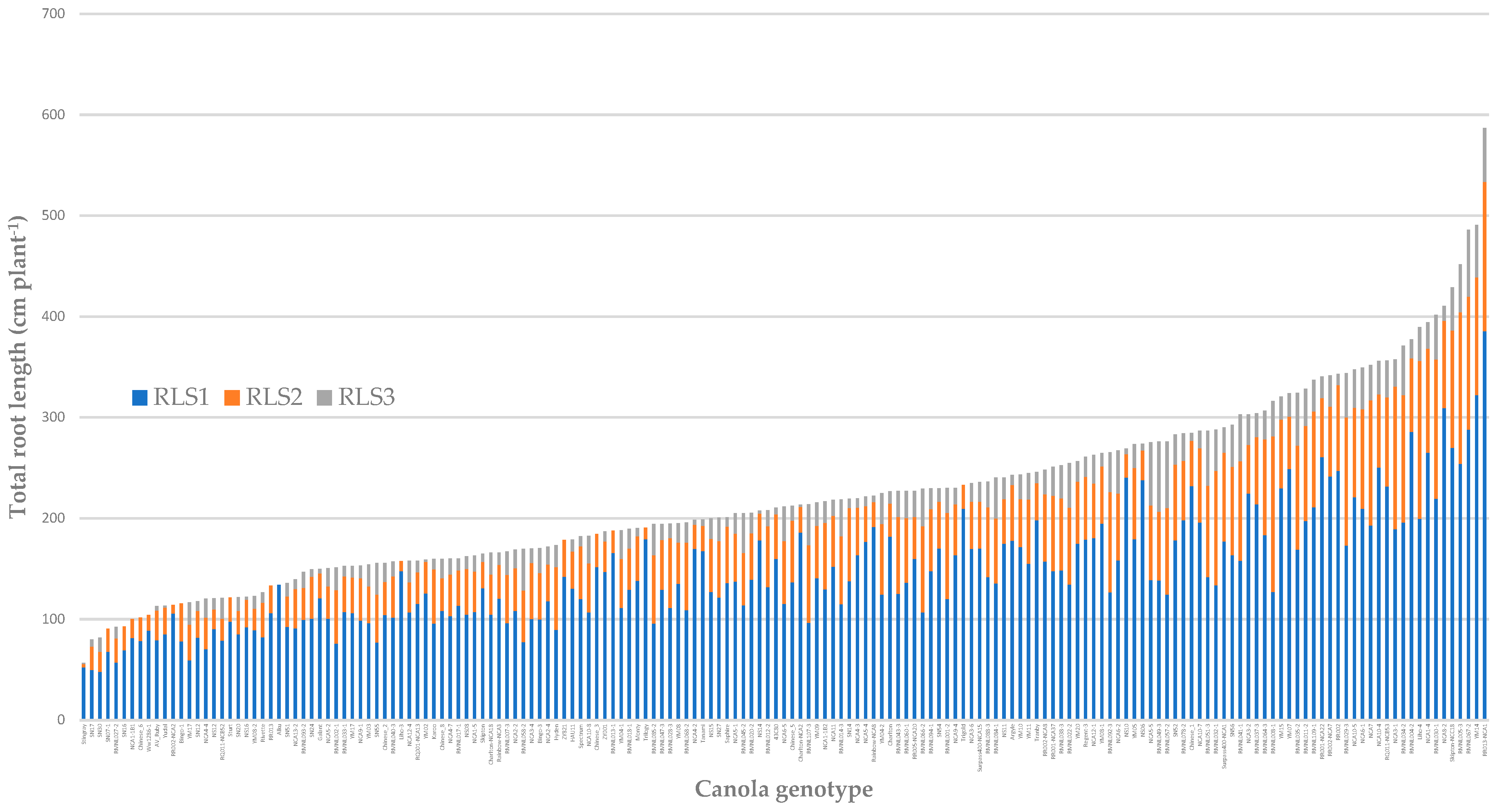
3.1. Global Root Traits
3.2. Local Root Traits
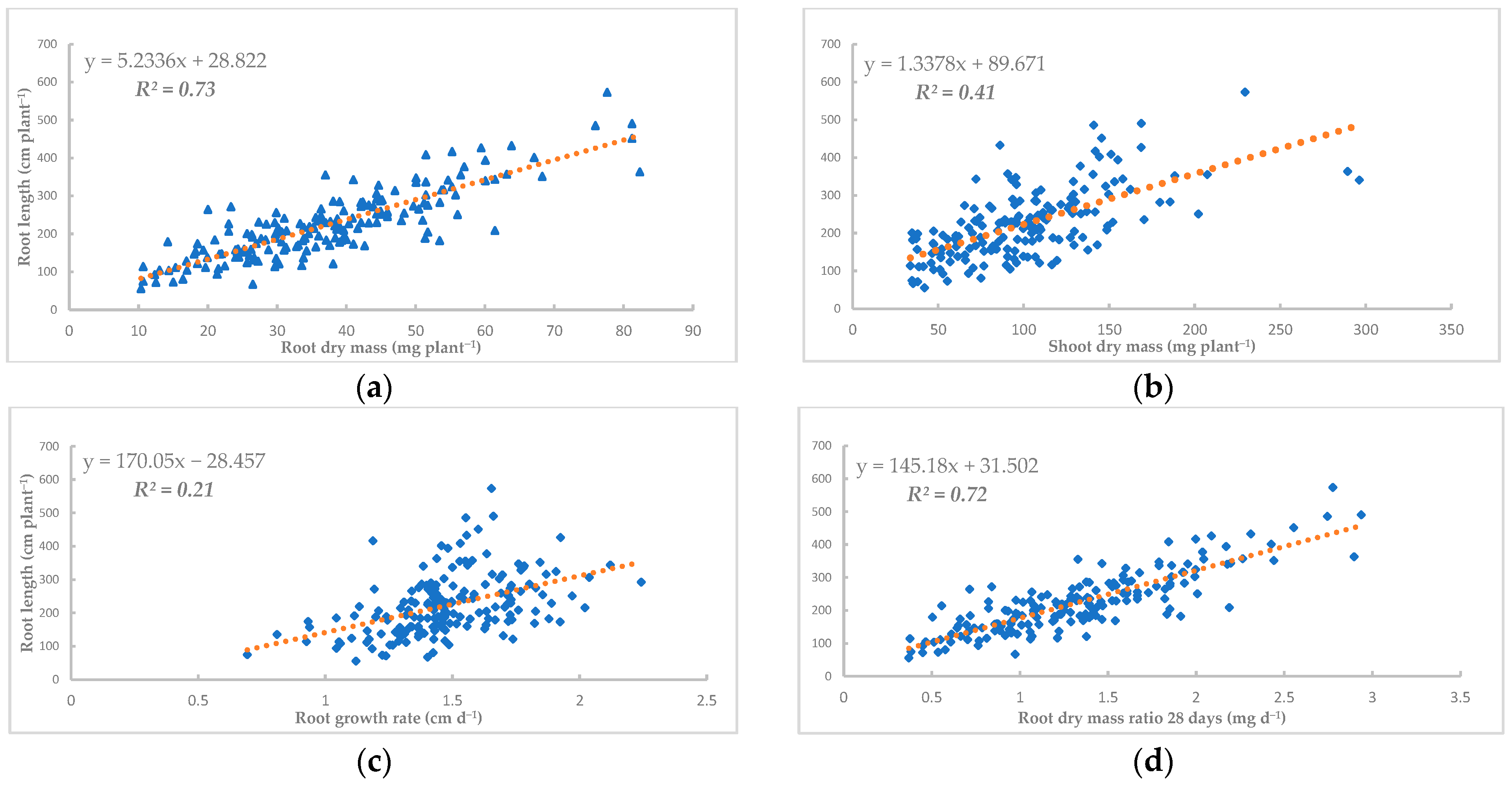
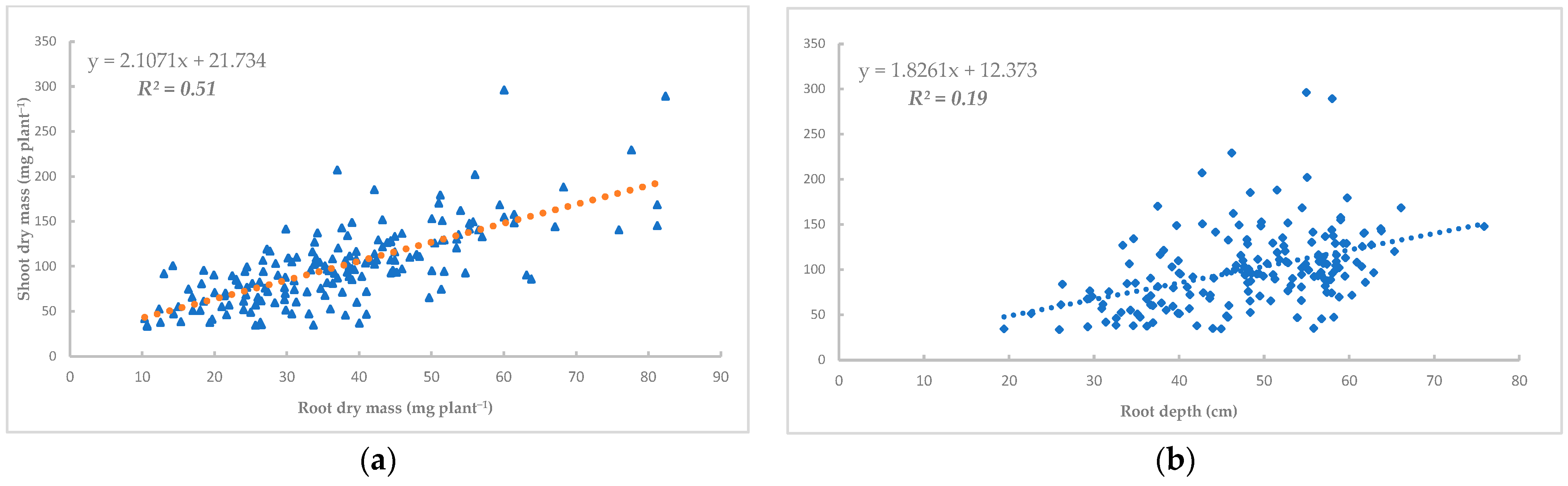
3.3. Correlations Between Different Traits
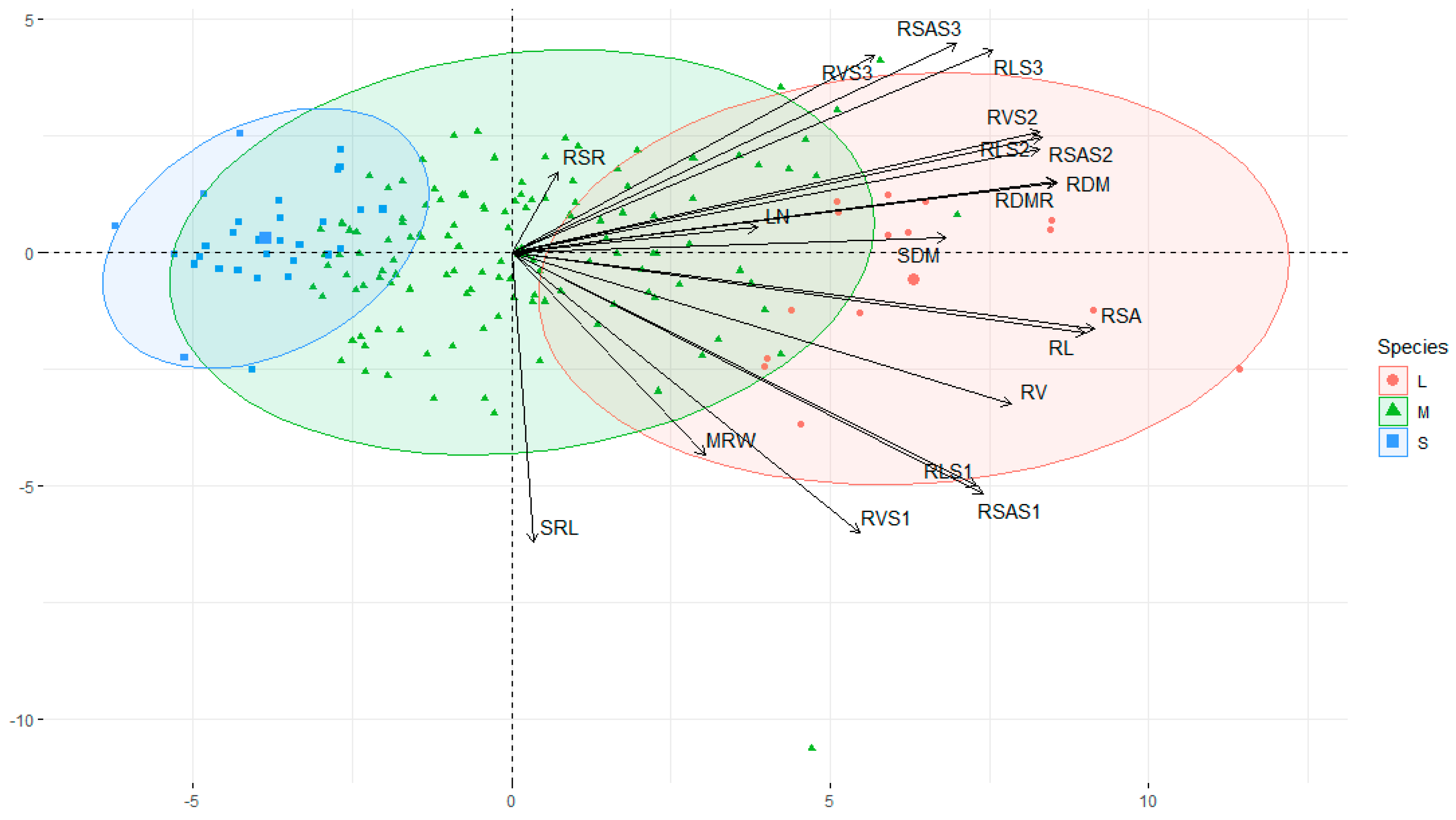
3.4. Principal Component Analysis for High Coefficient of Variation Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirkegaard, J.A.; Lilley, J.M.; Berry, P.M.; Rondanini, D.P. Canola. In Crop Physiology Case Histories for Major Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 518–549. [Google Scholar]
- Kirkegaard, J.A.; Lilley, J.M.; Morrison, M.J. Drivers of trends in Australian canola productivity and future prospects. Crop Pasture Sci. 2016, 67, i–ix. [Google Scholar] [CrossRef]
- Verdon-Kidd, D.; Kiem, A.; Moran, R. Links between the Big Dry in Australia and hemispheric multi-decadal climate variability–implications for water resource management. Hydrol. Earth Syst. Sci. 2014, 18, 2235–2256. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar]
- Porterfield, D.M. Environmental sensing and directional growth of plant roots. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 733–758. [Google Scholar]
- Gao, H.-J.; Yang, H.-Q.; Wang, J.-X. Arginine metabolism in roots and leaves of apple (Malus domestica Borkh.): The tissue-specific formation of both nitric oxide and polyamines. Sci. Hortic. 2009, 119, 147–152. [Google Scholar]
- Yu, Z.; Fredua-Agyeman, R.; Hwang, S.-F.; Strelkov, S.E. Molecular genetic diversity and population structure analyses of rutabaga accessions from Nordic countries as revealed by single nucleotide polymorphism markers. BMC Genom. 2021, 22, 442. [Google Scholar]
- Wang, J.; Kuang, L.; Wang, X.; Liu, G.; Dun, X.; Wang, H. Temporal genetic patterns of root growth in Brassica napus L. revealed by a low-cost, high-efficiency hydroponic system. Theor. Appl. Genet. 2019, 132, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Bengough, A.G.; Grinev, D.; Schmidt, S.; Thomas, W.B.T.; Wojciechowski, T.; Young, I.M. Root phenomics of crops: Opportunities and challenges. Funct. Plant Biol. 2009, 36, 922–929. [Google Scholar] [PubMed]
