Use of Phage Cocktail for Improving the Overall Microbiological Quality of Sprouts—Two Methods of Application
Abstract
:1. Introduction
2. Material and Methods
2.1. Total Number of Bacteria (TNB) in Sprouts
2.2. Bacteria Isolation from Sprouts
2.3. The Phages Isolation—A Phage Stock
2.4. The Multiplication of Bacteriophages
2.5. The Lytic Activity
2.6. Phage Titer
2.7. The Resistance to Chloroform
2.8. Rate of Attachment of Phage to Cells
2.9. Single-Step Growth Curve
2.10. Transmission Electron. Microscopy
2.11. Application of the Phage Cocktail to the Analyzed Products
2.12. Statistical Analyses
3. Results
3.1. Microbiological Quality of Sprouts
3.2. Bacteriophages Characteristics
3.3. Effect of Phage Cocktail Method of Application
4. Discussion
4.1. Microbiological Quality of Sprouts
4.2. Characteristic of Bacteriophages
4.3. Effect of Phage Cocktail Application Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermeulen, A.; Devlieghere, F.; Ragaert, P. Optimal packaging design and innovative packaging technologies for minimally processed fresh produce. In Quantitative Methods for Food Safety and Quality in the Vegetable Industry; Pérez-Rodríguez, F., Skandamis, P., Valdramidis, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 193–212. [Google Scholar]
- Lewis, R.; Bolocan, A.S.; Draper, L.A.; Ross, R.P.; Hill, C. The effect of a commercially available bacteriophage and bacteriocin on Listeria monocytogenes in Coleslaw. Viruses 2019, 11, 977. [Google Scholar] [CrossRef] [Green Version]
- Barak, J.; Whitehand, L.; Charkowski, A. Differences in Attachment of Salmonella enterica Serovars and Escherichia coli O157:H7 to Alfalfa Sprouts. Appl. Environ. Microb. 2002, 68, 4758–4763. [Google Scholar] [CrossRef] [Green Version]
- Keshri, J.; Krouptiski, Y.; Abu-Fani, L.; Achmon, Y.; Bauer, T.S.; Zarka, O.; Maler, I.; Pinto, R.; Saldinger, S.S. Dynamics of bacterial communities in alfalfa and mung bean sprouts during refrigerated conditions. Food Microbiol. 2019, 84, 103261. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; MacKinon, L.C.; Goulding, J.S.; Slutsker, L. Surveillance for foodborne disease outbreaks–United States, 1993–1997. Morb. Mortal. Wkly. Rep. 2000, 49, 1–51. [Google Scholar]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microb. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Liu, H.K.; Li, Z.H.; Zhang, X.W.; Liu, Y.P.; Hu, J.P.; Yang, C.W.; Zhao, X.Y. The effects of ultrasound on the growth, nutritional quality and microbiological quality of sprouts. Trends Food Sci. Technol. 2021, 111, 292–300. [Google Scholar] [CrossRef]
- FUSION. Reducing Food Waste through Social Innovation. Estimates of European Food Waste Levels; FUSION: Stockholm, Sweden, 2016. [Google Scholar]
- Kumara, S.; Gautam, S. A combination process to ensure microbiological safety, extend storage life and reduce anti-nutritional factors in legume sprouts. Food Biosci. 2019, 27, 18–29. [Google Scholar] [CrossRef]
- Lund, B.M. Bacterial spoilage. In Post Harvest Pathology of Fruits and Vegetables; Dennis, C., Ed.; Academic Press: London, UK, 1983; pp. 219–257. [Google Scholar]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcicki, M.; Błażejak, S.; Gientka, I.; Brzezicka, K. The concept of using bacteriophages to improve the microbiological quality of minimally-processed foods. Acta Sci. Pol. Technol. Aliment. 2019, 18, 373–383. [Google Scholar] [CrossRef]
- Lewis, R.; Hill, C. Overcoming barriers to phage application in food and feed. Curr. Opin. Biotechnol. 2020, 61, 38–44. [Google Scholar] [CrossRef]
- Microbiology of Food and Animal Feeding Stuffs. Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination–Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. International Standard 2003, ISO 6887-1. Available online: https://www.iso.org/standard/63335.html (accessed on 10 May 2021).
- Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO 2013, 4833–1. Available online: https://www.iso.org/standard/53728.html (accessed on 22 April 2021).
- Kropinski, M.A.; Mazzocco, A.; Waddell, T.E.; Linghor, E.; Johnson, R.P. Enumeration of Bacteriophages by Double Agar Overlay Plaque Assay. In Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions; Clokie, M.R.J., Kropinski, A., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501. [Google Scholar]
- Mirzaei, M.K.; Nilsson, A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Lee, J.H.; Shin, H.; Kim, M.; Choi, J.; Kang, D.H.; Heu, S.; Ryu, S. Characterization and comparative genomic analysis of a novel bacteriophage, SFP10, simultaneously inhibiting both Salmonella enterica and Escherichia coli O157:H7. Appl. Environ. Microbiol. 2012, 78, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Deveau, J.; Labrie, S.J.; Chopin, M.C.; Moineau, S. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 2006, 72, 4338–4346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, H.-W. Basic Phage Electron Microscopy. In Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions; Clokie, M.R.J., Kropinski, A., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501. [Google Scholar]
- Nguyen-the, C.; Carlin, F. The microbiology of minimally processed fresh fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1994, 34, 371–401. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, M.; Torriano, S.; Orsi, C.; Macchiarolo, F.; Scolari, G. Application of antimicrobial-producing lactic acid bacteria to control pathogens in ready-to-use vegetables. J. Appl. Bacteriol. 1996, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W. 5500 Phages examined in the electron microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Tavares, P. The Bacteriophage Head-to-Tail Interface. In Virus Protein and Nucleoprotein Complexes. Subcellular Biochemistry; Harris, J., Bhella, D., Eds.; Springer: Singapore, 2018; Volume 88. [Google Scholar] [CrossRef]
- King, M.Q.A.; Adams, M.J.; Carstens, E.B.; Lefkowitz, J.E. Virus Taxonomy Classification and Nomenclature of Viruses Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Oxford, UK, 2012. [Google Scholar]
- De Paepe, M.; Taddei, F. Viruses’ Life History: Towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol. 2006, 4, e0040193. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, M.G.; Peduzzi, P. Frequency, size and distribution of bacteriophages in different marine bacterial morphotypes. Marine Ecol. Prog. 1994, 108, 11–20. [Google Scholar] [CrossRef]
- Gabriel, A.A.; Berja, M.C.; Estrada, A.P.; Lopez, M.A.; Nery, J.B.; Villaflor, E.B. Microbiology of retail mung bean sprouts vended in public markets of National Capital Region, Philippines. Food Control 2007, 18, 1307–1313. [Google Scholar] [CrossRef]
- Michalczyk, M.; Kowalińska, J. Zanieczyszczenie mikrobiologiczne kiełkowalnych nasion dostępnych w handlu. Żywność. Nauka. Technol. Jakość 2009, 3, 32–39. [Google Scholar]
- Lewicki, P.P. Kiełki nasion jako źródło cennych składników odżywczych. Żywność. Nauka. Technol. Jakość 2010, 73, 18–33. [Google Scholar]
- Seo, Y.H.; Jang, J.H.; Moon, K.D. Microbial evaluation of minimally processed vegetables and sprouts produced in Seoul, Korea. Food Sci. Biotechnol. 2010, 19, 1283–1288. [Google Scholar] [CrossRef]
- Althaus, D.; Hofer, E.; Corti, S.; Julmi, A.; Stephan, R. Bacteriological survey of ready-to-eat lettuce, fresh-cut fruit, and sprouts collected from the Swiss market. J. Food Prot. 2012, 75, 1338–1341. [Google Scholar] [CrossRef]
- Kim, S.A.; Kim, O.M.; Rhee, M.S. Changes in microbial contamination levels and prevalence of foodborne pathogens in alfalfa (Medicago sativa) and rapeseed (Brassica napus) during sprout production in manufacturing plants. Lett. Appl. Microbiol. 2013, 56, 30–36. [Google Scholar] [CrossRef]
- D’Sa, E.; Paulin, S.; Anderson, G.; Xavier, R.; Castle, M.; Cook, R.A. Microbiological Survey of Seed Sprouts (and Shoots) Available in New Zealand MPI Technical Report-Paper. 2015. Available online: https://www.mpi.govt.nz/dmsdocument/12939/direct (accessed on 15 April 2021).