- Liao, H.; Yan, X.; Rubio, G.; Beebe, S.E.; Blair, M.W.; Lynch, J.P. Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Funct. Plant Biol. 2004, 31, 959–970. [Google Scholar] [CrossRef]
- Nelson, S.K.; Oliver, M.J. A soil-plate based pipeline for assessing cereal root growth in response to polyethylene glycol (PEG)-induced water deficit stress. Front. Plant Sci. 2017, 8, 1272. [Google Scholar]
- Wu, Q.; Wu, J.; Zheng, B.; Guo, Y. Optimizing soil-coring strategies to quantify root-length-density distribution in field-grown maize: Virtual coring trials using 3-D root architecture models. Ann. Bot. 2018, 121, 809–819. [Google Scholar]
- Benjamin, J.; Nielsen, D. A method to separate plant roots from soil and analyze root surface area. Plant Soil 2004, 267, 225–234. [Google Scholar]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.; Rengel, Z. Development of a novel semi-hydroponic phenotyping system for studying root architecture. Funct. Plant Biol. 2011, 38, 355–363. [Google Scholar]
- Chen, Y.; Zhou, T.; Siddique, K.H. Method for characterization of root traits in chickpea germplasm for legume genomics and breeding. In Legume Genomics: Methods and Protocols; Humana: New York, NY, USA, 2020; pp. 269–275. [Google Scholar]
- He, F. Effects of Root Temperature on Food Quality of Horticultural Crops. Ph.D. Thesis, Universitäts-und Landesbibliothek Bonn, Bonn, Germany, 2021. [Google Scholar]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Lynch, J.P. Roots of the second green revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar]
- Hashimoto, M.; Kisseleva, L.; Sawa, S.; Furukawa, T.; Komatsu, S.; Koshiba, T. A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol. 2004, 45, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar]
- Nguyen, V.L.; Luu, H.N.; Phan, T.H.N.; Nguyen, V.L.; Chu, D.H.; Bertero, D.; Curti, N.; McKeown, P.C.; Spillane, C. Genotype by environment interaction across water regimes in relation to cropping season response of quinoa (Chenopodium quinoa). PLoS ONE 2024, 19, e0309777. [Google Scholar]
- Wade, L.J.; Bartolome, V.; Mauleon, R.; Vasant, V.D.; Prabakar, S.M.; Chelliah, M.; Kameoka, E.; Nagendra, K.; Reddy, K.K.; Varma, C.M.K. Environmental response and genomic regions correlated with rice root growth and yield under drought in the OryzaSNP panel across multiple study systems. PLoS ONE 2015, 10, e0124127. [Google Scholar]
- Ayalew, H.; Liu, H.; Yan, G. Identification and validation of root length QTLs for water stress resistance in hexaploid wheat (Titicum aestivum L.). Euphytica 2017, 213, 126. [Google Scholar]
- Wilson, P.; Rebetzke, G.; Condon, A. Pyramiding greater early vigour and integrated transpiration efficiency in bread wheat; trade-offs and benefits. Field Crops Res. 2015, 183, 102–110. [Google Scholar] [CrossRef]
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought response in wheat: Key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef]
- Lynch, J.P.; Wojciechowski, T. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Roumet, C.; Urcelay, C.; Díaz, S. Suites of root traits differ between annual and perennial species growing in the field. New Phytol. 2006, 170, 357–368. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, B.; Ge, X.; Cao, Y.; Brunner, I.; Shi, J.; Li, M.-H. Species-specific responses of root morphology of three co-existing tree species to nutrient patches reflect their root foraging strategies. Front. Plant Sci. 2021, 11, 618222. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Richards, R.; Chatrath, R.; Misra, S.; Prasad, S.S.; Rebetzke, G.; Kirkegaard, J.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Akhatar, J.; Banga, S.S. Genome-wide association mapping for grain yield components and root traits in Brassica juncea (L.) Czern & Coss. Mol. Breed. 2015, 35, 48. [Google Scholar]
- Choi, H.-S.; Cho, H.-T. Root hairs enhance Arabidopsis seedling survival upon soil disruption. Sci. Rep. 2019, 9, 11181. [Google Scholar] [CrossRef]