- Iacumin, L.; Comi, G. Microbial quality of raw and ready-to-eat mung bean sprouts produced in Italy. Food Microbiol. 2019, 82, 371–377. [Google Scholar] [CrossRef]
- Leszczyńska-Fik, A.; Fik, M. Kiełki roślinne: Jakość mikrobiologiczna skiełkowanych nasion. Przem. Ferm. Owo-Warz. 2003, 47, 29–31. [Google Scholar]
- Buck, J.W.; Walcott, R.R.; Beuchat, L.R. Recent trends in microbiological safety of fruits and vegetables. Online. Plant Health 2003, 4. [Google Scholar] [CrossRef] [Green Version]
- Wójcicki, M.; Żuwalski, A.; Gientka, I.; Błażejak, S. Ocena Jakości Mikrobiologicznej Żywności Minimalnie Przetworzonej Podczas Przechowywania. In Problematyka nauk technicznych i przyrodniczych-Tom 1, XXII Międzynarodowa Konferencja Studenckich Kół Naukowych; Dyjakon, A., Krzyś, A., Eds.; Uniwersytet Przyrodniczy we Wrocławiu: Wroclaw, Poland, 2017. [Google Scholar]
- Jang, M.I.; Kim, S.Y.; Ricka, S.C.; Rhee, M.S.; Kim, S.A. Microbial ecology of alfalfa, radish, and rapeseed sprouts based on culture methods and 16S rRNA microbiome sequencing. Food Res. Int. 2021, 144. [Google Scholar] [CrossRef] [PubMed]
- Fett, W.F. Intervetions to ensure the microbial safety of sprouts. In Microbiology of Fruits and Vegetables; Sapers, G.M., Gorny, J.R., Yousef, A.E., Eds.; Taylor & Francis: Bocaraton, FL, USA, 2006; pp. 187–209. [Google Scholar]
- Seow, J.; Agoston, R.; Phua, L.; Yuk, H.G. Microbiological quality of fresh vegetables and fruits sold in Singapore. Food Control 2012, 25, 39–44. [Google Scholar] [CrossRef]
- Robertson, L.J.; Johannessen, G.S.; Gjerde, B.K.; Loncarevic, S. Microbiological analysis of seed sprouts in Norway. Int. J. Food 2002, 75, 119–126. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Garbowska, M.; Stefańska, I.; Pluta, A. Microbiological quality of selected ready-to-eat leaf vegetables, sprouts and non-pasteurized fresh fruit-vegetable juices including the presence of Cronobacter spp. Food Microb. 2017, 65, 221–230. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Kłak, M.; Jończyk-Matysiak, E.; Bubak, B.; Wójcik, A.; Kaszowska, M.; Weber-Dąbrowska, B.; Łobocka, M.; Górski, A. Means to facilitate the overcoming of gastric juice barier by a therapeutic staphylococcal bacteriophage A5/80. Front. Microbiol. 2017, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.; Carrascosa, J.L. Bacteriophages: Structure; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 1–12. [Google Scholar] [CrossRef]