- Zarzyńska, K.; Boguszewska-Mańkowska, D.; Nosalewicz, A. Differences in size and architecture of the potato cultivars root system and their tolerance to drought stress. Plant Soil Environ. 2017, 63, 159–164. [Google Scholar]
- Pongrac, P.; Castillo-Michel, H.; Reyes-Herrera, J.; Hancock, R.D.; Fischer, S.; Kelemen, M.; Thompson, J.A.; Wright, G.; Likar, M.; Broadley, M.R. Effect of phosphorus supply on root traits of two Brassica oleracea L. genotypes. BMC Plant Biol. 2020, 20, 368. [Google Scholar]
- Ahmed, H.G.M.-D.; Sajjad, M.; Li, M.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H.; Khan, S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar]
- Chen, Y.; Palta, J.; Prasad, P.V.; Siddique, K.H. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar]
- Li, P.; Chen, J.; Wu, P.; Zhang, J.; Chu, C.; See, D.; Brown-Guedira, G.; Zemetra, R.; Souza, E. Quantitative trait loci analysis for the effect of Rht-B1 dwarfing gene on coleoptile length and seedling root length and number of bread wheat. Crop Sci. 2011, 51, 2561–2568. [Google Scholar]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Zaman, M.A.U.; Rahman, M. A comparative study on root traits of spring and winter canola (Brassica napus L.) under controlled and water stressed conditions. J. Agric. Sci. 2017, 9, 58. [Google Scholar]
- Pires, M.V.; de Castro, E.M.; de Freitas, B.S.M.; Lira, J.M.S.; Magalhães, P.C.; Pereira, M.P. Yield-related phenotypic traits of drought resistant maize genotypes. Environ. Exp. Bot. 2020, 171, 103962. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar]
- Curlango-Rivera, G.; Hawes, M.C. Root tips moving through soil: An intrinsic vulnerability. Plant Signal. Behav. 2011, 6, 726–727. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G.; Loades, K.; McKenzie, B.M. Root hairs aid soil penetration by anchoring the root surface to pore walls. J. Exp. Bot. 2016, 67, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L.; Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Yang, C.; Fredua-Agyeman, R.; Hwang, S.-F.; Gorim, L.Y.; Strelkov, S.E. Optimizing the evaluation of root system architectural traits in Brassica napus. Can. J. Plant Sci. 2024, 104, 265–269. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Oladzadabbasabadi, A.; McClean, P.; Rahman, M. Shovelomics for phenotyping root architectural traits of rapeseed/canola (Brassica napus L.) and genome-wide association mapping. Mol. Genet. Genom. 2019, 294, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.; Rengel, Z. Assessing variability in root traits of wild Lupinus angustifolius germplasm: Basis for modelling root system structure. Plant Soil 2012, 354, 141–155. [Google Scholar] [CrossRef]
- Liu, S.; Begum, N.; An, T.; Zhao, T.; Xu, B.; Zhang, S.; Deng, X.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H. Characterization of root system architecture traits in diverse soybean genotypes using a semi-hydroponic system. Plants 2021, 10, 2781. [Google Scholar] [CrossRef]
- Salim, M.; Chen, Y.; Ye, H.; Nguyen, H.T.; Solaiman, Z.M.; Siddique, K.H. Screening of soybean genotypes based on root morphology and shoot traits using the semi-hydroponic phenotyping platform and rhizobox technique. Agronomy 2021, 12, 56. [Google Scholar] [CrossRef]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H. Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy 2018, 8, 109. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, Y.; Zhang, Y.; Ai, Y.; Feng, Y.; Moody, D.; Diggle, A.; Damon, P.; Rengel, Z. Phenotyping and validation of root morphological traits in barley (Hordeum vulgare L.). Agronomy 2021, 11, 1583. [Google Scholar] [CrossRef]
- Sui, J.; Tian, H.; Ding, Z.; Kong, X. Crop Designs: The ideal root architecture for future crop breeding. New Crops 2024, 1, 100030. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 117, 21242–21250. [Google Scholar] [CrossRef]
- Tene, M.; Adhikari, E.; Cobo, N.; Jordan, K.W.; Matny, O.; del Blanco, I.A.; Roter, J.; Ezrati, S.; Govta, L.; Manisterski, J.; et al. GWAS for Stripe Rust Resistance in Wild Emmer Wheat (Triticum dicoccoides) Population: Obstacles and Solutions. Crops 2022, 2, 42–61. [Google Scholar] [CrossRef]
- Potts, J.; Jangra, S.; Michael, V.N.; Wu, X. Speed Breeding for Crop Improvement and Food Security. Crops 2023, 3, 276–291. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef]
- Rana, M.M.; Takamatsu, T.; Baslam, M.; Kaneko, K.; Itoh, K.; Harada, N.; Sugiyama, T.; Ohnishi, T.; Kinoshita, T.; Takagi, H.; et al. Salt Tolerance Improvement in Rice through Efficient SNP Marker-Assisted Selection Coupled with Speed-Breeding. Int. J. Mol. Sci. 2019, 20, 2585. [Google Scholar] [CrossRef]

| Traits | Abbreviations | Trait Description | Units |
|---|---|---|---|
| Shoot dry mass | SDM | Total shoot dry mass per plant | mg |
| Root dry mass | RDM | Total root dry mass per plant | mg |
| Root depth | RD | Total root depth per plant | cm |
| Maximal root width | MRW | Maximal root width per plant | cm |
| Root angle | RA | Root angle | degree |
| Total root length | RL | Root length | cm |
| Root length section 1 | RLS 1 | Root length 0–20 cm | cm |
| Root length section 2 | RLS 2 | Root length 20–40 cm | cm |
| Root length section 3 | RLS 3 | Root length 40 cm and beyond | cm |
| Total Root surface area | RSA | Root surface area | cm2 |
| Root surface area section 1 | RSAS 1 | Root surface area 0–20 cm | cm2 |
| Root surface area section 2 | RSAS 2 | Root surface area 20–40 cm | cm2 |
| Root surface area section 3 | RSAS 3 | Root surface area 40 and beyond | cm2 |
| Total average root diameter | ARD | Average root diameter | mm |
| Average root diameter section 1 | ARDS 1 | Average root diameter 0–20 cm | mm |
| Average root diameter section 2 | ARDS 2 | Average root diameter 20–40 cm | mm |
| Average root diameter section 3 | ARDS 3 | Average root diameter 40 cm and beyond | mm |
| Total root volume | RV | Whole root volume | cm3 |
| Root volume section 1 | RVS 1 | Root volume top 0–20 cm | cm3 |
| Root volume section 2 | RVS 2 | Root volume middle 20–40 cm | cm3 |
| Root volume section 3 | RVS 3 | Root volume bottom 40 cm and beyond | cm3 |
| Specific root length | SRL | Root mass/Root length | cm mg−1 |
| Root–shoot ratio | RSR | Root mass/Shoot mass | |
| Root growth rate | RGR | Root growth rate | cm d−1 |