- Prigent, M.; Leroy, M.; Confalonieri, F.; Dutertre, M.; DuBow, M.S. A diversity of bacteriophages forms and genomes can be isolated from the surface sands of Sahara Desert. Extremophiles 2005, 9, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lasobras, J.; Muniesa, M.; Lucena, F.; Jofre, J. Relationship between the morphology of bacteriophages and their persistence in the environment. Water Sci. Technol. 1997, 35, 129–132. [Google Scholar] [CrossRef]
- Ackermann, H.W.; Tremblay, D.; Moineau, S. Long-term bacteriophage preservation. WFCC Newslett. 2004, 38, 35–40. [Google Scholar]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Kannoly, S.; Wang, I.N. Effects of bacteriophage traits on plaque formation. BMC Microbiol. 2011, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Evaluation of the safety and efficacy of Listex P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, e04565. [Google Scholar]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Control. 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Beuchat, L.R. Comparison of chemical treatments to kill Salmonella on alfalfa seeds destined for sprout production. Int. J. Food Microbiol. 1997, 34, 329–333. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Soffer, N.; Woolston, J.; Li, M.; Das, C.; Sulakvelidze, A. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS ONE 2017, 12, e0175256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, K.; Cazarez-Montoya, C.; Lopez-Moreno, H.S.; Castro-del Campo, N. Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PLoS ONE 2018, 13, e0195023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, A.P.; Ehrenfeld, E.E. Microbial loads of bean sprouts: Coliform, E. coli and Salmonella. In Proceedings of the 98th General Meeting of the American Society for Microbiology, Atlanta, GA, USA, 17–21 May 1998. [Google Scholar]
- Puligundla, P.; Kim, J.W.; Mok, C. Broccoli sprout washing with electrolyzed water: Effects on microbiological and physicochemical characteristics. LWT Food Sci. Technol. 2018, 92, 600–606. [Google Scholar] [CrossRef]
- Worley-Morse, T.O.; Zhang, L.; Gunsch, C.K. The long-term effects of phage concentration on the inhibition of planktonic bacterial cultures. Environ. Sci. Process. Impacts 2013, 16, 81–87. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24301469 (accessed on 20 April 2021). [CrossRef]
- Hooton, S.P.; Atterbury, R.J.; Connerton, I.F. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 2011, 151, 157–163. [Google Scholar] [CrossRef]