| Root dry mass ratio | RDMR | Root dry mass ratio | mg d−1 |
| Root length ratio of top 20 cm/total root length | RLR/TRLs | Root length ratio of top 20 cm/Total root length | |
| Leaf number | LN | Number of leaves per plant | |
| Abbreviation | Minimum | Maximum | Mean | Median | Std. Deviation | CV | p |
|---|---|---|---|---|---|---|---|
| SDM | 33.67 | 296.19 | 100.20 | 95.71 | 43.78 | 0.44 | 0.000 |
| RDM | 10.33 | 82.33 | 37.24 | 36.31 | 14.84 | 0.40 | 0.000 |
| RD | 19.40 | 75.88 | 48.09 | 49.24 | 10.36 | 0.22 | 0.000 |
| MRW | 2.56 | 17.96 | 7.10 | 6.60 | 2.59 | 0.36 | 0.000 |
| RA | 61.15 | 128.77 | 98.48 | 99.48 | 12.75 | 0.13 | 0.016 |
| RL | 55.96 | 573.61 | 223.72 | 214.48 | 91.06 | 0.41 | 0.000 |
| SRL 1 | 47.69 | 385.48 | 146.62 | 135.79 | 57.85 | 0.39 | 0.000 |
| RLS 2 | 0.00 | 156.63 | 56.22 | 48.52 | 31.42 | 0.56 | 0.000 |
| RLS 3 | 0.00 | 69.80 | 22.95 | 20.41 | 14.15 | 0.62 | 0.000 |
| RSA | 2.74 | 33.86 | 12.10 | 11.04 | 5.27 | 0.44 | 0.000 |
| RSAS 1 | 2.54 | 24.22 | 7.91 | 7.44 | 3.35 | 0.42 | 0.000 |
| RSAS 2 | 0.00 | 8.91 | 2.97 | 2.61 | 1.73 | 0.58 | 0.000 |
| RSAS 3 | 0.00 | 6.30 | 1.33 | 1.15 | 0.95 | 0.71 | 0.000 |
| ARD | 0.07 | 0.21 | 0.15 | 0.15 | 0.02 | 0.15 | 0.039 |
| RARD 1 | 0.13 | 0.26 | 0.16 | 0.16 | 0.01 | 0.09 | 0.050 |
| ARDS 2 | 0.00 | 0.18 | 0.15 | 0.15 | 0.01 | 0.08 | 0.072 |
| ARDS 3 | 0.00 | 0.35 | 0.16 | 0.16 | 0.03 | 0.19 | 0.102 |
| RV | 0.01 | 0.22 | 0.05 | 0.04 | 0.02 | 0.49 | 0.000 |
| RVS 1 | 0.01 | 0.21 | 0.03 | 0.03 | 0.02 | 0.59 | 0.000 |
| RVS 2 | 0.00 | 0.03 | 0.02 | 0.01 | 0.01 | 0.52 | 0.000 |
| RVS 3 | 0.00 | 0.05 | 0.01 | 0.01 | 0.00 | 0.89 | 0.000 |
| RSR | 0.13 | 1.20 | 0.43 | 0.39 | 0.19 | 0.44 | 0.000 |
| SRL | 2.55 | 13.24 | 6.19 | 6.08 | 2.44 | 0.40 | 0.000 |
| RGR | 0.69 | 2.24 | 1.48 | 1.46 | 0.25 | 0.17 | 0.000 |
| RDMR | 0.37 | 2.94 | 1.32 | 1.29 | 0.53 | 0.40 | 0.000 |
| RLR/TRL | 0.45 | 1.00 | 0.79 | 0.80 | 0.09 | 0.12 | 0.002 |
| LN | 0.50 | 2.67 | 1.50 | 1.50 | 0.46 | 0.30 | 0.000 |
| Trait | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 |