- Bigwood, T.; Hudson, J.A.; Billington, C. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol. Lett. 2010, 291, 59–64. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Honjoh, K.I.; Miyamoto, T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT 2018, 91, 353–360. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepúlveda, D.R.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Sáenz-Mendoza, A.I.; Acosta-Muñiz, C.H. The role of food compounds and emerging technologies on phage stability. Innov. Food Sci. Emerg. Technol. 2020, 64, 102436. [Google Scholar] [CrossRef]
| Sprouts Type | log CFU/g | ||
|---|---|---|---|
| 4 °C | 20 °C | 30 °C | |
| Alfalfa | 6.92 ± 0.22 a | 8.18 ± 0.12 b,c | 8.14 ± 0.39 b,c |
| Kale | 7.31 ± 0.16 b | 8.01 ± 0.25 b,c | 8.06 ± 0.23 b,c |
| Lentil | 7.42 ± 0.13 b | 7.69 ± 0.20 b | 7.67 ± 0.25 b |
| Sunflower | 6.20 ± 0.15 a | 7.00 ± 0.01 b | 6.62 ± 0.03 a |
| Radish | 6.11 ± 0.22 a | 6.96 ± 0.30 a,b | 7.03 ± 0.40 b |
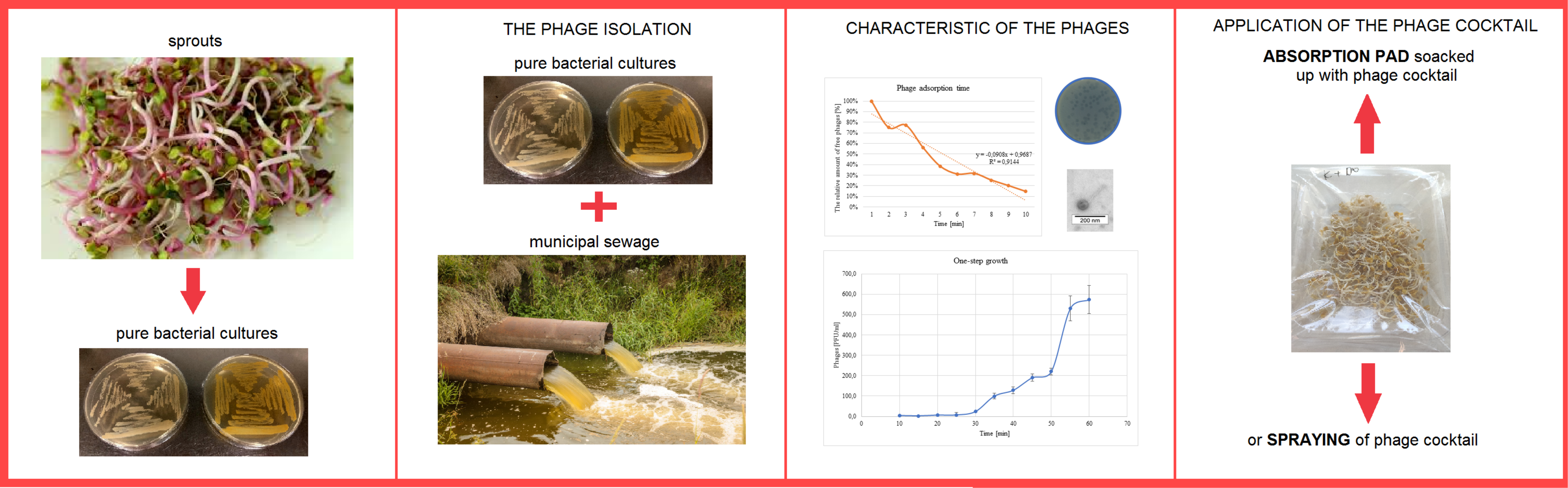
| Phage Name | TEM Photography | Family of Bacteriophages | Originating of the Target Bacteria | Morphology of Plaque |
|---|---|---|---|---|
| bAs |  |  Siphoviridae | Alfalfa Sprouts G(+) |  |
| bKs |  | 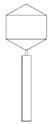 Siphoviridae | Kale Sprouts G(+) | 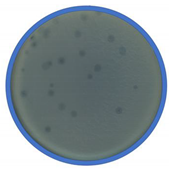 |
| bLs | 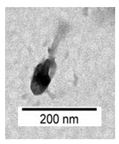 | 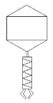 Myoviridae | Lentil Sprouts G(−) | 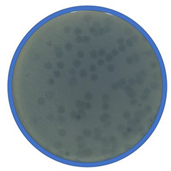 |
| bSs |  |  Siphoviridae | Sunflower Sprouts G(+) |  |
| bRs |  | 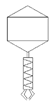 Myoviridae | Radish sprouts G(−) |  |
| Phage | Resistance to Chloroform | Rate of Attachment (k) | Adsorption Time | Latency Time | Burst Size | |
|---|---|---|---|---|---|---|
| a | b | |||||
| log PFU/mL | log PFU/mL | mL/min | min | min | ||
| bAs | 9.15 ± 0.10 | 6.24 ± 0.11 | 4.86 × 10−9 | 4 | 21 | 135 |
| bKs | 7.73 ± 0.05 | 7.29 ± 0.01 | 1.61 × 10−9 | 8 | 22 | 27 |
| bLs | 7.49 ± 0.22 | 7.95 ± 0.31 | 7.53 × 10−10 | 10 | 20 | 50 |
| bSs | 9.58 ± 0.10 | 9.45 ± 0.05 | 3.48 × 10−9 | 4 | 26 | 79 |
| bRs | 8.02 ± 0.51 | 8.71 ± 0.31 | 1.25 × 10−9 | 8.5 | 27 | 7 |
| Sprouts | Time h | Control (c) | Control (a) | Spraying (A) | Control (b) | Adsorption Pad (B) | ||
|---|---|---|---|---|---|---|---|---|
| log cfu/g | log cfu/g | log cfu/g | Reduction Level (c-A) | log cfu/g | log cfu/g | Reduction Level (c-B) | ||
| Alfalfa | 0 | 8.35 ± 0.12 a | 8.35 ± 0.12 a | 8.35 ± 0.12 a | 8.35 ± 0.12 a | 8.35 ± 0.12 a | ||
| 24 | 9.81 ± 0.48 b | n.d. | 9.56 ± 0.08 b | Δ24 = 0.25 | n.d. | 9.84 ± 0.09 b | Δ24 = 0.03↑ | |
| 48 | 9.89 ± 0.20 b | 9.83 ± 0.31 b | 9.60 ± 0.10 b | Δ48 = 0.29 | 9.69 ± 0.11 b | 9.38 ± 0.08 b | Δ48 = 0.51 | |
| Kale | 0 | 8.35 ± 0.10 a | 8.35 ± 0.10 a | 8.35 ± 0.10 a | 8.35 ± 0.10 a | 8.35 ± 0.10 a | ||
| 24 | 9.11 ± 0.39 b | n.d. | 8.82 ± 0.12 a | Δ24 = 0.29 | n.d. | 8.92 ± 0.11 a,b | Δ24 = 0.19 | |
| 48 | 9.37 ± 0.20 b | 9.52 ± 0.15 b | 8.27 ± 0.19 a | Δ48 = 1.10 | 9.22 ± 0.14 b | 8.97 ± 0.05 a,b | Δ48 = 0.40 | |
| Lentil | 0 | 7.83 ± 0.14 a | 7.83 ± 0.14 a | 7.83 ± 0.14 a | 7.83 ± 0.12 a | 7.83 ± 0.14 a | ||
| 24 | 8.41 ± 0.03 b | n.d. | 8.27 ± 0.29 a,b | Δ24 = 0.14 | n.d. | 7.89 ± 0.14 a | Δ24 = 0.52 | |
| 48 | 8.92 ± 0.02 b | 9.21 ± 0.02 c | 8.53 ± 0.02 b | Δ48 = 0.39 | 8.95 ± 0.12 b | 8.62 ± 0.19 b | Δ48 = 0.30 | |
| Sunflower | 0 | 7.07 ± 0.12 a | 7.07 ± 0.12 a | 7.07 ± 0.12 a | 7.07 ± 0.12 a | 7.07 ± 0.12 | ||
| 24 | 8.35 ± 0.21 c | n.d. | 7.68 ± 0.21 b | Δ24 = 0.67 | n.d. | 7.56 ± 0.20 b | Δ24 = 0.79 | |
| 48 | 8.61 ± 0.12 c | 8.48 ± 0.09 c | 8.02 ± 0.05 c | Δ48 = 0.59 | 8.73 ± 0.12 c | 8.34 ± 0.10 c | Δ48 = 0.27 | |
| Radish | 0 | 8.26 ± 0.14 b | 8.26 ± 0.12 b | 8.26 ± 0.14 b | 8.26 ± 0.12 b | 8.26 ± 0.14 b | ||
| 24 | 9.05 ± 0.10 c | n.d. | 7.92 ± 0.02 a | Δ24 = 1.13 | n.d. | 8.97 ± 0.09 b | Δ24 = 0.08 | |
| 48 | 8.99 ± 0.02 c | 8.84 ± 0.12 b,c | 7.49 ± 0.29 a | Δ48 = 1.50 | 8.82 ± 0.06 b,c | 8.81 ± 0.08 b,c | Δ48 = 0.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gientka, I.; Wójcicki, M.; Żuwalski, A.W.; Błażejak, S. Use of Phage Cocktail for Improving the Overall Microbiological Quality of Sprouts—Two Methods of Application. Appl. Microbiol. 2021, 1, 289-303. https://doi.org/10.3390/applmicrobiol1020021
Gientka I, Wójcicki M, Żuwalski AW, Błażejak S. Use of Phage Cocktail for Improving the Overall Microbiological Quality of Sprouts—Two Methods of Application. Applied Microbiology. 2021; 1(2):289-303. https://doi.org/10.3390/applmicrobiol1020021
Chicago/Turabian StyleGientka, Iwona, Michał Wójcicki, Aleksander W. Żuwalski, and Stanisław Błażejak. 2021. "Use of Phage Cocktail for Improving the Overall Microbiological Quality of Sprouts—Two Methods of Application" Applied Microbiology 1, no. 2: 289-303. https://doi.org/10.3390/applmicrobiol1020021
APA StyleGientka, I., Wójcicki, M., Żuwalski, A. W., & Błażejak, S. (2021). Use of Phage Cocktail for Improving the Overall Microbiological Quality of Sprouts—Two Methods of Application. Applied Microbiology, 1(2), 289-303. https://doi.org/10.3390/applmicrobiol1020021