|---|---|---|---|---|---|
| SDM | 0.719 | −0.033 | −0.047 | −0.410 | −0.416 |
| RDM | 0.904 | −0.158 | 0.318 | −0.049 | −0.136 |
| MRW | 0.320 | 0.458 | 0.103 | 0.311 | −0.630 |
| RL | 0.949 | 0.183 | 0.048 | 0.028 | −0.024 |
| RLS1 | 0.770 | 0.529 | 0.169 | 0.044 | −0.085 |
| RLS2 | 0.874 | −0.233 | −0.008 | −0.009 | 0.127 |
| RLS3 | 0.797 | −0.458 | −0.266 | 0.097 | −0.011 |
| RSA | 0.965 | 0.175 | −0.072 | 0.027 | 0.015 |
| RSAS1 | 0.78 | 0.547 | 0.018 | 0.045 | −0.009 |
| RSAS2 | 0.879 | −0.260 | −0.06 | −0.011 | 0.092 |
| RSAS3 | 0.737 | −0.471 | −0.373 | 0.179 | −0.037 |
| RV | 0.827 | 0.344 | −0.081 | 0.041 | 0.269 |
| RVS1 | 0.577 | 0.636 | 0.002 | −0.004 | 0.296 |
| RVS2 | 0.874 | −0.272 | 0.026 | −0.043 | 0.154 |
| RVS3 | 0.602 | −0.445 | −0.399 | 0.288 | −0.014 |
| RSR | 0.076 | −0.182 | 0.661 | 0.624 | 0.281 |
| SRL | 0.036 | 0.657 | −0.56 | 0.119 | 0.209 |
| RDMR | 0.901 | −0.159 | 0.314 | −0.06 | −0.135 |
| LN | 0.407 | −0.057 | 0.271 | −0.587 | 0.298 |
| Variation proportion Eigenvalue | 12.994 | 0.801 | 0.064 | 0.630 | 0.244 |
| Variance (%) | 54.3 | 14.3 | 7.7 | 6.1 | 5.5 |
| Cumulative variability (%) | 54.3 | 68.6 | 76.3 | 82.4 | 87.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Chen, A.; Chen, S.; Chen, Y. Genotypic Variability in Root Morphological Traits in Canola (Brassica napus L.) at the Seedling Stage. Crops 2025, 5, 18. https://doi.org/10.3390/crops5020018
Peng Y, Chen A, Chen S, Chen Y. Genotypic Variability in Root Morphological Traits in Canola (Brassica napus L.) at the Seedling Stage. Crops. 2025; 5(2):18. https://doi.org/10.3390/crops5020018
Chicago/Turabian StylePeng, Yongkang, Andrew Chen, Sheng Chen, and Yinglong Chen. 2025. "Genotypic Variability in Root Morphological Traits in Canola (Brassica napus L.) at the Seedling Stage" Crops 5, no. 2: 18. https://doi.org/10.3390/crops5020018
APA StylePeng, Y., Chen, A., Chen, S., & Chen, Y. (2025). Genotypic Variability in Root Morphological Traits in Canola (Brassica napus L.) at the Seedling Stage. Crops, 5(2), 18. https://doi.org/10.3390/crops5020018







